Abstract
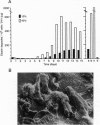
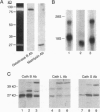
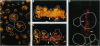
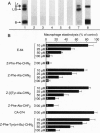
Human macrophages are believed to damage host tissues in chronic inflammatory disease states, but these cells have been reported to express only modest degradative activity in vitro. However, while examining the ability of human monocytes to degrade the extracellular matrix component elastin, we identified culture conditions under which the cells matured into a macrophage population that displayed a degradative phenotype hundreds of times more destructive than that previously ascribed to any other cell population. The monocyte-derived macrophages synthesized elastinolytic matrix metalloproteinases (i.e., gelatinase B and matrilysin) as well as cysteine proteinases (i.e., cathepsins B, L, and S), but only the cathepsins were detected in the extracellular milieu as fully processed, mature enzymes by either vital fluorescence or active-site labeling. Consistent with these observations, macrophage-mediated elastinolytic activity was not affected by matrix metalloproteinase inhibitors but could be almost completely abrogated by inhibiting cathepsins L and S. These data demonstrate that human macrophages mobilize cysteine proteinases to arm themselves with a powerful effector mechanism that can participate in the pathophysiologic remodeling of the extracellular matrix.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achkar C., Gong Q. M., Frankfater A., Bajkowski A. S. Differences in targeting and secretion of cathepsins B and L by BALB/3T3 fibroblasts and Moloney murine sarcoma virus-transformed BALB/3T3 fibroblasts. J Biol Chem. 1990 Aug 15;265(23):13650–13654. [PubMed] [Google Scholar]
- Baricos W. H., Cortez S. L., Le Q. C., Wu L. T., Shaw E., Hanada K., Shah S. V. Evidence suggesting a role for cathepsin L in an experimental model of glomerulonephritis. Arch Biochem Biophys. 1991 Aug 1;288(2):468–472. doi: 10.1016/0003-9861(91)90222-5. [DOI] [PubMed] [Google Scholar]
- Busiek D. F., Ross F. P., McDonnell S., Murphy G., Matrisian L. M., Welgus H. G. The matrix metalloprotease matrilysin (PUMP) is expressed in developing human mononuclear phagocytes. J Biol Chem. 1992 May 5;267(13):9087–9092. [PubMed] [Google Scholar]
- Busiek D. F., Ross F. P., McDonnell S., Murphy G., Matrisian L. M., Welgus H. G. The matrix metalloprotease matrilysin (PUMP) is expressed in developing human mononuclear phagocytes. J Biol Chem. 1992 May 5;267(13):9087–9092. [PubMed] [Google Scholar]
- Buttle D. J., Bonner B. C., Burnett D., Barrett A. J. A catalytically active high-Mr form of human cathepsin B from sputum. Biochem J. 1988 Sep 15;254(3):693–699. doi: 10.1042/bj2540693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttle D. J., Bonner B. C., Burnett D., Barrett A. J. A catalytically active high-Mr form of human cathepsin B from sputum. Biochem J. 1988 Sep 15;254(3):693–699. doi: 10.1042/bj2540693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttle D. J., Handley C. J., Ilic M. Z., Saklatvala J., Murata M., Barrett A. J. Inhibition of cartilage proteoglycan release by a specific inactivator of cathepsin B and an inhibitor of matrix metalloproteinases. Evidence for two converging pathways of chondrocyte-mediated proteoglycan degradation. Arthritis Rheum. 