Abstract
During the course of a screening for novel anti-infective agents from cultures of tropical Xylariaceae originating from French Guiana and Thailand, pronounced antifungal activity was noted in extracts of cultures of Hypoxylon monticulosum. A bioassay-guided fractionation led to the known metabolite sporothriolide as active principle. In addition, three new derivatives of sporothriolide were isolated, for which we propose the trivial names sporothric acid, isosporothric acid and dihydroisosporothric acid. Their chemical structures were elucidated by high-resolution electrospray mass spectrometry in conjunction with two-dimensional nuclear magnetic resonance (2D-NMR) spectroscopy. From earlier studies on the biogenesis of the chemically similar canadensolides, we postulate that the new compounds were shunt products, rather than biogenetic precursors of sporothriolide. Interestingly, this compound class, as well as strong antifungal activities, was only observed in multiple cultures of H. monticulosum, but not in several hundreds of Hypoxylon cultures studied previously or concurrently. Therefore, sporothriolide production may constitute a species-specific feature with respect to Hypoxylon and the Xylariaceae, although the compound was previously reported from non-related fungal taxa.
Keywords: antibiotics, secondary metabolites, screening, chemotaxonomy, Hypoxylon, furofurandiones
Introduction
Due to the ongoing development of resistances of human pathogens against common antibiotics, new drugs are urgently needed (Cooper and Shlaes 2011). Besides various chemical approaches, the exploration of natural sources for discovery of new anti-infective lead structures still seems most promising, because natural products have historically been the most prolific source for anti-infective drugs (Newman and Cragg 2012). Prominent examples are the beta-lactam antibiotics and many other useful compounds, which were mostly found during the course of empirical screening programs using fungal soil isolates.
Through the availability of molecular phylogenetic and genomic data in conjunction with high-performance liquid chromatography (HPLC) profiling studies, ‘hotspots’ of metabolic diversity now can be identified within the fungal kingdom that can be circumscribed by taxonomic terms. One of the most prolific fungal families of secondary metabolite producers are the Xylariaceae, which are known for their high morphological and chemical diversity. Whalley and Edwards (1995 and references therein) already provided evidence for the high chemical diversity of secondary metabolites in this fungal family and pointed out their chemotaxonomic significance. Up to date, ca. 500 different secondary metabolites have been discovered, most of them exhibit some kind of biological activity. Interestingly, expression of secondary metabolism of these fungi may change completely as their life cycle’s progress (Stadler et al. 2006). The stromata, which grow mostly on dead wood, contain various cytotoxic agents, e.g., cohaerin azaphilones or tetramic acid hypoxyvermelhotin A (Figure 1; Surup et al. 2013, Kuhnert, Heitkämper et al. 2014) whereas the cultured mycelia produce various other metabolites, such as the potent antifungal agents hypoxysordarin and xylaral, or the antiparasitics PF-1022A and nodulisporic acid (Figure 1; Stadler and Hellwig 2005, Bills et al. 2012). Besides their bioactivity, secondary metabolites of Xylariaceae were proven to be a valuable tool for taxonomic purpose (Stadler and Hellwig 2005; Stadler and Fournier 2006; Stadler 2011). Some of these compounds are so unique in nature, that they are only produced by a certain species. Prominent examples are sassafrins in Creosphaeria sassafras, carneic acids in Hypoxylon carneum or hypoxyvermelhotins in H. lechatii (Figure 1; Quang et al. 2005, Quang et al. 2006, Kuhnert, Heitkämper et al. 2014). By means of high-performance liquid chromatography coupled with a diode array detector and a mass spectrometer (HPLC-DAD/MS) extracts from small amounts of stromata are sufficient to identify known compounds (Bitzer et al. 2007). The thereby generated chemoprofiles are specific for certain taxonomic levels down to species rank. However, some species within the Xylariaceae are devoid of secondary metabolites in fruiting bodies or produce only the ubiquitous BNT, e.g., all species of the genera Camillea and Biscogniauxia or some Hypoxylon species like Hypoxylon monticulosum. In these cases, chemotaxonomy is restricted to HPLC profiles derived from culture extracts. Despite the unlimited availability of strains and the very good possibility of upscaling, the evaluation of secondary metabolites from the Xylariaceae has been restricted so far to small molecules or those with conspicuous bioactivity. Bitzer et al. (2008) already showed that secondary metabolites derived from cultures are a valuable tool to resolve infrageneric and intergeneric relationships among various species within the Xylariaceae. However, the variety of taxonomic relevant marker metabolites is limited. The most striking one so far, nodulisporic acid produced by Hypoxylon pulicicidum, led to the development of a product candidate for an insecticidal drug used to treat animals (Bills et al. 2012). They also showed that nodulisporic acid appears restricted to H. pulicicidum and therefore serves as a taxonomic marker at the species level.
Figure 1.
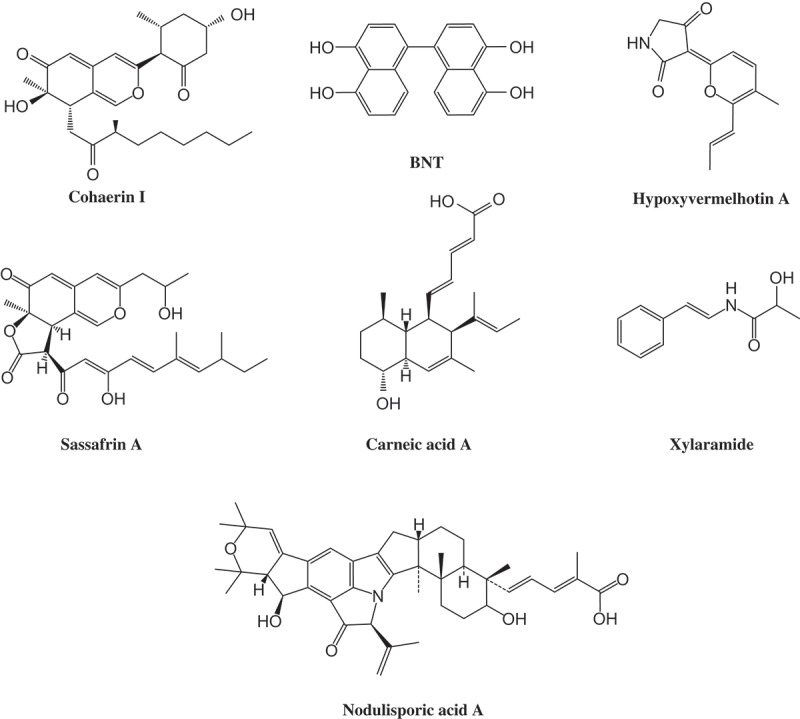
Structures of prominent secondary metabolites from Xylariaceae.
