Significance
The importance of antigenic peptides with low-affinity HLA binding in human autoimmune disease remains unclear. Studies in the nonobese diabetic mouse demonstrate recognition of a crucial insulin epitope presented in a weakly bound register. This work details a direct study of responses to this insulin B-chain peptide in humans. Responses were readily detected in subjects with type 1 diabetes. Furthermore, T-cell clones were shown to recognize the peptide presented in a weakly bound alternative register. These findings confirm the relevance of immune recognition of this segment of the insulin B-chain in human disease and highlight a mechanism shared by mouse and man through which T cells that recognize a weakly bound peptide can circumvent tolerance mechanisms.
Keywords: antigen presentation, self-antigen, MHCII tetramers
Abstract
Previous studies in type 1 diabetes (T1D) in the nonobese diabetic mouse demonstrated that a crucial insulin epitope (B:9-23) is presented to diabetogenic CD4 T cells by IAg7 in a weakly bound register. The importance of antigenic peptides with low-affinity HLA binding in human autoimmune disease remains less clear. The objective of this study was to investigate T-cell responses to a low-affinity self-epitope in subjects with T1D. HLA-DQ8 tetramers loaded with a modified insulin peptide designed to improve binding the low-affinity register were used to visualize T-cell responses following in vitro stimulation. Positive responses were only detectable in T1D patients. Because the immunogenic register of B:9-23 presented by DQ8 has not been conclusively demonstrated, T-cell assays using substituted peptides and DQ8 constructs engineered to express and present B:9-23 in fixed binding registers were used to determine the immunogenic register of this peptide. Tetramer-positive T-cell clones isolated from T1D subjects that responded to stimulation by B:11-23 peptide and denatured insulin protein were conclusively shown to recognize B:11-23 bound to HLA-DQ8 in the low-affinity register 3. These T cells also responded to homologous peptides derived from microbial antigens, suggesting that their initial priming could occur via molecular mimicry. These results are in accord with prior observations from the nonobese diabetic mouse model, suggesting a mechanism shared by mouse and man through which T cells that recognize a weakly bound peptide can circumvent tolerance mechanisms and play a role in the initiation of autoimmune diseases, such as T1D.
Type 1 diabetes (T1D) is a polygenic T-cell–mediated autoimmune disease with strong genetic linkages to the MHC class II and insulin promoter regions (1). Class II molecules are fundamental for CD4+ T-cell activation, whereas the insulin promoter polymorphism can modulate insulin levels in the thymus thereby influencing the threshold of selection for insulin-specific autoreactive T cells (2, 3). In the nonobese diabetic (NOD) mouse model of autoimmune mediated diabetes, insulin-specific IAg7-restricted CD4+ T cells have been strongly implicated in β-cell destruction. In prediabetic NOD mice, 50% of the T-cell clones established from islet-infiltrating lymphocytes were insulin-specific and the majority of these clones recognized the insulin B:9-23 (B:9-23 SHLVEALYLVCGERG) epitope (4, 5). Moreover, substitution of a single residue in the B:9-23 region abrogated development of diabetes in a transgenic mouse model (6, 7). Thus, in the murine NOD model, the IAg7-restricted B:9-23 epitope is considered to be pivotal in the development of diabetes.
The position or “register” that B:9-23 occupies within the IAg7 binding groove has been a controversial but important question. At least three binding registers (R) have been considered, defined here by which B:9-23 amino acid occupies the first binding pocket (p1) position in the IAg7 binding groove: R1, p1 V12; R2, p1 E13; and R3, p1 A14. Each binding register creates a unique set of upward pointing amino acid side-chains for T-cell recognition and also exhibits a distinct binding affinity for MHC class II conferred by the downward-facing amino acids that occupy its binding pockets. The Unanue group first reported that B:9-23 can be presented by IAg7 in either R1 or R2 and that each register is recognized by a different set of B:9-23–spepcifc CD4+ T cells (8). In contrast, a separate study (9, 10) reported that T cells recognize the B:9-23 peptide bound to IAg7 in R3, the weakest binding of the three registers. Mutation of R22 to the highly p9 favorable E greatly improved the binding of the peptide to IAg7 and its presentation to CD4 T cells. Disulfides introduced between the peptide and IAg7 confirmed the R3 binding. These data support the relevance of antigenic peptides with low-affinity HLA binding in the NOD model of diabetes.
In humans, the HLA-DQB1*0302 allele confers high disease risk. The DQ8 molecule, which is comprised of the DQA1*0301 and DQB1*0302 chains, is the human homolog for IAg7. In particular, both IAg7 and DQ8 have a basic pocket 9 (p9, because of having a non-Asp β57 residue in the class II β-chain) that accommodates and is stabilized by peptides with acidic residues at p9 (11). Insulin autoreactive antibodies precede the onset of disease and have been used for risk stratification in susceptible individuals (12, 13). CD8+ T cells that are specific for insulin have also been detected (14, 15), and it has been documented that they are capable of lysing islets (16, 17) and there is one report of human CD4+ T cells responsive to an insulin A-chain peptide presented by HLA-DR4 (18). These data all suggest that insulin is also an important autoantigen in human T1D.
In the light of the accumulated data from both murine and human studies, it is logical to postulate that DQ8-restricted B:9-23–specific CD4+ T cells are present in human subjects with T1D and that this peptide may also be recognized in a weakly bound register. Although recognition of B:9-23 has long been suspected, direct evidence for the presence and importance of DQ8-restricted B:9-23–specific CD4+ T cells in human subjects with T1D is limited and no study has definitively established the immunogenic register of B:9-23 as presented by DQ8. Alleva et al. showed that T cells from the peripheral blood of DR4-DQ8 T1D subjects had measurable proliferative and IFN responses to the B:9-23 peptide (19). In addition, the proliferation of a B:9-23–specific T-cell line could be blocked through addition of an anti-DQ Ab (19). Eerligh et al. also reported the isolation of a DQ8 restricted Ins B:6-22 T-cell clone from a T1D subject (20). However, more detailed analysis of these responses, for example by direct visualization and cloning of multiple DQ8/B:9-23–specific cells, has remained a desirable but elusive goal at least in part because of the technical challenge of interrogating DQ8 restricted autoreactive T cells.