1993 Dec;36(12):1709–1717. doi: 10.1002/art.1780361210. [DOI] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Reilly J. J., Jr, Kobzik L. Role of plasminogen activator in degradation of extracellular matrix protein by live human alveolar macrophages. Am Rev Respir Dis. 1988 Feb;137(2):412–419. doi: 10.1164/ajrccm/137.2.412. [DOI] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Reilly J. J., Jr, Yee R., Grubb A. Identification of cystatin C, a cysteine proteinase inhibitor, as a major secretory product of human alveolar macrophages in vitro. Am Rev Respir Dis. 1990 Mar;141(3):698–705. doi: 10.1164/ajrccm/141.3.698. [DOI] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Stone O. L. Comparison of live human neutrophil and alveolar macrophage elastolytic activity in vitro. Relative resistance of macrophage elastolytic activity to serum and alveolar proteinase inhibitors. J Clin Invest. 1984 Nov;74(5):1693–1700. doi: 10.1172/JCI111586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Stone O. L., Vavrin Z. Degradation of fibrin and elastin by intact human alveolar macrophages in vitro. Characterization of a plasminogen activator and its role in matrix degradation. J Clin Invest. 1984 Mar;73(3):806–815. doi: 10.1172/JCI111275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Circolo A., Welgus H. G., Pierce G. F., Kramer J., Strunk R. C. Differential regulation of the expression of proteinases/antiproteinases in fibroblasts. Effects of interleukin-1 and platelet-derived growth factor. J Biol Chem. 1991 Jul 5;266(19):12283–12288. [PubMed] [Google Scholar]
- Corticchiato O., Cajot J. F., Abrahamson M., Chan S. J., Keppler D., Sordat B. Cystatin C and cathepsin B in human colon carcinoma: expression by cell lines and matrix degradation. Int J Cancer. 1992 Oct 21;52(4):645–652. doi: 10.1002/ijc.2910520425. [DOI] [PubMed] [Google Scholar]
- Cullen B. M., Halliday I. M., Kay G., Nelson J., Walker B. The application of a novel biotinylated affinity label for the detection of a cathepsin B-like precursor produced by breast-tumour cells in culture. Biochem J. 1992 Apr 15;283(Pt 2):461–465. doi: 10.1042/bj2830461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. M., Halliday I. M., Kay G., Nelson J., Walker B. The application of a novel biotinylated affinity label for the detection of a cathepsin B-like precursor produced by breast-tumour cells in culture. Biochem J. 1992 Apr 15;283(Pt 2):461–465. doi: 10.1042/bj2830461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaissé J. M., Ledent P., Vaes G. Collagenolytic cysteine proteinases of bone tissue. Cathepsin B, (pro)cathepsin L and a cathepsin L-like 70 kDa proteinase. Biochem J. 1991 Oct 1;279(Pt 1):167–174. doi: 10.1042/bj2790167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers P. E., Mookhtiar K., Van Wart H. E., Hasty K. A., Weiss S. J. Proteolytic inactivation of alpha 1-proteinase inhibitor and alpha 1-antichymotrypsin by oxidatively activated human neutrophil metalloproteinases. J Biol Chem. 1992 Mar 5;267(7):5005–5012. [PubMed] [Google Scholar]
- Desrochers P. E., Mookhtiar K., Van Wart H. E., Hasty K. A., Weiss S. J. Proteolytic inactivation of alpha 1-proteinase inhibitor and alpha 1-antichymotrypsin by oxidatively activated human neutrophil metalloproteinases. J Biol Chem. 1992 Mar 5;267(7):5005–5012. [PubMed] [Google Scholar]
- Dong J. M., Prence E. M., Sahagian G. G. Mechanism for selective secretion of a lysosomal protease by transformed mouse fibroblasts. J Biol Chem. 1989 May 5;264(13):7377–7383. [PubMed] [Google Scholar]
- Dong J. M., Prence E. M., Sahagian G. G. Mechanism for selective secretion of a lysosomal protease by transformed mouse fibroblasts. J Biol Chem. 1989 May 5;264(13):7377–7383. [PubMed] [Google Scholar]
- Esser R. E., Angelo R. A., Murphey M. D., Watts L. M., Thornburg L. P., Palmer J. T., Talhouk J. W., Smith R. E. Cysteine proteinase inhibitors decrease articular cartilage and bone destruction in chronic inflammatory arthritis. Arthritis Rheum. 1994 Feb;37(2):236–247. doi: 10.1002/art.1780370213. [DOI] [PubMed] [Google Scholar]