Due to the fact that the diversity of species within the Xylariaceae is the highest in tropical regions, the probability of finding producers of new bioactive secondary metabolites in these areas is considerably higher than in temperate regions. We therefore screened for the bioactivity of extracts prepared from around 50 submerged cultures derived from stromata of various Xylariaceae species originating from French Guiana. In this context, we found strong antifungal activity in extracts of H. monticulosum. The isolation and structural elucidation of the bioactive compound are reported here, and the chemotaxonomic relevance of these findings is discussed.
Material & methods
General
If not indicated otherwise, solvents were obtained in analytical grade from J.T.Baker (Deventer, Netherlands) or Merck (Darmstadt, Germany). Bold numbering refers to the chemical structures depicted in Figure 2. All scientific names of fungi follow the entries in Mycobank (www.mycobank.org). Reference specimens are housed in LIP (University of Lille, France) or Mae Fah Luang University (MFLU) herbarium and corresponding reference cultures have been deposited with MUCL (Louvain, Belgium), MFLU or DSMZ (Braunschweig, Germany). Acronyms of herbaria and culture collections are given as recommended in Index Herbariorum (http://sciweb.nybg.org/science2/IndexHerbariorum.asp).
Figure 2.
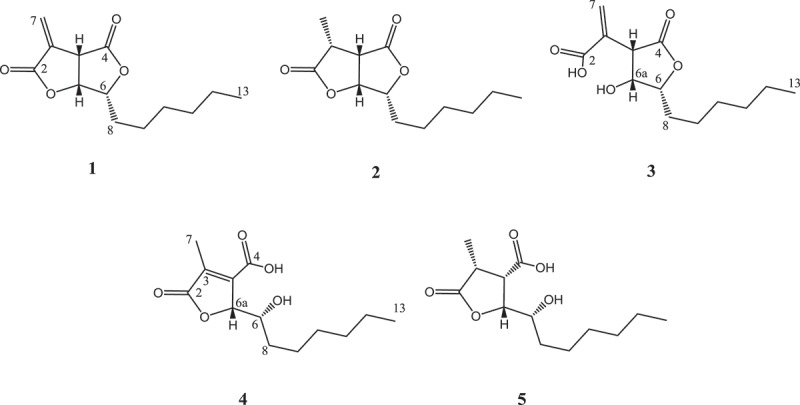
Structures of sporothriolide (1), dihydrosporothriolide (2), sporothric acid (3), isosporothric acid (4) and dihydroisosporothric acid (5) isolated from submerged cultures of Hypoxylon monticulosum (MUCL 54604).
Fungal material
The screening was carried out on cultures derived from 27 strains of various Xylariaceae species (LIP), including 12 Hypoxylon species, 7 Annulohypoxylon species, 2 Phylacia species and 1 Biscogniauxia species, which were collected in 2012 in French Guyana. Among these the H. monticulosum strain GYJF12243 (Figure 3) and two additional specimens of the same species collected in 2013 in Thailand (MFLU 13–0356, MFLU 13–0358) were chosen for further evaluation. Cultures (MUCL 54604, MFLUCC 13–0593, MFLUCC 13–0595) were derived from multispore isolation of the respective fruiting bodies as described in Kuhnert, Fournier et al. (2014). Strains were determined by morphological characters and supported by molecular data of the ITS region (Genbank Acc. No.: KJ810554, KJ810555, KJ810556) according to Kuhnert, Fournier et al. (2014). To clarify the degree of relationship between H. monticulosum and the original producer strain of sporothriolide, ‘Sporothrix sp.’ (DSM 28461, Krohn et al. 1994), the ITS region of the latter was also sequenced.
Figure 3.

Stroma of Hypoxylon monticulosum GYJF12243 on natural substrate. Scale is indicated by bar: 1 mm.
Cultivation and extraction
All strains were grown in submerged cultures (500-ml Erlenmeyer flasks) containing 200 ml of YMG media (10 g l−1 malt, 4 g l−1 glucose, 4 g l−1 yeast extract, pH 6.3). For scale-up, a batch of 19 shake flasks of strain MUCL 54604 was inoculated. Furthermore, the latter strain was also cultivated in 20 flasks, each containing 200 ml of ZM½ media (5 g l−1 molasses, 5 g l−1 oat meal, 1.5 g l−1 glucose, 4 g l−1 sucrose, 4 g l−1 mannitol, 0.5 g l−1 edamine, 0.5 g l−1(NH4)2SO4, 1.5 g l−1 CaCO3, pH 7.2). Fungal cultures were incubated at 23°C on a rotary shaker at 140 min−1. Fermentations were aborted after free glucose was consumed (Stadler et al. 2001).
The culture broth of the large scale production was (20 × 200 ml) combined and afterwards biomass was separated from the media by vacuum filtration. The mycelia were extracted in both scales with 200 and 1000 ml acetone, respectively. The organic extract was then filtered and evaporated. The remaining water phase was extracted with the same amount of ethyl acetate in a separating funnel. The organic phase was then mixed with sodium sulfate, filtered and again evaporated to yield dry mycelia extracts (ME). In small scale, the supernatant was mixed with 200 ml ethyl acetate for 15 min on a magnetic stirrer and additionally extracted in an ultrasonic bath for 15 min at 40°C. The organic phase was separated and proceeded as for the ME. In the case of the 4-l fermentation, the supernatant was mixed with 80 g adsorbent resin (Amberlite XAD™-16N) and incubated overnight. The Amberlite was then filtered and eluted with 800 ml methanol. The extract was also evaporated until a water phase remained. The aqueous phase was extracted with ethyl acetate and processed as described for the ME. The extracts of the 29 strains were tested for activity against Bacillus subtilis (DSM 10), Escherischia coli (DSM 1116), Pichia anomala (DSM 6766) and Mucor plumbeus (MUCL 49355) as described by Halecker et al. (2014).