In the current study, our objective was to extend these previous observations by visualizing DQ8-restricted T-cell responses to B:9-23 in human subjects with T1D and determining the immunogenic register of the DQ8/B:9-23 complex. In particular, we wished to investigate responses to B:9-23 as a possible example of T-cell responses to a self-epitope with low-affinity MHC binding. We used DQ8/insulin tetramers to detect B:11-23–specific T cells in peripheral blood samples from T1D subjects and compared the frequency of these responses in T1D subjects and healthy controls with DQ8 haplotypes. After directly cloning these T cells, we investigated their responsiveness to stimulation with insulin peptides, denatured insulin protein, and homologous peptides derived from bacterial antigens. By assessing proliferation in response to peptides with alanine or phenylalanine substitutions, we identified a clear immunogenic register within B:11-23 that is bound by DQ8 with low affinity and recognized by human T cells.
Results
DQ8-Restricted Responses to B:11-23 Are Detectable in Subjects with T1D but Not Healthy Controls.
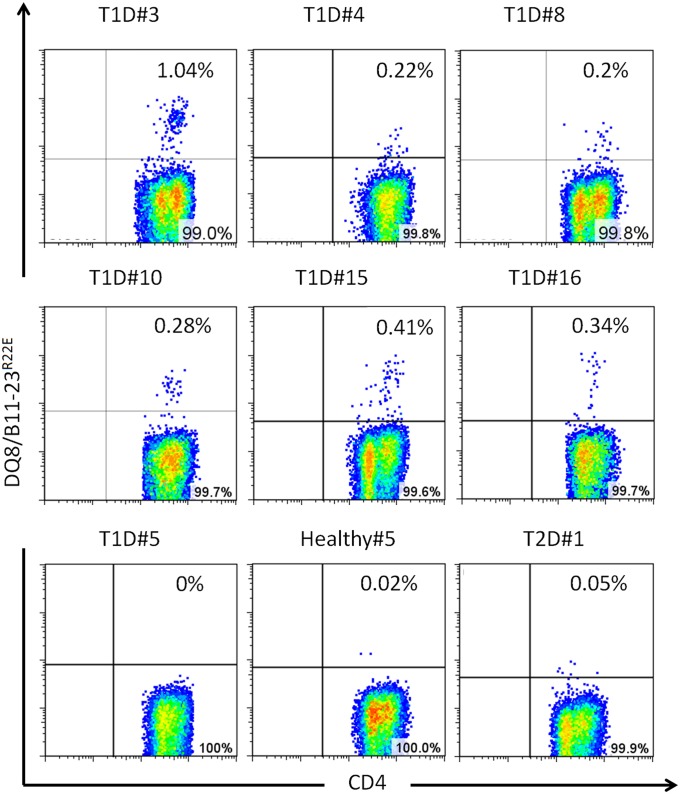
We first used an in vitro tetramer assay to directly visualize DQ8-restricted T-cell responses to insulin B:11-23 in subjects with T1D and controls. A B:9-23 peptide with a R22E substitution (B:9-23R22E) was previously shown to be more potent in activating a set of B:9-23 hybridomas from NOD mice compared with the wild-type B:9-23 peptide (9, 10). This modification enhances binding to IAg7 in the R3 register by about 50-fold (9). Because the human and mouse B:11-23 amino acid sequences are identical, we used an HLA-DQ8 tetramer loaded with the B:11-23R22E peptide to enhance expansion and detection of responsive T cells. Samples from 16 T1D patients, 10 healthy controls, and 2 control subjects with type 2 diabetes (who needed daily insulin injections)—all with DQ8 haplotypes—were evaluated for insulin responses. Peripheral blood mononuclear cell from these subjects were stimulated with either B:11-23R22E or B:11-23 peptide. The cells were cultured for 2 wk and assayed for the presence of B:11-23–specific T cells using DQ8/B:11-23 and DQ8/B:11-23R22E tetramers. Specific responses as assayed by DQ8/B:11-23R22E tetramers were significantly more prevalent in subjects with diabetes than in healthy controls, with detectable responses in 6 of 16 T1D subjects (Fig. 1) and 0 of 12 HLA-DQ8–matched non-T1D controls (Fisher’s exact test, P = 0.0237). Among the positive responses, Five were from B:11-23R22E stimulation and one from B:11-23 stimulation. Staining results for DQ8/B:11-23 tetramer were negative in this set of experiments, suggesting that the R22E substitution is necessary to produce an effective tetramer. Responses in DQ8+ control subjects with type 2 diabetes were negative, suggesting that injection with exogenous insulin was not sufficient to elicit a response to B:11-23R22E stimulation. Interestingly, five of the six subjects who responded to B:11-23 had the susceptible VNTR 1/1 genotype that has been associated with reduced insulin levels in the thymus (2) (Table S1).
Fig. 1.
Detection of insulin-specific T cells following in vitro expansion. CD4+ T cells from T1D patients, type 2 diabetes (T2D) patients, and healthy control subjects with DQ8 haplotypes were individually stimulated with B:11-23 or modified B:11-23R22E for 14 d. Antigen-specific T cells were then detected by staining with HLA-DQ8/B:11-23R22E tetramer. Each panel depicts a representative subject (patient ID listed above each panel) which had a positive tetramer staining result. Cells from subject T1D#10 were stimulated with B:11-23 peptide and the rest were stimulated with B:11-23R22E. Subjects T1D#5, Healthy#5, and T2D#1 provide examples of negative tetramer staining.
Characterization of DQ8-Restricted Insulin-Reactive CD4 T Cells.
