Abstract
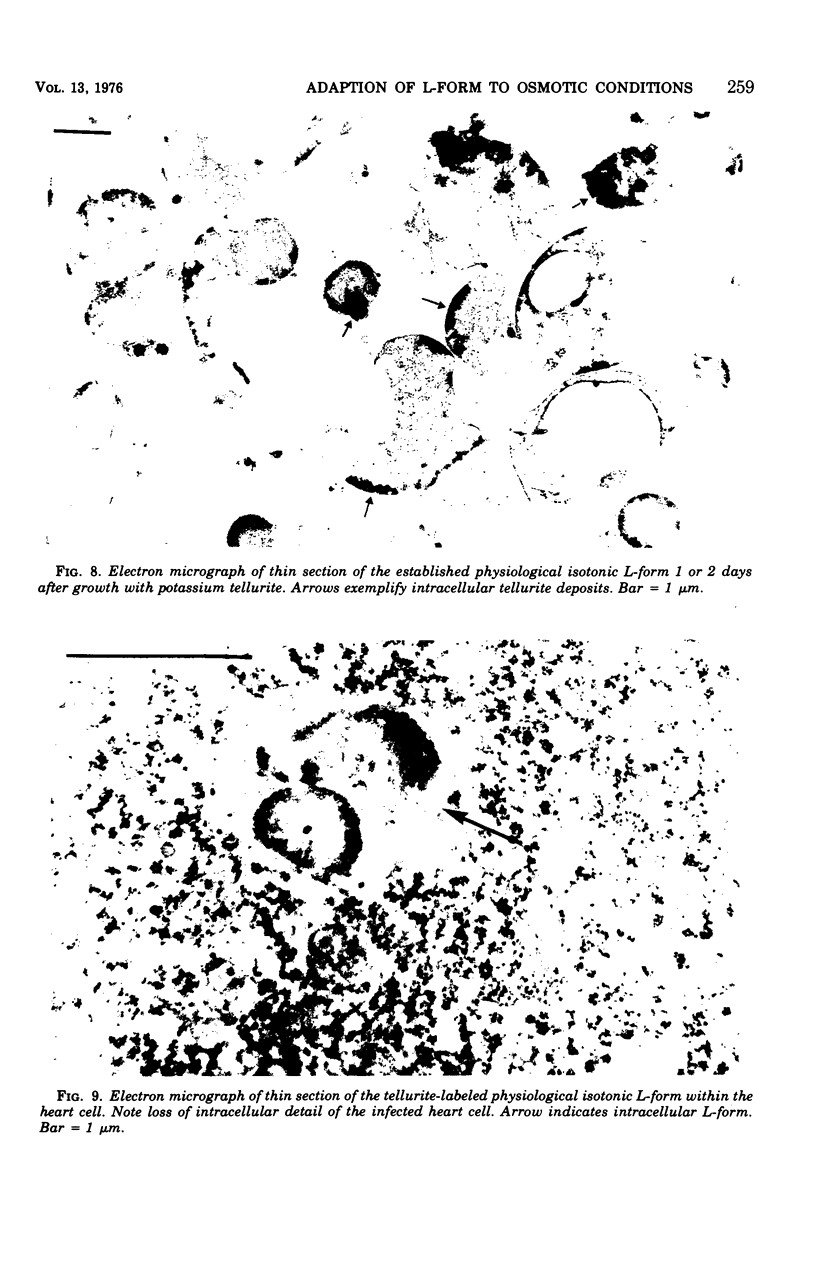
An osmotically fragile L-form of Streptococcus pyogenes, type 12, was quickly rendered osmotically stable by decreasing the sodium chloride content of the growth medium and with the temporary use of oleic acid. The change from osmotic fragility to stability was accompanied by changes in cell yield, generation time, saturated/unsaturated fatty acid ratio of the membrane, and cytoplasmic protein composition. Finally, this resulting osmotically stable L-form survived and was capable of rapidly destroying Girardi human heart cells in tissue culture.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beeley J. A. Separation of human salivary proteins by iso-electric focusing in polyacrylamide gels. Arch Oral Biol. 1969 May;14(5):559–561. doi: 10.1016/0003-9969(69)90150-2. [DOI] [PubMed] [Google Scholar]
- Bentwich Z., Silberstein Z., Boss J. H., Ginsburg I. Cardiac and muscular lesions in mice and rabbits injected with group A streptococcal products. Pathol Microbiol (Basel) 1968;31(4):233–242. doi: 10.1159/000162023. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Clasener H. A., Ensering H. L., Hijmans W. Persistance in mice of the L-phase of three streptococcal strains adapted to physiological osmotic conditions. J Gen Microbiol. 1970 Aug;62(2):195–202. doi: 10.1099/00221287-62-2-195. [DOI] [PubMed] [Google Scholar]
- Dale G., Latner A. L. Isoelectric focusing in polyacrylamide gels. Lancet. 1968 Apr 20;1(7547):847–848. doi: 10.1016/s0140-6736(68)90303-6. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Kaye D., O'Leary W. M. Serum lipids in infection. N Engl J Med. 1969 Nov 13;281(20):1081–1086. doi: 10.1056/NEJM196911132812001. [DOI] [PubMed] [Google Scholar]
- Gilpin R. W., Young F. E., Chatterjee A. N. Characterization of a stable L-form of Bacillus subtilis 168. J Bacteriol. 1973 Jan;113(1):486–499. doi: 10.1128/jb.113.1.486-499.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. T., Heidger P. M., Jr, Domingue G. Demonstration of the phenomena of microbial persistence and reversion with bacterial L-forms in human embryonic kidney cells. Infect Immun. 1974 Oct;10(4):889–914. doi: 10.1128/iai.10.4.889-914.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrikson C. V., Panos C. Fatty acid composition, distribution, and requirements of two nonsterol-requiring mycoplasmas from complex but defatted growth media. Biochemistry. 