Abstract
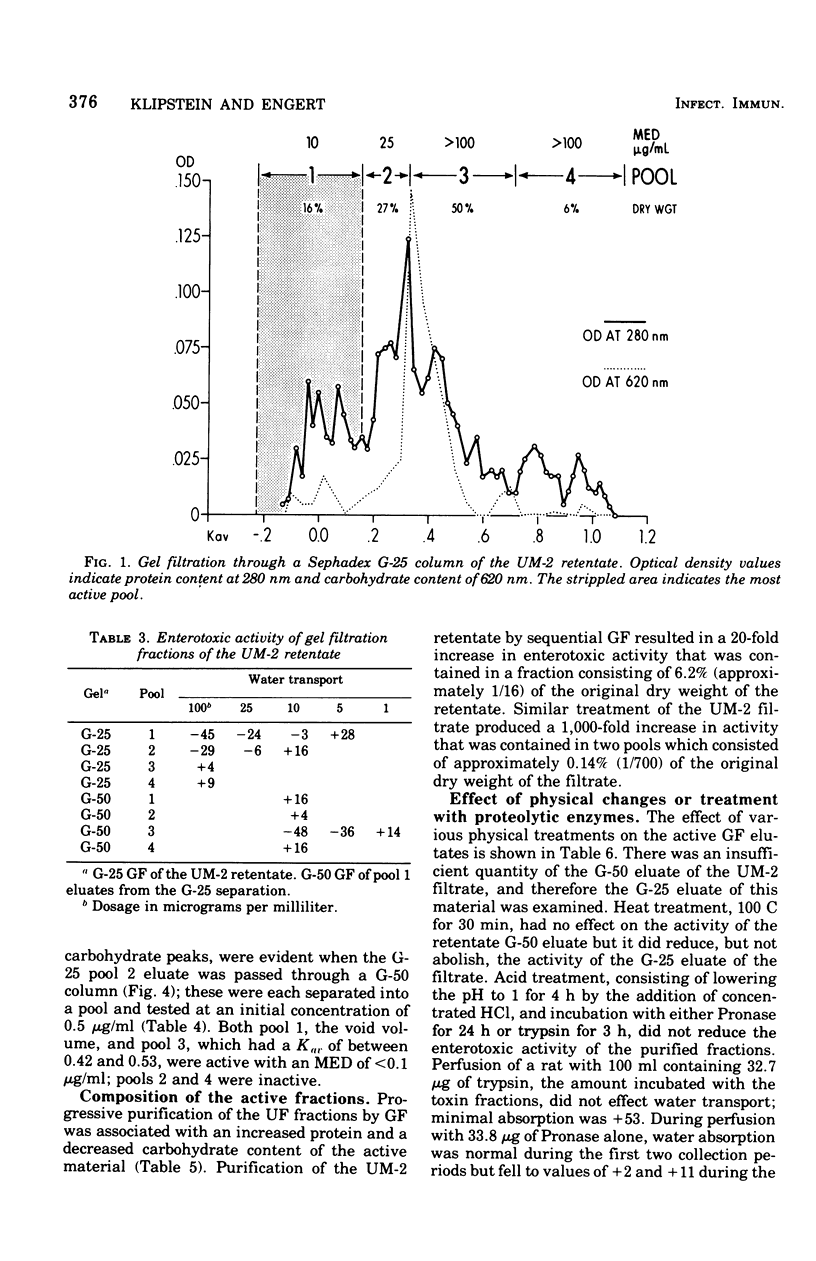
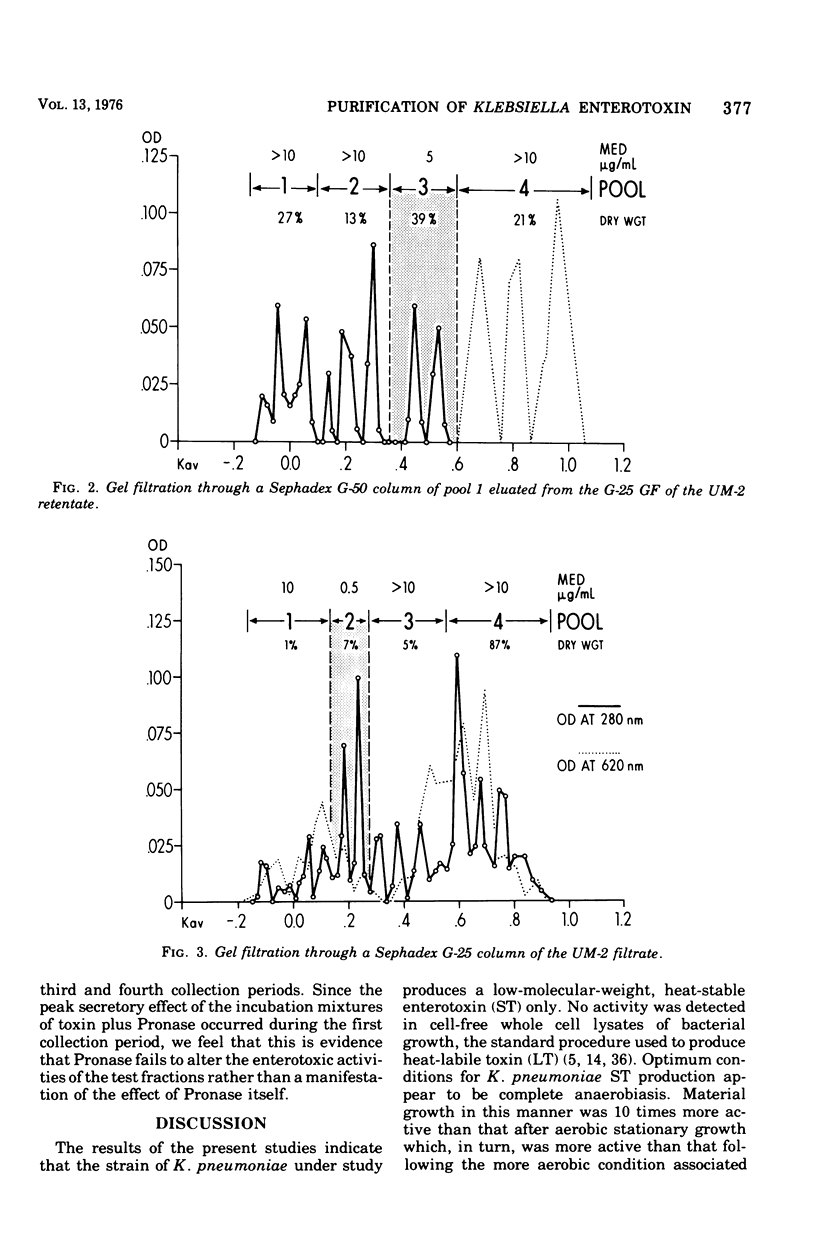
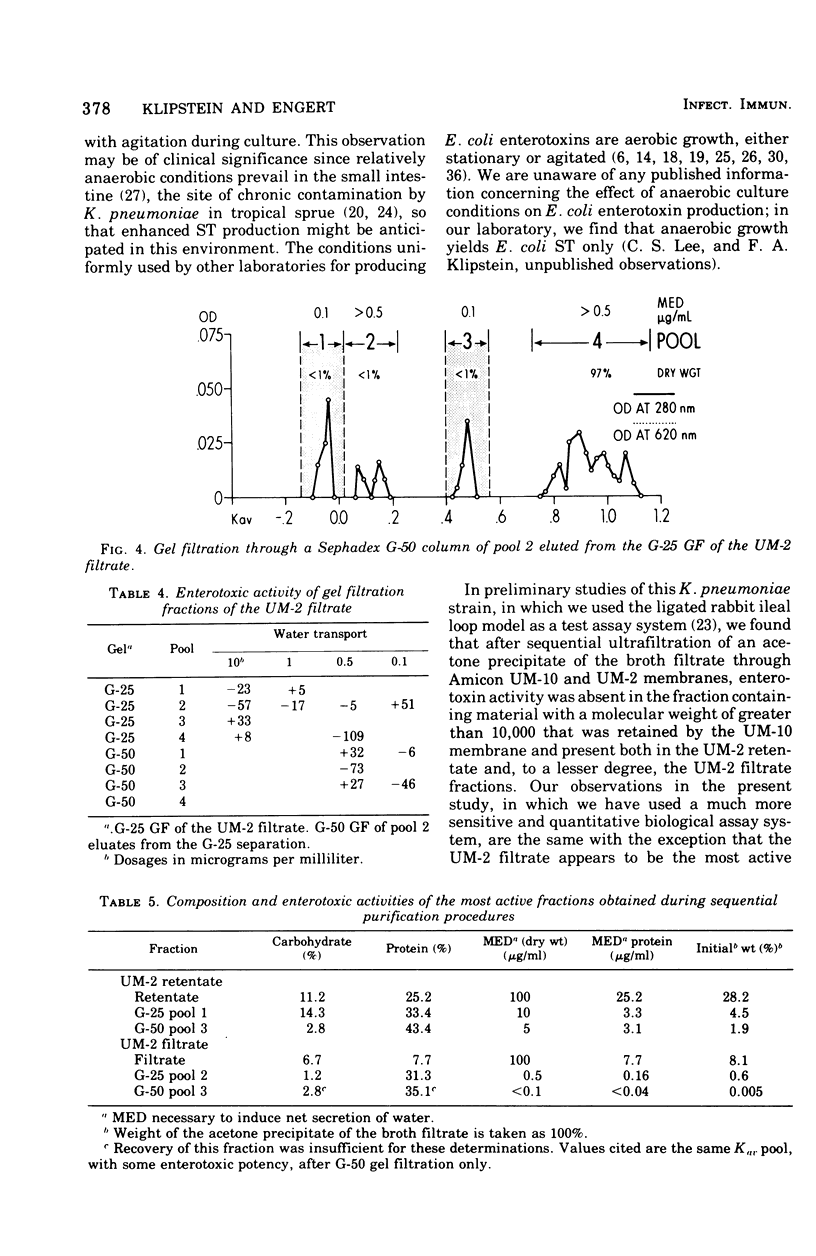
The enterotoxic material in cell-free growth preparations of Klebsiella pneumoniae serotype 5 was purified by sequential ultrafiltration and gel filtration (GF) procedures and the fractions were assayed for enterotoxic activity by determining their ability to induce in vivo net water secretion in the rat jejunum. Whole-cell lysates were inactive. Anaerobic broth culture conditions yielded a 10-fold increase in toxin production over aerobic conditions. Enterotoxic activity was absent in the UM-10 retentate of the broth filtrate but present in both the retentate and filtrate of the UM-2 membrane. GF of the two UM-2 ultrafiltration fractions through a Sephadex G-25 column yielded an active eluate, whose potency was increased by 10- or 200-fold, in or adjacent to the void volume. When subsequently passed through a G-50 column, these pools eluted at a Kav of between 0.4 and 0.6 and were further increased in potency by two- or fivefold. A second equally potent fraction was also recovered in the void volume of the G-50 eluate of the UM-2 filtrate; this may represent a polymer. Progressive