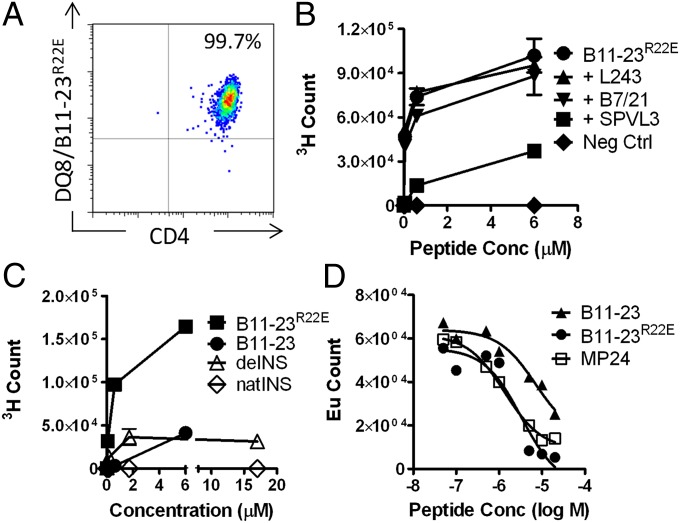
A total of 26 T-cell clones were obtained from five different subjects with T1D by single-cell sorting and expanding of DQ8/B:11-23R22E tetramer-positive cells. Eight of these clones were used for subsequent experiments. Fig. 2 shows representative results for one clone (results for additional clones were all similar). T-cell clones stained brightly with tetramer (Fig. 2A) and proliferated vigorously in response to B:11-23R22E peptide (Fig. 2 B and C). That proliferation was inhibited by anti-DQ blocking antibody (SPVL3), indicating an HLA-DQ–restricted response (Fig. 2B). Functional analysis of the clones by intracellular cytokine staining indicated that IFN-γ, IL-2, and TNF-α were the predominant cytokines produced (Fig. S1 and Table S2). Several clones also produced lesser amounts of IL-13 and IL-4, a few clones also had some IL-10, and one clone was positive for IL-5. The clones responded to stimulation with B:11-23 peptide and denatured insulin (deINS) protein (Fig. 2C). deINS delivered a stronger stimulatory activity at 1.7 μM than at 17 μM, probably because protein aggregates were inhibitory or toxic at 17-μM concentration (Fig. 2C). The clones failed to proliferate in response to native insulin protein (Fig. 2C). The responsiveness of eight different clones from four different subjects to B:11-23R22E peptide, B:11-23 peptide, and deINS protein are summarized in Table 1. These data demonstrate that T cells isolated with DQ8/B:11-23R22E tetramer are also specific for wild-type insulin B:11-23 peptide and respond to deINS. The stimulation index for clones stimulated with B:11-23 was approximately sevenfold lower than clones stimulated with an equal concentration of B:11-23R22E peptide. This finding is in agreement with our observation that B:11-23R22E (IC50 = 1.6 nM) has a sevenfold higher affinity than B:11-23 (IC50 = 10.4 nM) to the HLA-DQ8 molecule as measured using an in vitro peptide binding assay (Fig. 2D). Interestingly, when antigen-presenting cells (APCs) were primed with deINS protein (∼1.7 μM) or B:11-23 (6 μM), deINS protein elicited a similar stimulation index compared with B:11-23 peptide (Table 1). This result indicates that an epitope that corresponds to the B:11-23 peptide can be processed from deINS protein and presented effectively on HLA-DQ8 to activate insulin-specific T cells.
Fig. 2.
HLA-DQ8–restricted T-cell clones isolated using B:11-23R22E tetramers respond to wild-type insulin peptide and denatured insulin. (A) DQ8/B:11-23R22E tetramer staining of a representative T-cell clone isolated from subject T1D#3. (B) 3H-Thymidine incorporation of T-cell clone T1D#3 C8 following stimulation with B:1-23R22E peptide or irrelevant control peptide stimulation in the absence or presence of 5 μg/mL of L243 (HLA-DR blocking antibody), B7/21 (HLA-DP blocking antibody), or SPVL3 (HLA-DQ blocking antibody). Data are mean ± SD of duplicates. (C) Stimulation of a representative T-cell clone T1D#3 C8 by B:11-23R22E, B:11-23, denatured or native insulin. Data are mean ± SD of duplicates. (D) In vitro binding of B:11-23, B:11-23R22E, and influenza matrix protein (MP) peptides to DQ8. Competitive binding assays were carried out using a biotinylated reference GAD65 peptide and increasing concentrations of nonbiotinylated B:11-23, B:11-23R22E, or MP24 (MP185–204 TAKAMEQMAGSSEQAAEAME). Based on the binding curves, the IC50 values were 10.4 μM for B:11-23, 1.6 μM for B:11-23R22E, and 1.4 μM for MP24, respectively.
Table 1.
Stimulation index of insulin specific T-cell clones in response to peptides and denatured protein
| Clone no. | B:11-23R22E | B:11-23 | deINS |
| T1D#3 C5 | 485 | 122 | 190 |
| T1D#3 C8 | 327 | 5.3 | 110 |
| T1D#4 C5 | 189.6 | 77.7 | 54.2 |
| T1D#4 C6 | 212.0 | 37.1 | NT |
| T1D#10 C1 | 278.2 | 9.6 | 48 |
| T1D#10 C3 | 302.2 | 10.2 | 46.5 |
| T1D#10 C8 | 634.5 | 83 | 149 |
| T1D#15 C2 | 346.9 | 31.4 | NT |
| Average ± SD | 347 ± 147 | 47 ± 43 | 100 ± 60 |
Reported values are stimulation index in response to stimulation with B:11-23R22E peptide (6 μM), B:11-23 peptide (6 μM), or deINS protein (1.7 μM) versus an unstimulated control.
Determining the Immunogenic Register of Insulin B:11-23 Bound to HLA-DQ8.