Isolation of the bioactive compounds
The extract derived from the supernatant of the strain MUCL 54604 grown in YMG media showed the strongest antifungal activity and was therefore first processed. The extract was filtered through a RP solid phase cartridge (Strata-X 33 µm, Polymeric Reversed Phase; Phenomenex, Aschaffenburg, Germany) to yield 152 mg crude extract. The bioactive compound was identified by means of bioassay-guided fractionation and later isolated by preparative reversed phase liquid chromatography (PLC 2020, Gilson, Middleton, USA) under the following conditions: A VP Nucleodur 100–5 C18 ec column (250 × 21 mm, 5 µm; Macherey-Nagel) was used as stationary phase. The mobile phase was composed of deionised water (Milli-Q, Millipore, Schwalbach, Germany) with 0.05% TFA (solvent A; Roth) and acetonitrile with 0.1% acetic acid (solvent B). A flow rate of 15 ml min−1 was used for the following gradient: 40–80% solvent B in 30 min, afterwards linear gradient to 100% solvent B in 10 min, thereafter isocratic conditions at 100% for 10 min. UV detection was carried out at 210 nm and 254 nm and fractions were collected and combined according to the observed peaks. In addition to the active compound 1 (54.7 mg) at a retention time (Rt) of 23–25 min, compound 4 was yielded in pure amounts (2.7 mg) at Rt = 15 min. The ME derived from both media was fractionized as described for the supernatant extract. Compound 5 was isolated in pure state (0.5 mg) at Rt = 12 min from ME of the YMG fermentation. Compound 3 (1.0 mg, Rt = 11 min) and compound 2 (4.2 mg, Rt = 23 min) were yielded from ME of the ZM½ fermentation.
Sporothriolide (1): colorless oil; [α]25 D −144 (c 3.3, CHCl3); 1H NMR, 13C NMR see Tables 1 and 2; high-resolution electrospray mass spectrometry (HR-ESI-MS) m/z 239.1274 [M + H] + (calcd for C13H19O4, 239.1278); spectral data are in good agreement with Krohn et al. (1994).
Table 1.
1H NMR data for metabolites 1–5 (CDCl3, 700 MHz).
| #a | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 3 | 3.05, dq (10.1, 7.5) | 2.99, dq (11.0, 7.1) | |||
| 3a | 4.00, dt (6.7, 2.1) | 3.44, dd (10.1, 6.0) | 3.82, dd (3.9, 1.4) | 3.23, dd (11.0, 9.0) | |
| 6 | 4.64, ddd (8.0, 6.3, 4.6) | 4.50, ddd (8.0, 6.2, 3.9) | 4.51, ddd (8.6, 5.2, 1.4) | 4.25, dt (7.0, 1.5) | 3.71, m |
| 6a | 5.14, dd (6.7, 4.6) | 5.01, dd (6.0, 3.9) | 4.30, dd (3.9, 1.3) | 5.06, qd (2.1, 1.5) | 4.47, dd (9.0, 2.2) |
| 7 | 6.46, d (2.1) 6.15, d (2.1) | 1.47, d (7.5) | 6.78, s 5.95, s | 2.24, d (2.1) | |
| 8 | 1.86, m | 1.92, m; 1.81, m | 1.80, m; 1.69, m | 1.73, m | 1.82, m |
| 9 | 1.50, m | 1.50, m | 1.54, m; 1.38, m | 1.50, m | 1.46, m |
| 10 | 1.37, m | 1.37, m | 1.32, m | 1.34, m | 1.29, m |
| 11 | 1.30, m | 1.30, m | 1.27, m | 1.28, m | 1.25, m |
| 12 | 1.31, m | 1.31, m | 1.28, m | 1.29, m | 1.26, m |
| 13 | 0.88, t (7.0) | 0.88, t (7.0) | 0.88, t (7.0) | 0.88, t (7.0) | 0.88, t (7.0) |
Note: aFor clarity the numbering of 3–5 was based on compound 1.
Table 2.
13C NMR data for metabolites 1–5 (CDCl3, 175 MHz).
| #a | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 2 | 167.5 | 176.2 | 163.5 | 173.1 | 176.7 |
| 3 | 129.9 | 36.8 | 128.5 | 140.2 | 39.2 |
| 3a | 46.2 | 44.7 | 50.0 | 144.7 | 48.2 |
| 4 | 172.1 | 172.1 | 171.3 | 164.8 | 171.9 |
| 6 | 82.8 | 81.7 | 79.1 | 70.1 | 71.0 |
| 6a | 77.2 | 78.1 | 66.5 | 83.1 | 80.6 |
| 7 | 127.4 | 11.1 | 134.6 | 11.2 | 14.8 |
| 8 | 28.9 | 28.9 | 31.0 | 34.4 | 34.0 |
| 9 | 25.4 | 25.3 | 25.1 | 25.8 | 25.7 |
| 10 | 29.0 | 29.0 | 29.0 | 29.1 | 29.0 |
| 11 | 31.6 | 31.6 | 31.6 | 31.7 | 31.8 |
| 12 | 22.5 | 22.6 | 22.6 | 22.6 | 22.6 |
| 13 | 14.1 | 14.2 | 14.1 | 14.1 | 14.2 |
Note: aFor clarity the numbering of 3–5 was based on compound 1.
Dihydrosporothriolide (2): colorless oil; [α]25 D +116 (c 0.1, CHCl3); 1H NMR, 13C NMR see Tables 1 and 2; HR-ESI-MS m/z 241.1429 [M + H] + (calcd for C13H21O4, 241.1434); spectral data are in good agreement with Krohn et al. (1994).