purification by GF was associated with an increased protein and decreased carbohydrate content of the most active fractions. The most active G-50 eluate of the UM-2 retentate had a minimal effective enterotoxic dose of 5 mug/ml and that of the filtrate was less than 0.1 mug/ml. Heating the active GF eluates to 100 C for 30 min did not abolish enterotoxic activity and lowering the pH to 1 or incubation with either Pronase or trypsin had no effect on activity. These observations indicate that K. pneumoniae heat-stable enterotoxin is probably a single toxin with an apparent molecular weight in the range of 5,000. The elution characteristics during GF as well as the chemical composition of the most purified enterotoxin fractions indicate that the toxin is not associated with endotoxin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEER H., STAEHELIN T., DOUGLAS H., BRAUDE A. I. RELATIONSHIP BETWEEN PARTICLE SIZE AND BIOLOGICAL ACTIVITY OF E. COLI BOIVIN ENDOTOXIN. J Clin Invest. 1965 Apr;44:592–602. doi: 10.1172/JCI105172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywater R. J. Dialysis and ultrafiltration of heat-stable enterotoxin from Escherichia coli. J Med Microbiol. 1972 Aug;5(3):337–343. doi: 10.1099/00222615-5-3-337. [DOI] [PubMed] [Google Scholar]
- Coello-Ramirez P., Lifshitz F. Enteric microflora and carbohydrate intolerance in infants with diarrhea. Pediatrics. 1972 Feb;49(2):233–242. [PubMed] [Google Scholar]
- DuPont H. L., Formal S. B., Hornick R. B., Snyder M. J., Libonati J. P., Sheahan D. G., LaBrec E. H., Kalas J. P. Pathogenesis of Escherichia coli diarrhea. N Engl J Med. 1971 Jul 1;285(1):1–9. doi: 10.1056/NEJM197107012850101. [DOI] [PubMed] [Google Scholar]
- Etkin S., Gorbach S. L. Studies on enterotoxin from Escherichia coli associated with acute diarrhea in man. J Lab Clin Med. 1971 Jul;78(1):81–87. [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Pierce N. F. Differences in the response of rabbit small intestine to heat-labile and heat-stable enterotoxins of Escherichia coli. Infect Immun. 1973 Jun;7(6):873–880. doi: 10.1128/iai.7.6.873-880.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Evans D. G., Gorbach S. L. Polymyxin B-Induced Release of Low-Molecular-Weight, Heat-Labile Enterotoxin from Escherichia coli. Infect Immun. 1974 Nov;10(5):1010–1017. doi: 10.1128/iai.10.5.1010-1017.