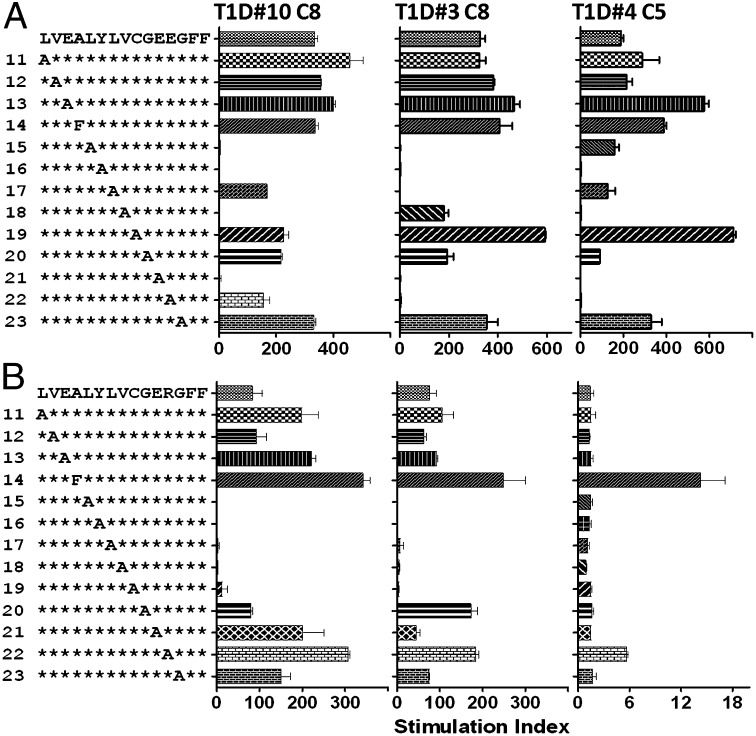
To define the register of the B:11-23 peptide that is recognized as an immunogenic epitope by human T cells, two libraries of alanine substituted peptides with either B:11-23R22E or B:11-23 as the prototypic peptide template were synthesized and the relative capacities of the substituted peptides to stimulate T-cell clones were compared. Fig. 3A shows the stimulation indexes of each peptide in the library using B:11-23R22E as prototype for three clones (clone T1D#10 C8 was generated from wild-type B:11-23 stimulation, and clone T1D#3 C8 and T1D#4 C5 generated from B:11-23R22E stimulation). Overall, this alanine substituted library induced a similar response pattern for all three T-cell clones examined. The major influence of alanine substitution on stimulatory activities was present between residues A15 to E22. This finding suggests the region of the peptide that is recognized by these T cells is the A15 to E22 region. Further analysis revealed that substitutions of A14F, L17A, C19A, and G20A had minimal effect on stimulatory activity for T1D#10 C8 and T1D#4 C5 clones. Although substitutions of Y16A and E21A markedly reduced the stimulatory activity for all three clones, substitutions of L15A and V18A reduced the stimulatory activity of two of three clones. Based on this pattern of peptide stimulatory activity, it is likely that B:11-23R22E bound to DQ8 using R3. In this scenario, residues A14, L17, and C19 correspond to the p1, p4, and p6 anchoring residues, respectively. The side chains of these anchor residues are buried within the HLA binding pockets and substitution of these residues would have limited effects on T-cell receptor (TCR) recognition. In contrast, residues L15, Y16, V18, and E21 would correspond to positions 2, 3, 5, and 8 of the HLA binding register, which are potential TCR contact residues; alanine substitutions at TCR contact positions would be expected to affect T-cell recognition, as was observed.
Fig. 3.
Determining the immunogenic register of B:11-23 that is recognized by DQ8-restricted T cells. (A) Stimulation index for T-cell clones activated in vitro using B:11-23R22E or its substituted derivatives (all clones were stimulated with 3-μM peptides). The B:11-23R22E peptide sequence is listed on the top of the y axis, followed by substituted peptides, with identical residues marked using the asterisk symbol. Alanine was used to replace each residue of the peptide with the exception of A14 where the wild-type alanine was replaced with F. Clone T1D#10 C8 was generated from wild-type B:11-23 stimulated cultures, and clone T1D#3 C8 and T1D#4 C5 were generated from B:11-23R22E-stimulated cultures. Data are mean ± SD of duplicates. (B) Stimulation index for T-cell clones activated in vitro using B:11-23 or its substituted derivatives (all clones were stimulated with 30-μM peptides). The B:11-23 peptide sequence is listed on the top of the y axis, followed by substituted peptides, with identical residues marked using the asterisk. Alanine was used to replace each residue of the peptide with the exception of A14 where the wild-type alanine was replaced with F. Data are mean ± SD of duplicates.
Clone T1D#4 C5 also showed increased responses to the E13A substituted peptide compared with wild-type peptide. E is a naturally preferred p1 anchor for DQ8, as described by Suri et al. (11). Therefore, replacement of E13 to A would eliminate the preferred p1 anchor of R2, increasing the likelihood that the peptide would anchor to other DQ8 registers such as in R3, thereby enhancing stimulatory activity. Clone T1D#3 C8 showed a slightly different reactivity profile compared with the other two clones. For this clone, L17A substitution abrogated activation, whereas V18A substitution had minimal effect. This finding could be interpreted to suggest that R2 is the immunogenic register recognized by this clone. In this scenario, L15, Y16, L17, and E22 would be potential T-cell contact residues (Fig. 3A). However, it is more likely that this clone still recognizes B:11-23R22E presented in R3, because the overall pattern of stimulatory activity was very similar to the other two clones. This conclusion was further supported by the next set of data.
When T-cell clones were stimulated with the library using B:11-23 as the prototypic peptide, the stimulation activities were enhanced for all clones with peptides having substitutions of A14F and R22A. These data suggest that A14 is the p1 anchor residue, because F has been shown to serve as a better p1 anchor than A (21). Correspondingly, A22 would be the p9 residue, where A would be better accommodated than a basic R. These data do not support E13 as the p1 anchor for the recognition by these T-cell clones, because if E13 were anchored in p1, A14 would be at position 2 of the binding register and would likely influence TCR recognition. However, the results show that an A14F substitution did not decrease stimulatory activity for any of the T-cell clones. Instead this substitution enhanced the stimulatory activity for all clones. Substituting R22 to A also enhanced stimulatory activity for all clones that could also potentiate binding in R3, because R is a relatively poor p9 anchor (compared with an A residue at p9) for DQ8 binding.