- Esser R. E., Angelo R. A., Murphey M. D., Watts L. M., Thornburg L. P., Palmer J. T., Talhouk J. W., Smith R. E. Cysteine proteinase inhibitors decrease articular cartilage and bone destruction in chronic inflammatory arthritis. Arthritis Rheum. 1994 Feb;37(2):236–247. doi: 10.1002/art.1780370213. [DOI] [PubMed] [Google Scholar]
- Everts V., Delaissé J. M., Korper W., Niehof A., Vaes G., Beertsen W. Degradation of collagen in the bone-resorbing compartment underlying the osteoclast involves both cysteine-proteinases and matrix metalloproteinases. J Cell Physiol. 1992 Feb;150(2):221–231. doi: 10.1002/jcp.1041500202. [DOI] [PubMed] [Google Scholar]
- Everts V., Delaissé J. M., Korper W., Niehof A., Vaes G., Beertsen W. Degradation of collagen in the bone-resorbing compartment underlying the osteoclast involves both cysteine-proteinases and matrix metalloproteinases. J Cell Physiol. 1992 Feb;150(2):221–231. doi: 10.1002/jcp.1041500202. [DOI] [PubMed] [Google Scholar]
- Goretzki L., Schmitt M., Mann K., Calvete J., Chucholowski N., Kramer M., Günzler W. A., Jänicke F., Graeff H. Effective activation of the proenzyme form of the urokinase-type plasminogen activator (pro-uPA) by the cysteine protease cathepsin L. FEBS Lett. 1992 Feb 3;297(1-2):112–118. doi: 10.1016/0014-5793(92)80339-i. [DOI] [PubMed] [Google Scholar]
- Huet G., Flipo R. M., Colin C., Janin A., Hemon B., Collyn-d'Hooghe M., Lafyatis R., Duquesnoy B., Degand P. Stimulation of the secretion of latent cysteine proteinase activity by tumor necrosis factor alpha and interleukin-1. Arthritis Rheum. 1993 Jun;36(6):772–780. doi: 10.1002/art.1780360606. [DOI] [PubMed] [Google Scholar]
- Huet G., Flipo R. M., Colin C., Janin A., Hemon B., Collyn-d'Hooghe M., Lafyatis R., Duquesnoy B., Degand P. Stimulation of the secretion of latent cysteine proteinase activity by tumor necrosis factor alpha and interleukin-1. Arthritis Rheum. 1993 Jun;36(6):772–780. doi: 10.1002/art.1780360606. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr Current concepts: immunology. Monocytes and macrophages. N Engl J Med. 1988 Mar 24;318(12):747–752. doi: 10.1056/NEJM198803243181205. [DOI] [PubMed] [Google Scholar]
- Kane S. E., Gottesman M. M. The role of cathepsin L in malignant transformation. Semin Cancer Biol. 1990 Apr;1(2):127–136. [PubMed] [Google Scholar]
- Keppler D., Waridel P., Abrahamson M., Bachmann D., Berdoz J., Sordat B. Latency of cathepsin B secreted by human colon carcinoma cells is not linked to secretion of cystatin C and is relieved by neutrophil elastase. Biochim Biophys Acta. 1994 May 25;1226(2):117–125. doi: 10.1016/0925-4439(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Kindzelskii A. L., Xue W., Todd R. F., 3rd, Boxer L. A., Petty H. R. Aberrant capping of membrane proteins on neutrophils from patients with leukocyte adhesion deficiency. Blood. 1994 Mar 15;83(6):1650–1655. [PubMed] [Google Scholar]
- Kobayashi H., Moniwa N., Sugimura M., Shinohara H., Ohi H., Terao T. Effects of membrane-associated cathepsin B on the activation of receptor-bound prourokinase and subsequent invasion of reconstituted basement membranes. Biochim Biophys Acta. 1993 Jul 28;1178(1):55–62. doi: 10.1016/0167-4889(93)90109-3. [DOI] [PubMed] [Google Scholar]
- Kobayashi J., Wigle D., Childs T., Zhu L., Keeley F. W., Rabinovitch M. Serum-induced vascular smooth muscle cell elastolytic activity through tyrosine kinase intracellular signalling. J Cell Physiol. 1994 Jul;160(1):121–131. doi: 10.1002/jcp.1041600115. [DOI] [PubMed] [Google Scholar]