Sporothric acid (3): colorless oil; [α]25 D +14 (c 0.1, CHCl3); 1H NMR, 13C NMR see Tables 1 and 2; IR (KBr) 2957, 2928, 2858, 1762, 1738, 1627, 1384, 1272, 1189 cm−1; HR-ESI-MS m/z 257.1382 [M + H] + (calcd for C13H21O5, 257.1384).
Isosporothric acid (4): colorless oil; [α]25 D −18 (c 0.5, CHCl3); 1H NMR, 13C NMR see Tables 1 and 2; IR (KBr) 2957, 2927, 2857, 1749, 1728, 1213, 1114, 719 cm−1; HR-ESI-MS m/z 257.1384 [M + H] + (calcd for C13H21O5, 257.1384).
Dihydroisosporothric acid (5): colorless oil; [α]25 D +16 (c 0.04, CHCl3); 1H NMR, 13C NMR see Tables 1 and 2; IR (KBr) 2956, 2929, 2858, 1723, 1384, 1294, 1185, 1027 cm−1; HR-ESI-MS m/z 259.1544 [M + H] + (calcd for C13H23O5, 259.1540), 281.1363 [M + Na] + (calcd for C13H22O5Na, 281.1359).
Hydrogenation of sporothriolide (1) to dihydrosporothriolide (2)
A solution of 12.5 mg of sporothriolide (1) in 2 ml of methanol was stirred in the presence of 5 mg of 10% Pd/charcoal under an atmosphere of hydrogen (1 atm) at ambient temperature for 2 h. The solution was centrifuged (14600 rpm, 5 min) and purified by preparative HPLC to yield 6.9 mg (55%) of 2.
Structure elucidation
Optical rotations were determined with a PerkinElmer 241 polarimeter, UV–Vis spectra were recorded with a Shimadzu (Kyoto, Japan) UV–Vis spectrophotometer UV-2450, IR spectra were measured with Spectrum 100 FTIR spectrometer (Perkin Elmer). NMR spectra were recorded with a Bruker (Bremen, Germany) Ascend 700 spectrometer with a 5 mm TXI cryoprobe (1H 700 MHz, 13C 175 MHz). Electrospray ionization mass spectrometry spectra were obtained with an ion trap MS (Amazon, Bruker), high resolution electrospray ionization mass spectrometry spectra were obtained with a time-of-flight MS (Maxis, Bruker) as described by Pažoutová et al. (2013). For HPLC-based dereplication, data from the cited previous studies on Xylariaceae, recorded by the method described in general by Bitzer et al. (2007), including various standards of previously published metabolites, were used in conjunction with the Dictionary of Natural Products (Dictionary… 2013).
Bioactivity assay
Minimum inhibitory concentrations (MIC) were recorded in a serial dilution assay with pure substances using various test organisms for antibacterial and antifungal activity (Halecker et al. 2014). Cytotoxicity was assayed in a similar manner as described by Herrmann et al. (2012) employing the cell lines HCT-116 (human colon carcinoma), CHO-K1 (Chinese hamster ovary) and U-2 OS (human bone osteosarcoma).
Results
Structure elucidation
The bioactive metabolite 1 was isolated from the crude extract by preparative HPLC. The molecular formula of C13H18O4 was deduced from an [M + H] + molecular ion at m/z 239.1274 in the HR-ESI-MS spectrum. The proton and heteronuclear single quantum coherence NMR spectra of 1 revealed the presence of an exocyclic methylene (δ 6.46, 6.15), three coupled methines (δ 5.14, 4.64, 4.00), five methylenes (δ 1.30–1.86) and a methyl group (δ 0.88). A literature search within the Chapman and Hall Dictionary of Natural Products (Dictionary… 2013) and Chemical Abstracts Plus (SciFinder Scholar) databases unambiguously confirmed that the compound 1 is identical to sporothriolide (1) (Krohn et al. 1994).
As by-products of the HPLC fractionation multiple derivatives of 1 were obtained. The first is a metabolite with a molecular formula of C13H20O4. The proton NMR of 2 was very similar to the one of 1 except the replacement of the exomethylene by a methyl group and the appearance of an additional methine signal. The data are consistent with the formal hydrogenation of 1 to its 3,7-dihydro analogue. As demonstrated by the observed chemical shifts and coupling constants for H-3 (δ 3.05, dq, J 3,3a = 10.1 Hz, J 3,7 = 7.5 Hz) and H-3a (δ 3.44, dd, J 3,3a = 10.1 Hz, J 3a,6a = 6.0 Hz), the stereo configuration of 2 was assigned as (3R,3aS,6R,6aR), which is the same as those reported for the synthetic dihydrosporothriolide (Krohn et al. 1994), differing from the previously isolated dihydrosporothriolide from Xylaria (Isaka et al. 2010) in having a (3S,3aS,6R,6aR) stereochemistry. Rotating frame nuclear overhauser effect spectroscopy (ROESY) correlations from H-3 to H-3a and H-6a furthermore supported the cisoidal configuration of H-3, H-3a and H-6a. To obtain more material of this minor metabolite, main metabolite sporothriolide (1) was hydrogenated according to Krohn et al. (1994).
Metabolite 3 showed a [M + Na] + molecular ion at 257.1384, which indicated a molecular formula of C13H20O5. This was a formal addition of water compared to the major metabolite 1. The main spectral difference to sporothriolide (1) was the upfield shift of H-6a in the 1H NMR spectrum. This was consistent with an opening of the lactone ring bearing the exocyclic methylene moiety. A ROESY correlation between H-3a and H-6a indicates an cisoidal configuration between the two protons, therefore the structure was established as 2-[(3S,4R,5R)-5-hexyl-4-hydroxy-2-oxotetrahydrofuran-3-yl]prop-2-enoic acid. Due to its acidic nature the name sporothric acid was suggested for 3.