To further confirm that T-cell recognition of B:11-23 is specific to R3, T1D#10 C8, T1D#3 C8, and T1D#4 C5 T-cell clones were stimulated with DQ8 constructs engineered to express the insulin peptide covalently linked to the N terminus of DQB1*0302. The binding register of the peptide was fixed through formation of a disulfide bond between the DQ8 α-chain and the peptide (9). Two sets of such trapped constructs were generated, one with cysteine introduced at position 65 of the α-chain (V65C), designed to form a bond with a cysteine present at position p6 of the peptide, and another with an I72C mutation designed to bind to a cysteine at p11 (Fig. S2A). The peptides in different registers were also modified in such a fashion that both p1 and p9 would be an E; and any C that would not be at the p6 position was changed to an A. B6K10 cells were transduced with retroviral versions of these constructs for subsequent use as artificial APCs (22). The surface expression levels of DQ8/Ins complexes for both I72C and V65C series are similar except for the R4 variants of V65C, which showed a reduced surface expression (Fig. S2B). Western blot analysis of surface immunoprecipitates from the I72C series separated by SDS/PAGE under reducing or nonreducing conditions demonstrated correct formation of the peptide:DQ8 disulfide bond, thus confirming trapping of the insulin peptide in the defined register (R1, R2, R3, or R4) (Fig. S2C). Consistent with our previous results, the three representative T-cell clones tested were only responsive to B6K10 lines expressing DQ8/Ins complexes fixed in R3 (Table 2). Overall, the results of these experiments clearly demonstrate that B:11-23–specific human DQ8-restricted T-cell clones that we isolated respond to the peptide bound in the R3 register, and not in R1, R2, or R4.
Table 2.
T-cell clones respond to register-fixed DQ8/Ins complexes in R3
| DQ8-Ins | Peptide* | T1D#10 C8† | T1D#3 C8 | T1D#4 C5 |
| I72C-R1 | LEEALYLVAEEC | — | — | — |
| I72C-R2 | VEALYLVAGERC | — | — | — |
| I72C-R3 | VEELYLVAGEEGC | 1.5 | 31.6 | 4.9 |
| I72C-R4 | VEAEYLVAGEREGC | — | 0.4 | 0.1 |
| V65C-R1 | LEEALYCVAEER | — | — | — |
| V65C-R2 | VEALYLCAGERG | — | — | — |
| V65C-R3 | VEELYLVCGEEGG | 2.3 | 38.8 | 0.4 |
| V65C-R4 | VEAEYLVACEREGG | — | — | — |
The lowest concentration of IFN-γ standard was 0.017 ng/mL and readings below this level are indicated as with an “—.”
To “trap” each peptide in a specific register, corresponding p1 and p9 anchor residues of the peptide were mutated to E (in bold). To “fix” the peptide in a specific register by disulfide bonding, each set of peptides had a single C residue (underlined) at position p6 (to bind to V65C) or at p11 (to bind to I72C). The other C residue that would not be at p6 was changed to A (Italic).
T-cell clones were stimulated with B6K10 cells expressing the indicated register-fixed constructs and assayed for secreted human IFN-γ (ng/mL).
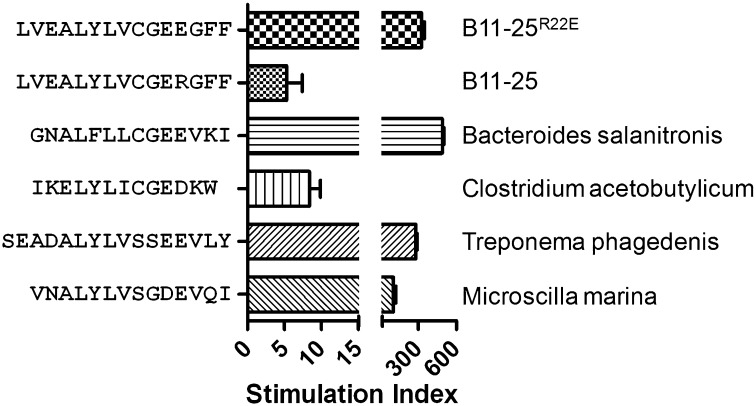
Insulin-Reactive T-Cell Clones Respond to the Microbially Derived Peptides.
Among the possible mechanisms that could initiate autoimmune diabetes, it has been postulated that adaptive responses that are primed by microbial antigens could cross-recognize homologous self-epitopes. To investigate whether B:11-23–specific T cells can be activated by such homologous peptides, we performed a sequence homology search for ALYLVCGEE among all bacteria, viruses, and fungi deposited within the National Center for Biotechnology Information protein database. We identified four microbial peptides with high-sequence homology to ALYLVCGEE (Table S3). These sequences were derived from Bacteroides salanitronis, a common inhabitant of chicken intestine/cecum (23), Clostridium acetobutylicum, a soil dwelling Clostridium species (24), Treponema phagedenis, a pathogen of papillomatous digital dermatitis isolated from Iowa dairy cattle (25), and Microscilla marina, a bacterial group frequently found on marine particulates (26). All of the T-cell clones tested responded to stimulation with these bacterial peptides and the responses, as evaluated by the stimulation index, were of comparable magnitude to the modified B:11-23R22E peptide (Fig. 4). These data imply that T cells that recognize B:11-23 on HLA-DQ8 could be effectively primed by microbial antigens.
Fig. 4.
Stimulation index for a representative T-cell clone activated in vitro using 3 μM of B:11-23R22E peptide, B:11-23 peptide, or homologous microbial peptides, respectively. Data are mean ± SD of duplicates.
Discussion
This study provides direct evidence that B:11-23 reactive CD4+ T cells are present in the peripheral blood of subjects with T1D and that the immunogenic register of this peptide has low-affinity binding to DQ8. Responses to B:11-23 by DQ8-restricted T cells were more readily detected in T1D subjects compared with healthy controls, visualizing these cells using DQ8/B:11-23R22E tetramers following in vitro expansion. We further verified the specificity and relevance of these T cells by isolating tetramer-positive clones and documenting their responsiveness to the wild-type peptide and denatured insulin protein. B:11-23 reactive cells appeared to be present at relatively low frequencies in the subjects studied, as responses were modest (less than 1% of expanded CD4+ T cells and of fairly low mean fluorescence intensity) and only detectable in 6 of 16 T1D subjects. Interestingly, five of the six subjects who responded to B:11-23 had the susceptible VNTR genotype, suggesting that levels of thymic insulin expression as dictated by the insulin VNTR polymorphism may influence the selection of B:11-23–specific T cells (27). In earlier experiments with the DR0401/preproinsulin tetramers, an average of 14% tetramer-positive T cells with a positive response rate of 75% in T1D subjects was observed under similar experimental conditions (28). This comparison of DR- and DQ-restricted insulin responses further underscores the apparent rarity of these DQ8 T cells in the periphery, although it could be argued that our tetramer reagents are unable to detect a subset of T cells that recognize this epitope with lower functional avidity. It is plausible that these T cells may exist at higher frequencies close to disease onset but are subsequently lost in patients because of insufficient levels of antigen to maintain their peripheral survival as a consequence of β-cell destruction (29–31). It is also possible that B:11-23–specific T cells that remain in subjects with long-standing disease preferentially reside in islets or secondary lymphoid tissue, and are therefore virtually undetectable in peripheral blood. Additional studies in at-risk subjects would be necessary to differentiate between these possibilities.