- Kominami E., Tsukahara T., Hara K., Katunuma N. Biosyntheses and processing of lysosomal cysteine proteinases in rat macrophages. FEBS Lett. 1988 Apr 11;231(1):225–228. doi: 10.1016/0014-5793(88)80736-1. [DOI] [PubMed] [Google Scholar]
- Kominami E., Tsukahara T., Hara K., Katunuma N. Biosyntheses and processing of lysosomal cysteine proteinases in rat macrophages. FEBS Lett. 1988 Apr 11;231(1):225–228. doi: 10.1016/0014-5793(88)80736-1. [DOI] [PubMed] [Google Scholar]
- Kreutz M., Andreesen R. Induction of human monocyte to macrophage maturation in vitro by 1,25-dihydroxyvitamin D3. Blood. 1990 Dec 15;76(12):2457–2461. [PubMed] [Google Scholar]
- Laszlo D. J., Henson P. M., Remigio L. K., Weinstein L., Sable C., Noble P. W., Riches D. W. Development of functional diversity in mouse macrophages. Mutual exclusion of two phenotypic states. Am J Pathol. 1993 Aug;143(2):587–597. [PMC free article] [PubMed] [Google Scholar]
- Mach L., Stüwe K., Hagen A., Ballaun C., Glössl J. Proteolytic processing and glycosylation of cathepsin B. The role of the primary structure of the latent precursor and of the carbohydrate moiety for cell-type-specific molecular forms of the enzyme. Biochem J. 1992 Mar 1;282(Pt 2):577–582. doi: 10.1042/bj2820577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach L., Stüwe K., Hagen A., Ballaun C., Glössl J. Proteolytic processing and glycosylation of cathepsin B. The role of the primary structure of the latent precursor and of the carbohydrate moiety for cell-type-specific molecular forms of the enzyme. Biochem J. 1992 Mar 1;282(Pt 2):577–582. doi: 10.1042/bj2820577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciewicz R. A., Wardale R. J., Etherington D. J., Paraskeva C. Immunodetection of cathepsins B and L present in and secreted from human pre-malignant and malignant colorectal tumour cell lines. Int J Cancer. 1989 Mar 15;43(3):478–486. doi: 10.1002/ijc.2910430323. [DOI] [PubMed] [Google Scholar]
- Maciewicz R. A., Wardale R. J., Etherington D. J., Paraskeva C. Immunodetection of cathepsins B and L present in and secreted from human pre-malignant and malignant colorectal tumour cell lines. Int J Cancer. 1989 Mar 15;43(3):478–486. doi: 10.1002/ijc.2910430323. [DOI] [PubMed] [Google Scholar]
- Mason R. W., Bartholomew L. T., Hardwick B. S. The use of benzyloxycarbonyl[125I]iodotyrosylalanyldiazomethane as a probe for active cysteine proteinases in human tissues. Biochem J. 1989 Nov 1;263(3):945–949. doi: 10.1042/bj2630945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W., Bartholomew L. T., Hardwick B. S. The use of benzyloxycarbonyl[125I]iodotyrosylalanyldiazomethane as a probe for active cysteine proteinases in human tissues. Biochem J. 1989 Nov 1;263(3):945–949. doi: 10.1042/bj2630945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W., Massey S. D. Surface activation of pro-cathepsin L. Biochem Biophys Res Commun. 1992 Dec 30;189(3):1659–1666. doi: 10.1016/0006-291x(92)90268-p. [DOI] [PubMed] [Google Scholar]
- Mason R. W., Massey S. D. Surface activation of pro-cathepsin L. Biochem Biophys Res Commun. 1992 Dec 30;189(3):1659–1666. doi: 10.1016/0006-291x(92)90268-p. [DOI] [PubMed] [Google Scholar]
- Mason R. W., Wilcox D., Wikstrom P., Shaw E. N. The identification of active forms of cysteine proteinases in Kirsten-virus-transformed mouse fibroblasts by use of a specific radiolabelled inhibitor. Biochem J. 1989 Jan 1;257(1):125–129. doi: 10.1042/bj2570125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy K., Musson R. A., Henson P. M. Protein synthesis-dependent and protein synthesis-independent secretion of lysosomal hydrolases from rabbit and human macrophages. J Reticuloendothel Soc. 1982 Feb;31(2):131–144. [PubMed] [Google Scholar]