For metabolite 4 a molecular formula of C13H20O5 was concluded from its [M + Na] + adduct at 257.1384. The proton NMR indicated a ring opening due to the high field shift of H-6 compared to sporothriolide (1). Furthermore, carbon and heteronuclear multiple-bond correlation NMR spectra demonstrated that C-3 and C-3a were quaternary sp2 atoms. Due to a common biosynthesis of 1–5 a 6R configuration can be assumed for 4 and 5, so the structure of metabolite 4 was determined to be (2R)-2-[(1R)-1-hydroxyheptyl]-4-methyl-5-oxo-2,5-dihydrofuran-3-carboxylic acid and named isosporothric acid.
Compound 5 possesses the molecular formula C13H22O5, which was deduced from its [M + Na] + adduct at 281.1363, is implying a formal addition of H2O compared to 2. The key difference of spectroscopic data, when compared to those of 2, was the upfield shift of H-6 (δ 3.71) in the 1H NMR spectrum. Therefore a ring opening of lactone ring could be concluded. ROESY correlations of H-3 with both H-3a and H-6a established the structure of 5 as (2R,3S,4R)-2-[(1R)-1-hydroxyheptyl]-4-methyl-5-oxotetrahydrofuran-3-carboxylic acid. The name dihydroisosporothric acid was proposed for 5.
Evaluation of sporothriolide as a marker metabolite for H. monticulosum
The H. monticulosum strains from Thailand (MFLUCC 13–0593, MFLUCC 13–0595) were evaluated for the presence of sporothriolide. HPLC profiling of the crude extracts from submerged cultures revealed significant amounts of sporothriolide in both strains. Furthermore, the crude extracts showed bioactivity against Mucor plumbeus. The original producer strain ‘Sporothrix sp.’ was also reevaluated for its ability to produce the compound by applying the same fermentation parameters as for the H. monticulosum strains. Sporothriolide could not be unambiguously detected. Furthermore, the molecular data of the ITS region reveal that the species belong most likely to the genus Emericellopsis (Hypocreales; asexual state Acremonium) rather than to the more distantly related genus Sporothrix (Ophiostomatales). Therefore, it is not clear if this strain is still the original producer strain or a contamination. Due to this fact the fermentation was not repeated with the parameters described by Krohn et al. (1994).
Bioactivity of sporothriolide
Sporothriolide (1) was found devoid of activity against Gram-positive and Gram-negative bacteria. Furthermore, no cytotoxic effects on the cell lines HCT-116, CHO-K1 and U-2 OS were observed. Strong antifungal activity was found on tested filamentous fungi and yeasts, including Candida albicans (Table 3). Metabolites 2 and 4 showed no activity, 3 and 5 were not tested due to the fact that only minor amounts were available. Our results of the bioactivity of sporothriolide (1) and dihydrosporothriolide (2) resemble the study of Krohn et al. (1994) which used an agar plate diffusion assay. The strong antifungal activity of furofurandiones has furthermore previously been described for canadensolide (6) (McCorkindale et al. 1968), ethiosolide (Atienza et al. 1992) and avenaciolide (Brookes et al. 1963).
Table 3.
Minimal inhibitory concentrations (MIC) of sporothriolide (1), dihydrosporothriolide (2) and isosporothric acid (4), all solved in MeOH, and control drugs. [a] Oxytetracyclin hydrochloride, [b] Gentamycin, [c] Nystatin, [d] Amphotericin B, [e] Polymyxin B sulphate; -: no inhibition; n.t.: not tested. The cell density was adjusted to 8 × 106 cells/ml. * spores from agar plate were applied without justification.
| Test organisms | MIC [µg/ml] |
|||
|---|---|---|---|---|
| 1 | 2 | 4 | Reference | |
| Mucor hiemalis* (DSM 2656) | 4.2 | n.t. | n.t. | 5.25 [c] |
| Candida albicans (DSM 1665) | 16.6 | — | — | 8.3–33.3 [c] |
| Nematospora coryli | 8.3 | n.t. | n.t. | 67.0 [c] |
| Pichia anomala (DSM 8766) | 33.3 | — | — | 8.3–33.3 [c] |
| Rhodotorula glutinis (DSM 10134) | 16.6 | n.t. | n.t | 16.7 [c] |
| Schizosaccharomyces pombe (DSM 70572) | 8.3 | n.t. | n.t. | 41.5 [c] |
| Trichosporon oleaginosus (DSM 11815) | 16.6 | n.t. | n.t. | 4.2 [c] |
| Bacillus subtilis (DSM 10) | — | — | — | 33.3 [a] |
| E. coli (DSM 1116) | — | — | — | 0.83 [a] |
| Pseudomonas aeruginosa (DSM 50071) | — | — | — | 16.6 [b] |
Discussion
Furofurandiones are a common class of fungal α–methylene-γ-butyrolactones (Kitson et al. 2009). The metabolites can retro-biosynthetically be cleaved and they are probably being generated from a fatty acid and a C3 moiety, which originates from oxaloacetate (Chesters and O´Hagan 1997). Those natural products resulting from condensation of the α-position of the fatty acid are referred as type A metabolites and include sporothriolide (Krohn et al. 1994), canadensolide (McCorkindale et al. 1968) and xylobovide (Abate et al. 1997). In contrast, in isomeric type B metabolites like ethisolide (Atienza et al. 1992) and avenaciolide (Brookes et al. 1963) the C3 unit is linked to the β-position of the fatty acid chain.
Although metabolites 3–5 might be artifacts from saponification of 1, their presence in the crude extract of H. monticulosum as well as previous reports on the chemically similar canadensolide derivatives indicate an origin as natural products. Apart from canadensolide and dihydrocandensoide, a number of related metabolites were isolated from cultures of Penicillium canadense (Hayes 1982, and references cited therein). The biosynthesis of canadensolide (6) and its derivatives dihydrocanadensolide (7), canadensic acid (8), isocanadensic acid and dihydroisocanadensic acid (9) was investigated with extensive labeling studies using radiolabeled or 13C-labeled precursors. These experiments indicated that 7–9 are not precursors of 6, but are generated from common precursors 10b and 11b. According to the structural similarity of sporothriolide to candensolide, we can deduce the biosynthetic relationship between metabolites 1–5 as shown in Figure 4. Therefore 2–5 can be considered as shunt products, but not as precursors of sporothriolide (1).