Although our T-cell clones responded to the denatured insulin protein and wild-type insulin peptide, none recognized any peptides from naturally processed native insulin. DeINS was generated in the presence of β-mercaptoethanol, which would be expected to reduce disulfide bonds and produce the free insulin A and B chains. Lack of reactivity to processed native insulin suggests that the disulfide bonds within the native structure of insulin protein may limit effective generation of the B:11-23 epitope. This behavior would be consistent with that of the “type B” T-cell clones described by Mohan et al., which respond only to peptides offered exogenously to the APC but not to naturally processed protein (32, 33). In any case, the binding register of B:11-23 that was recognized by our DQ8-restricted clones was not the high-affinity binding register. As more fully detailed in Results, the profound influence of substitutions between positions 14 and 22 (most notably residues 16, 18, and 21 for the B:11-23R22E peptide and residues 14 and 22 for the B:11-23 peptide) strongly implicate A14, L17, C19, and R22 as the immunogenic anchor residues and L15, Y16, V18, and E21 as important TCR contacts. Thus, the DQ8-restricted T cells we isolated appear to recognize B:11-23 presented in the suboptimal R3 register. This contention was further confirmed by experiments in which the insulin B:11-23 peptide was fixed in the R3 register by disulfide cross-linking between the linked peptide and DQ8 molecule. Thus, in both mice and humans, autoreactive T cells recognize the B:11-23 peptide presented in R3 (9, 10).
A long-standing puzzle in autoimmunity has been how the T cells that drive these diseases escape negative selection in the thymus and peripheral regulatory mechanisms to find an activating antigen in their target organs. It is likely that the interplay among genetic, epigenetic, and environmental factors ultimately play a role (34, 35). However, in most cases the mechanism does not seem to be via a general breakdown in thymic deletion or peripheral regulatory mechanisms, but rather is related to MHC allele-specific antigen presentation. In this regard, there have been several mechanisms suggested, including differential gene expression in the thymus versus periphery (36, 37), posttranslational modifications (38–40), and activation by molecular mimicry [e.g., high-affinity cross-reactive peptides from inflammatory microorganisms (41)]. Without excluding other mechanisms, our data raise the possibility that this third mechanism might be at work in T1D. We identified a number of environmental microbes expressing proteins that contain peptide sequences with high homology to the R3 core region of B:11-23R22E peptide (ALYLVCGEE). These microbes included species that are commonly isolated from poultry or dairy animals and environmental microbes that are commonly encountered (23–26). DQ8-restricted insulin-specific T-cell clones were activated by these homologous microbial peptides as effectively as the B:11-23R22E peptide. Thus, it is possible that responses to epitopes from these or other similar microbes could play a role in initiating the activation and expansion of insulin reactive T cells.
In total, our study verifies the importance of T-cell responses to antigenic peptides with low-affinity HLA binding in human autoimmune disease, documenting the presence of DQ8-restricted insulin B:11-23–specific T cells in subjects with T1D by directly visualizing these cells with DQ8/B:11-23R22E tetramers. These T cells were only detected in patients and also responded to stimulation with homologous peptides derived from commonly encountered microbial antigens. These observations highlight the possibility that self-reactive T cells could be activated by molecular mimicry early in the autoimmune process, leading to a subsequent breakdown in self-tolerance. Our results are in agreement with observations for the IAg7-restricted T cells of NOD mice (9, 10), highlighting a mechanism shared by mouse and man through which T cells that recognize a weakly bound peptide can circumvent tolerance mechanisms and play a role in the initiation of autoimmune diseases, such as T1D. Given that B:11-23–specific T cells can be successfully visualized using tetramers, tetramer-based approaches could be useful to monitor responses to this peptide in prediabetic subjects, over the course of immune-modulatory therapy, or following the administration of tolerogenic vaccines (42, 43).
Materials and Methods
Human Subjects.
Fresh blood samples were obtained from healthy individuals and subjects with T1D who had HLA-DQA1*03:01/DQB1*03:02 (DQ8) haplotypes as part of a Benaroya Research Institutional Review Board-approved study (R00123A) after obtaining written consent. The VNTR genotype was assigned based on the genotype at the −23 A/T single nucleotide polymorphism of the INS promoter (44). The attributes of the subjects recruited for the study are summarized in Table S1. Subjects with T1D were sampled within 4 y of diagnosis. Two DQ8 subjects with type 2 diabetes that received regular insulin injections to maintain glycemic control were also recruited.
Antibodies and Tetramer Reagents.
The following fluorescent antibodies were used: anti-human CD3-FITC and CD25-APC (eBioscience), CD4-PerCP (BD Biosciences), anti-HLA-DQ-PE (Leinco Technologies), and streptavidin-R-PE (Life Technology). HLA-DR and HLA-DQ blocking antibodies (L243 and SPVL3) were produced from hybridomas obtained from the ATCC. The B7/21 anti-DP producing hybridoma was a gift from Alessandro Sette (La Jolla Institute for Allergy and Immunology, La Jolla, CA). These antibodies were purified using protein A-Sepharose beads. Tetramers for staining T cells were generated as previously described (45, 46).
Peptides and deINS Protein.
Insulin B: 11-23 (B:11-23), LVEALYLVCGERG, its modified version, Insulin B:11-23, R22E (B:11-23R22E), LVEALYLVCGEEG, libraries of substituted peptides, and peptides derived from bacterial antigens (detailed in Figs. 3 and 4) were purchased from Mimotopes. Peptides were dissolved in DMSO at 20 mg/mL (∼12 mM). Insulin powder was purchased from Sigma-Aldrich. DeINS protein was prepared by dissolving 50 mg of insulin powder into 500 μL of 8 M Urea plus 10 μL of β-mercaptoethanol followed by dialysis into PBS in 0.5 mL capacity of Slide-A-Lyzer Dialysis Cassettes, 3K MWCO (Thermo Scientific). After dialysis, the denatured protein was collected and stored as aliquots at −20 °C.