1969 Feb;8(2):646–651. doi: 10.1021/bi00830a028. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maekawa S., Hayashi T. T. L-phase variants of group A hemolytic streptococci not requiring high osmolarity. Jpn J Microbiol. 1973 May;17(3):228–229. doi: 10.1111/j.1348-0421.1973.tb00732.x. [DOI] [PubMed] [Google Scholar]
- Panos C., Cohen M., Fagan G. Lipid alterations after cell wall inhibition. Fatty acid content of Streptococcus pyogenes and derived L-form. Biochemistry. 1966 May;5(5):1461–1468. doi: 10.1021/bi00869a003. [DOI] [PubMed] [Google Scholar]
- Panos C., Fagan G., Zarkadas C. G. Comparative electrophoretic and amino acid analyses of isolated membranes from Streptococcus pyogenes and stabilized L-form. J Bacteriol. 1972 Oct;112(1):285–290. doi: 10.1128/jb.112.1.285-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham-Huu-Trung, Schmitt-Slomska J., Boué A. Etude au microscope électronique des premiers stades de l'infection des cultures de cellules diploïdes humaines par des formes L du streptocoque du groupe A. Pathol Biol. 1968 Apr;16(7):431–437. [PubMed] [Google Scholar]
- Rottem S., Panos C. The effect of long chain fatty acid isomers on growth, fatty acid composition and osmotic fragility of Mycoplasma laidlawii A. J Gen Microbiol. 1969 Dec;59(3):317–328. doi: 10.1099/00221287-59-3-317. [DOI] [PubMed] [Google Scholar]
- SMITH P. F., MORTON H. E., LEBERMAN P. R. Susceptibilities of pleuropneumonia-like organisms to some selective bacteriostatic agents. Proc Soc Exp Biol Med. 1950 Jul;74(3):552–555. doi: 10.3181/00379727-74-17969. [DOI] [PubMed] [Google Scholar]
- SMITH P. F., ROTHBLAT G. H. Comparison of lipid composition of pleuropneumonia-like and L-type organisms. J Bacteriol. 1962 Mar;83:500–506. doi: 10.1128/jb.83.3.500-506.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt-Slomska J., Boué A., Caravano R. Induction of L-variants in human diploid cells infected by group A streptococci. Infect Immun. 1972 Mar;5(3):389–399. doi: 10.1128/iai.5.3.389-399.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt-Slomska J., Sacquet E., Caravano R. Group A streptococcal L forms. I. Persistence among inoculated mice. J Bacteriol. 1967 Jan;93(1):451–455. doi: 10.1128/jb.93.1.451-455.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]