Figure 4.
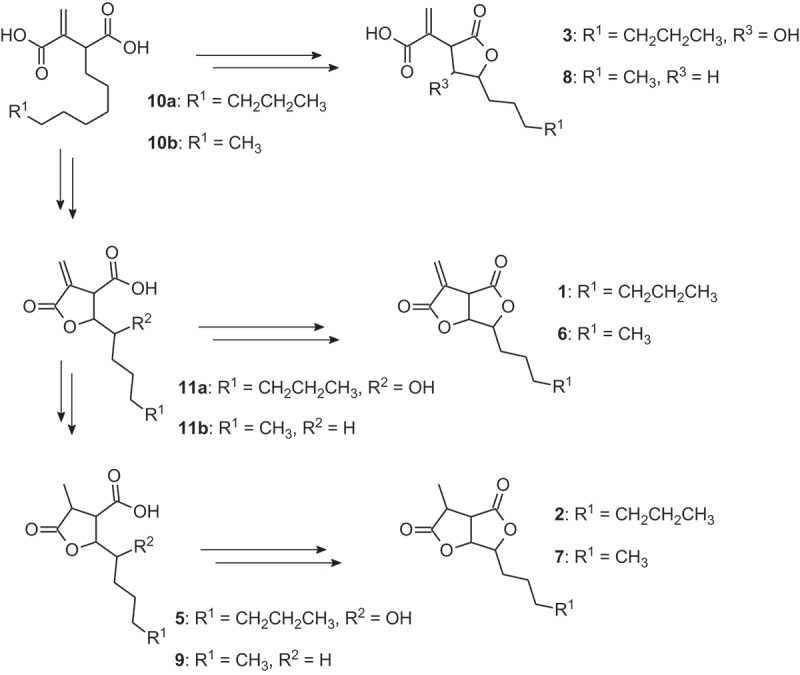
Hypothetical biosynthesis of sporothriolide (1), canadensolide (6) and their respective derivatives. Their relationship is indicated by feeding experiments with canadensolide derivatives by Hayes (1982).
Furofurandiones were previously described from two different strains of the genus Xylaria. Xylobovide was produced in cultures of Xylaria obovata (Abate et al. 1997), whereas a stereoisomer of dihydrosporothriolide was reported from cultures of two endophytes tentatively assigned to the genus Xylaria merely based on ITS sequencing (Isaka et al. 2010). However, this is the first report of these compounds being produced by Hypoxylon sp. So far the metabolite was only found in cultures of H. monticulosum. This observation becomes valuable due to the fact that stromatal extracts of the species contain only a minor unidentified compound, which is produced by young stromata. Older material is often devoid of this metabolite. Bitzer et al. (2008) already established strong correlations between chemotypes according to secondary metabolite production in culture and molecular phylogenetic data. They assigned H. monticulosum in the chemotype B, which is defined by the presence of 5-methymellein and the lack of other marker metabolites like mellein, 1–8-naphthol or isosclerones. However, the strain of H. monticulosum they used meanwhile turned out to be contaminated by an Annulohypoxylon species (M.S. & D. Persoh, unpublished data). Our new isolates of H. monticulosum were found devoid of 5-methylmellein.
From our experience, by far not all metabolites of Xylariaceae cultures can be used as chemotaxonomic markers, as the occurrence of certain compounds may be strongly dependent on the culture conditions, and some metabolites may even be strain specific. Therefore, we cannot exclude that the strains of species other than H. monticulosum we studied previously or concurrently do not contain traces of sporothriolide. However, sporothriolide was produced under various conditions in H. monticulosium, even when applying completely different media. Therefore, it might be a species-specific chemotaxonomic marker compound.
As the original producer strain was identified as Sporothix sp. a close relationship between this strain and H. monticulosum was expected due to the fact that sporothrix-like conidial states were defined as a subtype of nodulisporium-like asexual states of Hypoxylon sensu Ju and Rogers (1996). They also represent the asexual morphs of other genera in the hypoxyloid Xylariaceae, including Daldinia (Stadler et al. 2014), Phylacia (Bitzer et al. 2008), Rhopalostroma (Stadler, Fournier, Gardt, et al. 2010), Ruwenzoria (Stadler, Fournier, Læssøe, Decock, et al. 2010) and Thamnomyces (Stadler, Fournier, Læssøe, Chlebicki, et al. 2010). Several of these species are known to have asexual morphs reminiscent of Sporothrix. However, the molecular phylogenetic data generated from the original sporothriolide producer strain clearly revealed it to belong to the Hypocreales. Aside from the above cited papers on hypoxyloid genera, even the strains obtained by Bills et al. (2012), Fournier, Köpcke, et al. (2010), Fournier, Stadler et al. (2010), Fournier et al. (2011), Læssøe et al. (2013), Persoh et al. (2009) were all checked for antifungal effects and by chemotaxonomic methodology, but no sporothriolide was detected by HPLC–MS and prominent antifungal effects of the Xylaria sp. studied were always due to the overproduction of cytochalasins and other compounds.
Specific antifungal activity with lack of cytotoxicity on mammalian cell lines or antibacterial activity is a distinctive screening signal. Frequently, antifungal compounds affect the same targets in other eukaryotic cells (Roemer et al. 2011), and even in the case for a drug with a nonspecific mode-of-action like nystatin (Zheng and Audus 1994). Due to nystatin’s toxicity, it can only be applied as an oral or topical treatment. Forthcoming studies on the mode-of-action of sporothriolide might reveal a new target for antifungal treatment with reduced side effects.