Register-Fixed Constructs: Generation, Expression, and T-Cell Stimulation.
Murine stem cell virus-based retroviral expression vectors encoding the DQ8/Ins complexes were constructed using standard molecular biology procedures. The constructs encode the complexes as a single ORF, comprising the DQ8 α- and β-chains separated by a picornovirus 2A peptide (47), and an internal ribosome entry site-driven GFP. The insulin peptide is fused via a flexible linker to the N terminus of the mature β-chain (48) (Fig. S2A). B6K10 cells were transduced with the resulting recombinant retroviruses and used without further enrichment.
T-cell stimulation assays were performed by coculturing 105 T cells and 105 transduced B6K10 cells in 200 μL of medium for 16–24 h. Assays were performed in triplicate and 100 μL of the supernatant was assayed using the human IFN-γ ELISA Ready-SET-Go kit (eBioscience), according to the manufacturer’s recommendations.
Methods for peptide binding measurements, CD4 T in vitro stimulation, tetramer staining, insulin-specific CD4+ T-cell cloning, and T-cell proliferation assays are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank McKenzie Lettau, Jani Klein, and Gladys Doronio for helping to draw blood samples. This work was supported by National Institutes of Health Grant DK097653 (to W.W.K.), National Institutes of Health Grants DK032083 and DK052068 (to T.S. and H.W.D.), and the Juvenile Diabetes Research Foundation Research Grant 17-2012-609 (to T.S. and H.W.D.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1416864111/-/DCSupplemental.
References
- 1.Pociot F, McDermott MF. Genetics of type 1 diabetes mellitus. Genes Immun. 2002;3(5):235–249. doi: 10.1038/sj.gene.6363875. [DOI] [PubMed] [Google Scholar]
- 2.Pugliese A, et al. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet. 1997;15(3):293–297. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- 3.Vafiadis P, et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet. 1997;15(3):289–292. doi: 10.1038/ng0397-289. [DOI] [PubMed] [Google Scholar]
- 4.Daniel D, Gill RG, Schloot N, Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur J Immunol. 1995;25(4):1056–1062. doi: 10.1002/eji.1830250430. [DOI] [PubMed] [Google Scholar]
- 5.Wegmann DR, Norbury-Glaser M, Daniel D. Insulin-specific T cells are a predominant component of islet infiltrates in pre-diabetic NOD mice. Eur J Immunol. 1994;24(8):1853–1857. doi: 10.1002/eji.1830240820. [DOI] [PubMed] [Google Scholar]
- 6.Nakayama M, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435(7039):220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakayama M, et al. Priming and effector dependence on insulin B:9-23 peptide in NOD islet autoimmunity. J Clin Invest. 2007;117(7):1835–1843. doi: 10.1172/JCI31368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levisetti MG, Suri A, Petzold SJ, Unanue ER. The insulin-specific T cells of nonobese diabetic mice recognize a weak MHC-binding segment in more than one form. J Immunol. 2007;178(10):6051–6057. doi: 10.4049/jimmunol.178.10.6051. [DOI] [PubMed] [Google Scholar]
- 9.Stadinski BD, et al. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc Natl Acad Sci USA. 2010;107(24):10978–10983. doi: 10.1073/pnas.1006545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crawford F, et al. Specificity and detection of insulin-reactive CD4+ T cells in type 1 diabetes in the nonobese diabetic (NOD) mouse. Proc Natl Acad Sci USA. 2011;108(40):16729–16734. doi: 10.1073/pnas.1113954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suri A, Walters JJ, Gross ML, Unanue ER. Natural peptides selected by diabetogenic DQ8 and murine I-A(g7) molecules show common sequence specificity. J Clin Invest. 2005;115(8):2268–2276. doi: 10.1172/JCI25350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Achenbach P, et al. Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes. 2004;53(2):384–392. doi: 10.2337/diabetes.53.2.384. [DOI] [PubMed] [Google Scholar]
- 13.Wasserfall CH, Atkinson MA. Autoantibody markers for the diagnosis and prediction of type 1 diabetes. Autoimmun Rev. 2006;5(6):424–428. doi: 10.1016/j.autrev.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Jarchum I, et al. In vivo cytotoxicity of insulin-specific CD8+ T-cells in HLA-A*0201 transgenic NOD mice. Diabetes. 2007;56(10):2551–2560. doi: 10.2337/db07-0332. [DOI] [PubMed] [Google Scholar]
- 15.Luce S, et al. Single insulin-specific CD8+ T cells show characteristic gene expression profiles in human type 1 diabetes. Diabetes. 2011;60(12):3289–3299. doi: 10.2337/db11-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knight RR, et al. Human β-cell killing by autoreactive preproinsulin-specific CD8 T cells is predominantly granule-mediated with the potency dependent upon T-cell receptor avidity. Diabetes. 2013;62(1):205–213. doi: 10.2337/db12-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kronenberg D, et al. Circulating preproinsulin signal peptide-specific CD8 T cells restricted by the susceptibility molecule HLA-A24 are expanded at onset of type 1 diabetes and kill β-cells. Diabetes. 2012;61(7):1752–1759. doi: 10.2337/db11-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kent SC, et al. Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an insulin epitope. Nature. 2005;435(7039):224–228. doi: 10.1038/nature03625. [DOI] [PubMed] [Google Scholar]
- 19.Alleva DG, et al. A disease-associated cellular immune response in type 1 diabetics to an immunodominant epitope of insulin. J Clin Invest. 2001;107(2):173–180. doi: 10.1172/JCI8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eerligh P, et al. Functional consequences of HLA-DQ8 homozygosity versus heterozygosity for islet autoimmunity in type 1 diabetes. Genes Immun. 2011;12(6):415–427. doi: 10.1038/gene.2011.24. [DOI] [PubMed] [Google Scholar]