- McCarthy K., Musson R. A., Henson P. M. Protein synthesis-dependent and protein synthesis-independent secretion of lysosomal hydrolases from rabbit and human macrophages. J Reticuloendothel Soc. 1982 Feb;31(2):131–144. [PubMed] [Google Scholar]
- Pawlowski N. A., Abraham E. L., Pontier S., Scott W. A., Cohn Z. A. Human monocyte-endothelial cell interaction in vitro. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8208–8212. doi: 10.1073/pnas.82.23.8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Erickson A. H., Kochan J., Ravetch J. V., Unkeless J. C. Cloning and characterization of a mouse cysteine proteinase. J Biol Chem. 1986 Nov 5;261(31):14697–14703. [PubMed] [Google Scholar]
- Portnoy D. A., Erickson A. H., Kochan J., Ravetch J. V., Unkeless J. C. Cloning and characterization of a mouse cysteine proteinase. J Biol Chem. 1986 Nov 5;261(31):14697–14703. [PubMed] [Google Scholar]
- Reilly J. J., Jr, Chen P., Sailor L. Z., Wilcox D., Mason R. W., Chapman H. A., Jr Cigarette smoking induces an elastolytic cysteine proteinase in macrophages distinct from cathepsin L. Am J Physiol. 1991 Aug;261(2 Pt 1):L41–L48. doi: 10.1152/ajplung.1991.261.2.L41. [DOI] [PubMed] [Google Scholar]
- Reilly J. J., Jr, Mason R. W., Chen P., Joseph L. J., Sukhatme V. P., Yee R., Chapman H. A., Jr Synthesis and processing of cathepsin L, an elastase, by human alveolar macrophages. Biochem J. 1989 Jan 15;257(2):493–498. doi: 10.1042/bj2570493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan A. D., Mason P., Mach L., Mort J. S. Rat procathepsin B. Proteolytic processing to the mature form in vitro. J Biol Chem. 1992 Aug 5;267(22):15993–15999. [PubMed] [Google Scholar]
- Rowan A. D., Mason P., Mach L., Mort J. S. Rat procathepsin B. Proteolytic processing to the mature form in vitro. J Biol Chem. 1992 Aug 5;267(22):15993–15999. [PubMed] [Google Scholar]
- Senior R. M., Connolly N. L., Cury J. D., Welgus H. G., Campbell E. J. Elastin degradation by human alveolar macrophages. A prominent role of metalloproteinase activity. Am Rev Respir Dis. 1989 May;139(5):1251–1256. doi: 10.1164/ajrccm/139.5.1251. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Fliszar C. J., Shapiro S. D., Goldberg G. I., Welgus H. G. Human 92- and 72-kilodalton type IV collagenases are elastases. J Biol Chem. 1991 Apr 25;266(12):7870–7875. [PubMed] [Google Scholar]
- Shapiro S. D., Campbell E. J., Welgus H. G., Senior R. M. Elastin degradation by mononuclear phagocytes. Ann N Y Acad Sci. 1991;624:69–80. doi: 10.1111/j.1749-6632.1991.tb17007.x. [DOI] [PubMed] [Google Scholar]
- Shaw E., Mohanty S., Colic A., Stoka V., Turk V. The affinity-labelling of cathepsin S with peptidyl diazomethyl ketones. Comparison with the inhibition of cathepsin L and calpain. FEBS Lett. 1993 Nov 22;334(3):340–342. doi: 10.1016/0014-5793(93)80707-2. [DOI] [PubMed] [Google Scholar]
- Shaw E., Mohanty S., Colic A., Stoka V., Turk V. The affinity-labelling of cathepsin S with peptidyl diazomethyl ketones. Comparison with the inhibition of cathepsin L and calpain. FEBS Lett. 1993 Nov 22;334(3):340–342. doi: 10.1016/0014-5793(93)80707-2. [DOI] [PubMed] [Google Scholar]
- Shi G. P., Chapman H. A., Bhairi S. M., DeLeeuw C., Reddy V. Y., Weiss S. J. Molecular cloning of human cathepsin O, a novel endoproteinase and homologue of rabbit OC2. FEBS Lett. 1995 Jan 3;357(2):129–134. doi: 10.1016/0014-5793(94)01349-6. [DOI] [PubMed] [Google Scholar]
- Shi G. P., Munger J. S., Meara J. P., Rich D. H., Chapman H. A. Molecular cloning and expression of human alveolar macrophage cathepsin S, an elastinolytic cysteine protease. J Biol Chem. 1992 Apr 15;267(11):7258–7262. [PubMed] [Google Scholar]