Acknowledgments
Financial support by the German Academic Exchange Service (DAAD) and the Thai Royal Golden Ph.D. Jubilee-Industry program (RGJ) for a joint TRF-DAAD PPP academic exchange grant to Kevin D. Hyde, Eric Kuhnert, Frank Surup and Marc Stadler is gratefully acknowledged. Regis Courtecuisse and Christian Lechat are thanked for their continuous support in facilitating field work and finding important specimens during the field trips in the Neotropics and elsewhere. The field sampling in French Guiana has also benefited from ‘Investissements d'Avenir’ grants of the ANR (CEBA ANR-10-LABX-0025, CNRS Cayenne and Toulouse) to Jacques Fournier and coworkers. We are grateful to Barbara Schulz for providing the original sporothriolide producing strain from her collection at TU Braunschweig. Furthermore, we gratefully acknowledge the help of our colleagues at the HZI, Jennifer Herrmann and Wera Collisi for conducting the bioassays and Christel Kakoschke for recording NMR spectra. For measurements of HPLC–MS data, we are grateful to Aileen Teichmann and Heinrich Steinmetz. Finally, we thank Evgeny Prusov for the support with the hydrogenation reaction.
References
- Abate D, Abraham W-R, Meyer H Cytochalasins and phytotoxins from the fungus Xylaria obovata . Phytochemistry. 1997;44:1443–1448. doi: 10.1016/S0031-9422(96)00780-7. [DOI] [Google Scholar]
- Atienza J, Hernandez E, Primo J Isolation and identification of ethisolide as an antibiotic product from Penicillium capsulatum . Appl Microbiol Biotechnol. 1992;37:298–300. doi: 10.1007/BF00210981. [DOI] [PubMed] [Google Scholar]
- Bills GF, González-Menéndez V, Martín J, Platas G, Fournier J, Peršoh D, Stadler M Hypoxylon pulicicidum sp. nov. (Ascomycota, Xylariales), a pantropical insecticide-producing endophyte. PLoS ONE. 2012;7:e46687. doi: 10.1371/journal.pone.0046687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzer J, Köpcke B, Stadler M, Hellwig V, Ju YM, Seip S, Henkel T Accelerated dereplication of natural products, supported by reference libraries. Chimia. 2007;61:332–338. doi: 10.2533/chimia.2007.332. [DOI] [Google Scholar]
- Bitzer J, Læssøe T, Fournier J, Kummer V, Decock C, Tichy HV, Piepenbring M, Peršoh D, Stadler M Affinities of Phylacia and the daldinoid Xylariaceae, inferred from chemotypes of cultures and ribosomal DNA sequences. Mycol Res. 2008;112:251–270. doi: 10.1016/j.mycres.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Brookes D, Tidd BK, Turner WB 1028. Avenaciolide, an antifungal lactone from Aspergillus avenaceus . J Chem Soc. 1963:5385–5391. doi: 10.1039/jr9630005385. [DOI] [Google Scholar]
- Chesters NCJE, O´Hagan D Biosynthesis of the fungal metabolite, piliformic acid (2-hexylidene-3-methylsuccinic acid) J Chem Soc. 1997;8:927–834. [Google Scholar]
- Cooper MA, Shlaes D Fix the antibiotics pipeline. Nature. 2011;472:32. doi: 10.1038/472032a. [DOI] [PubMed] [Google Scholar]
- Dictionary of natural products on DVD . Dictionary of natural Products on DVD. Boca Raton (FL): CRC Press; 2013. [Google Scholar]
- Fournier J, Flessa F, Peršoh D, Stadler M Three new Xylaria species from Southwestern Europe. Mycol Progr. 2011;10:33–52. doi: 10.1007/s11557-010-0671-8. [DOI] [Google Scholar]
- Fournier J, Köpcke B, Stadler M New species of Hypoxylon from Western Europe and Ethiopia. Mycotaxon. 2010;113:209–235. doi: 10.5248/113.209. [DOI] [Google Scholar]
- Fournier J, Stadler M, Hyde KD, Duong ML The new genus Rostrohypoxylon and two new Annulohypoxylon species from Northern Thailand. Fungal Divers. 2010;40:23–36. doi: 10.1007/s13225-010-0026-4. [DOI] [Google Scholar]
- Halecker S, Surup F, Kuhnert E, Mohr KI, Brock NL, Dickschat JS, Junker C, Schulz B, Stadler M Hymenosetin, a 3-decalinoyltetramic acid antibiotic from cultures of the ash dieback pathogen, Hymenoscyphus pseudoalbidus . Phytochemistry. 2014;100:86–91. doi: 10.1016/j.phytochem.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Hayes D . Intermediates in the biosynthesis of bislactone antibiotics. University of Glasgow; 1982. [PhD thesis] [Google Scholar]
- Herrmann J, Elnakady YA, Wiedmann RM, Ullrich A, Rohde M, Kazmaier U, Vollmar AM, Müller R Pretubulysin: from hypothetical biosynthetic intermediate to potential lead in tumor therapy. Plos ONE. 2012;7:e37416. doi: 10.1371/journal.pone.0037416.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaka M, Chinthanom P, Boonruangprapa T, Rungjindamai N, Pinruan U Eremophilane-type sesquiterpenes from the fungus Xylaria sp. BCC 21097. J Nat Prod. 2010;73:683–687. doi: 10.1021/np100030x. [DOI] [PubMed] [Google Scholar]
- Ju Y-M, Rogers JD . A revision of the genus Hypoxylon. St. Paul, MN: APS Press; 1996. p. 365. [Google Scholar]
- Kitson RRA, Millemaggi A, Taylor RJK The renaissance of α-methylene-γ-butyrolactones: new synthetic approaches. Angew Chem Int Ed. 2009;48:9426–9451. doi: 10.1002/anie.200903108. [DOI] [PubMed] [Google Scholar]
- Krohn K, Ludewig K, Aust H-J, Draeger S, Schulz B Biologically active metabolites from fungi. 3. sporothriolide, discosiolide, and 4-epi-ethisolide new furofurandiones from Sporothrix sp., Discosia sp., and Pezicula livida . J Antibiot. 1994;47:113–118. doi: 10.7164/antibiotics.47.113. [DOI] [PubMed] [Google Scholar]