- 21.Kwok WW, Domeier ME, Johnson ML, Nepom GT, Koelle DM. HLA-DQB1 codon 57 is critical for peptide binding and recognition. J Exp Med. 1996;183(3):1253–1258. doi: 10.1084/jem.183.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huseby ES, Crawford F, White J, Kappler J, Marrack P. Negative selection imparts peptide specificity to the mature T cell repertoire. Proc Natl Acad Sci USA. 2003;100(20):11565–11570. doi: 10.1073/pnas.1934636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gronow S, et al. Complete genome sequence of Bacteroides salanitronis type strain (BL78) Stand Genomic Sci. 2011;4(2):191–199. doi: 10.4056/sigs.1704212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nölling J, et al. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J Bacteriol. 2001;183(16):4823–4838. doi: 10.1128/JB.183.16.4823-4838.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trott DJ, et al. Characterization of Treponema phagedenis-like spirochetes isolated from papillomatous digital dermatitis lesions in dairy cattle. J Clin Microbiol. 2003;41(6):2522–2529. doi: 10.1128/JCM.41.6.2522-2529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hopkinson BM, Roe KL, Barbeau KA. Heme uptake by Microscilla marina and evidence for heme uptake systems in the genomes of diverse marine bacteria. Appl Environ Microbiol. 2008;74(20):6263–6270. doi: 10.1128/AEM.00964-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durinovic-Belló I, et al. Insulin gene VNTR genotype associates with frequency and phenotype of the autoimmune response to proinsulin. Genes Immun. 2010;11(2):188–193. doi: 10.1038/gene.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, et al. CD4+ T cells from type 1 diabetic and healthy subjects exhibit different thresholds of activation to a naturally processed proinsulin epitope. J Autoimmun. 2008;31(1):30–41. doi: 10.1016/j.jaut.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Brocker T. Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class II-expressing dendritic cells. J Exp Med. 1997;186(8):1223–1232. doi: 10.1084/jem.186.8.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirberg J, Berns A, von Boehmer H. Peripheral T cell survival requires continual ligation of the T cell receptor to major histocompatibility complex-encoded molecules. J Exp Med. 1997;186(8):1269–1275. doi: 10.1084/jem.186.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeda S, Rodewald HR, Arakawa H, Bluethmann H, Shimizu T. MHC class II molecules are not required for survival of newly generated CD4+ T cells, but affect their long-term life span. Immunity. 1996;5(3):217–228. doi: 10.1016/s1074-7613(00)80317-9. [DOI] [PubMed] [Google Scholar]
- 32.Mohan JF, Petzold SJ, Unanue ER. Register shifting of an insulin peptide-MHC complex allows diabetogenic T cells to escape thymic deletion. J Exp Med. 2011;208(12):2375–2383. doi: 10.1084/jem.20111502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohan JF, Unanue ER. Unconventional recognition of peptides by T cells and the implications for autoimmunity. Nat Rev Immunol. 2012;12(10):721–728. doi: 10.1038/nri3294. [DOI] [PubMed] [Google Scholar]
- 34.Yip L, Fathman CG. Type 1 diabetes in mice and men: Gene expression profiling to investigate disease pathogenesis. Immunol Res. 2014;58(2-3):340–350. doi: 10.1007/s12026-014-8501-8. [DOI] [PubMed] [Google Scholar]
- 35.Steck AK, Rewers MJ. Genetics of type 1 diabetes. Clin Chem. 2011;57(2):176–185. doi: 10.1373/clinchem.2010.148221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villaseñor J, Besse W, Benoist C, Mathis D. Ectopic expression of peripheral-tissue antigens in the thymic epithelium: Probabilistic, monoallelic, misinitiated. Proc Natl Acad Sci USA. 2008;105(41):15854–15859. doi: 10.1073/pnas.0808069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taniguchi RT, Anderson MS. The role of Aire in clonal selection. Immunol Cell Biol. 2011;89(1):40–44. doi: 10.1038/icb.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sollid LM. Coeliac disease: Dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002;2(9):647–655. doi: 10.1038/nri885. [DOI] [PubMed] [Google Scholar]
- 39.Dieterich W, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3(7):797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 40.Vander Cruyssen B, et al. Anti-citrullinated protein/peptide antibodies (ACPA) in rheumatoid arthritis: specificity and relation with rheumatoid factor. Autoimmun Rev. 2005;4(7):468–474. doi: 10.1016/j.autrev.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 41.Getts DR, Chastain EM, Terry RL, Miller SD. Virus infection, antiviral immunity, and autoimmunity. Immunol Rev. 2013;255(1):197–209. doi: 10.1111/imr.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fourlanos S, et al. Evidence that nasal insulin induces immune tolerance to insulin in adults with autoimmune diabetes. Diabetes. 2011;60(4):1237–1245. doi: 10.2337/db10-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fousteri G, et al. Virtual optimization of nasal insulin therapy predicts immunization frequency to be crucial for diabetes protection. Diabetes. 2010;59(12):3148–3158. doi: 10.2337/db10-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barratt BJ, et al. Remapping the insulin gene/IDDM2 locus in type 1 diabetes. Diabetes. 2004;53(7):1884–1889. doi: 10.2337/diabetes.53.7.1884. [DOI] [PubMed] [Google Scholar]
- 45.Novak EJ, et al. Tetramer-guided epitope mapping: Rapid identification and characterization of immunodominant CD4+ T cell epitopes from complex antigens. J Immunol. 2001;166(11):6665–6670. doi: 10.4049/jimmunol.166.11.6665. [DOI] [PubMed] [Google Scholar]
- 46.Yang J, et al. Islet-specific glucose-6-phosphatase catalytic subunit-related protein-reactive CD4+ T cells in human subjects. J Immunol. 2006;176(5):2781–2789. doi: 10.4049/jimmunol.176.5.2781. [DOI] [PubMed] [Google Scholar]
- 47.Holst J, et al. Generation of T-cell receptor retrogenic mice. Nat Protoc. 2006;1(1):406–417. doi: 10.1038/nprot.2006.61. [DOI] [PubMed] [Google Scholar]
- 48.Kozono H, White J, Clements J, Marrack P, Kappler J. Production of soluble MHC class II proteins with covalently bound single peptides. Nature. 1994;369(6476):151–154. doi: 10.1038/369151a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.