- Shi G. P., Munger J. S., Meara J. P., Rich D. H., Chapman H. A. Molecular cloning and expression of human alveolar macrophage cathepsin S, an elastinolytic cysteine protease. J Biol Chem. 1992 Apr 15;267(11):7258–7262. [PubMed] [Google Scholar]
- Shi G. P., Webb A. C., Foster K. E., Knoll J. H., Lemere C. A., Munger J. S., Chapman H. A. Human cathepsin S: chromosomal localization, gene structure, and tissue distribution. J Biol Chem. 1994 Apr 15;269(15):11530–11536. [PubMed] [Google Scholar]
- Shi G. P., Webb A. C., Foster K. E., Knoll J. H., Lemere C. A., Munger J. S., Chapman H. A. Human cathepsin S: chromosomal localization, gene structure, and tissue distribution. J Biol Chem. 1994 Apr 15;269(15):11530–11536. [PubMed] [Google Scholar]
- Sloane B. F., Moin K., Krepela E., Rozhin J. Cathepsin B and its endogenous inhibitors: the role in tumor malignancy. Cancer Metastasis Rev. 1990 Dec;9(4):333–352. doi: 10.1007/BF00049523. [DOI] [PubMed] [Google Scholar]
- Sloane B. F., Rozhin J., Krepela E., Ziegler G., Sameni M. The malignant phenotype and cysteine proteinases. Biomed Biochim Acta. 1991;50(4-6):549–554. [PubMed] [Google Scholar]
- Stephens R. W., Pöllänen J., Tapiovaara H., Leung K. C., Sim P. S., Salonen E. M., Rønne E., Behrendt N., Danø K., Vaheri A. Activation of pro-urokinase and plasminogen on human sarcoma cells: a proteolytic system with surface-bound reactants. J Cell Biol. 1989 May;108(5):1987–1995. doi: 10.1083/jcb.108.5.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami K., Kakegawa H., Kamioka H., Sumitani K., Kawata T., Lenarcic B., Turk V., Katunuma N. The mechanisms and regulation of procathepsin L secretion from osteoclasts in bone resorption. FEBS Lett. 1994 Apr 11;342(3):308–312. doi: 10.1016/0014-5793(94)80522-9. [DOI] [PubMed] [Google Scholar]
- Vaes G. Cell-to-cell interactions in the secretion of enzymes of connective tissue breakdown, collagenase and proteoglycan-degrading neutral proteases. A review. Agents Actions. 1980 Dec;10(6):474–485. doi: 10.1007/BF02024145. [DOI] [PubMed] [Google Scholar]
- Van Noorden C. J., Vogels I. M., Everts V., Beertsen W. Localization of cathepsin B activity in fibroblasts and chondrocytes by continuous monitoring of the formation of a final fluorescent reaction product using 5-nitrosalicylaldehyde. Histochem J. 1987 Sep;19(9):483–487. doi: 10.1007/BF01675418. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Wiederanders B., Brömme D., Kirschke H., von Figura K., Schmidt B., Peters C. Phylogenetic conservation of cysteine proteinases. Cloning and expression of a cDNA coding for human cathepsin S. J Biol Chem. 1992 Jul 5;267(19):13708–13713. [PubMed] [Google Scholar]
- Xin X. Q., Gunesekera B., Mason R. W. The specificity and elastinolytic activities of bovine cathepsins S and H. Arch Biochem Biophys. 1992 Dec;299(2):334–339. doi: 10.1016/0003-9861(92)90283-3. [DOI] [PubMed] [Google Scholar]
- Xin X. Q., Gunesekera B., Mason R. W. The specificity and elastinolytic activities of bovine cathepsins S and H. Arch Biochem Biophys. 1992 Dec;299(2):334–339. doi: 10.1016/0003-9861(92)90283-3. [DOI] [PubMed] [Google Scholar]
- Yusa T., Blood C. H., Zetter B. R. Tumor cell interactions with elastin: implications for pulmonary metastasis. Am Rev Respir Dis. 1989 Nov;140(5):1458–1462. doi: 10.1164/ajrccm/140.5.1458. [DOI] [PubMed] [Google Scholar]
- Yusa T., Blood C. H., Zetter B. R. Tumor cell interactions with elastin: implications for pulmonary metastasis. Am Rev Respir Dis. 1989 Nov;140(5):1458–1462. doi: 10.1164/ajrccm/140.5.1458. [DOI] [PubMed] [Google Scholar]