- Kuhnert E, Fournier J, Peršoh D, Luangsa-ard JJ, Stadler M New Hypoxylon species from Martinique and new evidence on the molecular phylogeny of Hypoxylon based on ITS rDNA and β-tubulin data. Fungal Divers. 2014;64:181–203. doi: 10.1007/s13225-013-0264-3. [DOI] [Google Scholar]
- Kuhnert E, Heitkämper S, Fournier J, Surup F, Stadler M Hypoxyvermelhotins A-C, new pigments from Hypoxylon lechatii sp. nov. Fungal Biol. 2014;118:242–252. doi: 10.1016/j.funbio.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Læssøe T, Srikitikulchai P, Luangsa-ard JJD, Stadler M Theissenia reconsidered, including molecular phylogeny of the type species T. pyrenocrata and a new genus Durotheca (Xylariaceae, Ascomycota) IMA Fungus. 2013;4:57–69. doi: 10.5598/imafungus.2013.04.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCorkindale NJ, Wright JLC, Brian PW, Clarke SM, Hutchinson SA Canadensolide – an antifungal metabolite of Penicillium canadense . Tetrahedron Lett. 1968;9:727–730. doi: 10.1016/S0040-4039(00)75621-8. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pažoutová S, Follert S, Bitzer J, Keck M, Surup F, Šrůtka P, Holuša J, Stadler M A new endophytic insect-associated Daldinia species, recognised from a comparison of secondary metabolite profiles and molecular phylogeny. Fungal Divers. 2013;60:107–123. doi: 10.1007/s13225-013-0238-5. [DOI] [Google Scholar]
- Peršoh D, Melcher M, Graf K, Fournier J, Stadler M, Rambold G Molecular and morphological evidence for the delimitation of Xylaria hypoxylon . Mycologia. 2009;101:256–268. doi: 10.3852/08-108. [DOI] [PubMed] [Google Scholar]
- Quang DN, Hashimoto T, Fournier J, Stadler M, Radulović N, Asakawa Y Sassafrins A-D, new antimicrobial azaphilones from the fungus Creosphaeria sassafras. Tetrahedron. 2005;61:1743–1748. doi: 10.1016/j.tet.2004.12.031. [DOI] [Google Scholar]
- Quang DN, Stadler M, Fournier J, Asakawa Y Carneic acids A and B, Chemotaxonomically significant antimicrobial agents from the Xylariaceous Ascomycete Hypoxyloncarneum . J Nat Prod. 2006;69:1198–1202. doi: 10.1021/np0602057. [DOI] [PubMed] [Google Scholar]
- Roemer T, Xu D, Singh SB, Parish CA, Harris G, Wang H, Davies JE, Bills GF Confronting the challenges of natural product-based antifungal discovery. Chem Biol. 2011;18:148–164. doi: 10.1016/j.chembiol.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Somerville AN Scifinder scholar. J Chem Educ. 1998;75(959):975–976. [Google Scholar]
- Stadler M Importance of secondary metabolites in the Xylariaceae as parameters for assessment of their taxonomy, phylogeny, and functional biodiversity. Curr Res Environ Appl Mycol J Fungal Biol. 2011;1:75–133. doi: 10.5943/cream/1/2/1. [DOI] [Google Scholar]
- Stadler M, Fournier J Pigment chemistry, taxonomy and phylogeny of the hypoxyloideae (Xylariaceae) Rev Iberoam Micol. 2006;23:160–170. doi: 10.1016/S1130-1406(06)70037-7. [DOI] [PubMed] [Google Scholar]
- Stadler M, Fournier J, Gardt S, Peršoh D The phylogenetic position of Rhopalostroma as inferred from a polythetic approach. Persoonia. 2010;25:11–21. doi: 10.3767/003158510X524231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler M, Fournier J, Lassoe T, Chlebicki A, Lechat C, Flessa F, Rambold G, Peršoh D Chemotaxonomic and phylogenetic studies of Thamnomyces (Xylariaceae) Mycoscience. 2010;51:189–207. doi: 10.1007/S10267-009-0028-9. [DOI] [Google Scholar]
- Stadler M, Fournier J, Læssøe T, Decock C, Peršoh D, Rambold G Ruwenzoria, a new genus of the Xylariaceae from central africa. Mycol Progr. 2010;9:169–179. doi: 10.1007/s11557-009-0623-3. [DOI] [Google Scholar]
- Stadler M, Hellwig V Chemotaxonomy of the Xylariaceae and remarkable bioactive compounds from Xylariales and their associated asexual stages. Recent Res Devel Phytochem. 2005;9:41–93. [Google Scholar]
- Stadler M, Laessoe T, Fournier J, Decock C, Schmieschek B, Tichy HV, Peršoh D A polyphasic taxonomy of Daldinia (Xylariaceae) Stud Mycol. 2014;77:1–143. doi: 10.3114/sim0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler M, Quang DN, Tomita A, Hashimoto T, Asakawa Y Changes in secondary metabolism during stromatal ontogeny of Hypoxylon fragiforme . Mycol Res. 2006;110:811–820. doi: 10.1016/j.mycres.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Stadler M, Wollweber H, Mühllbauer A, Henkel T, Asakawa Y, Hashimoto T, Rogers JD, Ju YM, Wetzstein HG, Tichy HV Secondary metabolite profiles, genetic fingerprints and taxonomy of Daldinia and allies. Mycotaxon. 2001;77:379–429. [Google Scholar]
- Surup F, Mohr KI, Jansen R, Stadler M Cohaerins G-K, azaphilone pigments from Annulohypoxylon cohaerens and absolute stereochemistry of cohaerins C-K. Phytochemistry. 2013;95:252–258. doi: 10.1016/j.phytochem.2013.07.027. [DOI] [PubMed] [Google Scholar]
- Whalley AJS, Edwards RL Secondary metabolites and systematic arrangement within the Xylariaceae. Can J Bot. 1995;73(Suppl S1):S802–S810. doi: 10.1139/b95-325. [DOI] [Google Scholar]
- Zheng H, Audus KL Cytotoxic effects of chlorhexidine and nystatin on cultured hamster buccal epithelial cells. Int J Pharm. 1994;101:121–126. doi: 10.1016/0378-5173(94)90083-3. [DOI] [Google Scholar]