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Atthasampunna P., Chulasamaya M., Charunmethee P. Pathogenesis of experimental cholera: biologic ativities of purified procholeragen A. J Immunol. 1966 Mar;96(3):440–449. [PubMed] [Google Scholar]
- Gorbach S. L., Banwell J. G., Chatterjee B. D., Jacobs B., Sack R. B. Acute undifferentiated human diarrhea in the tropics. I. Alterations in intestinal micrflora. J Clin Invest. 1971 Apr;50(4):881–889. doi: 10.1172/JCI106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach S. L., Banwell J. G., Jacobs B., Chatterjee B. D., Mitra R., Mazumder D. N., Sen N. N. Tropical sprue and malnutrition in West Bengal. I. Intestinal microflora and absorption. Am J Clin Nutr. 1970 Dec;23(12):1545–1558. doi: 10.1093/ajcn/23.12.1545. [DOI] [PubMed] [Google Scholar]
- Gorbach S. L., Kean B. H., Evans D. G., Evans D. J., Jr, Bessudo D. Travelers' diarrhea and toxigenic Escherichia coli. N Engl J Med. 1975 May 1;292(18):933–936. doi: 10.1056/NEJM197505012921801. [DOI] [PubMed] [Google Scholar]
- Gracey M., Suharjono, Sunoto, Stone D. E. Microbial contamination of the gut: another feature of malnutrition. Am J Clin Nutr. 1973 Nov;26(11):1170–1174. doi: 10.1093/ajcn/26.11.1170. [DOI] [PubMed] [Google Scholar]
- Gyles C. L., Barnum D. A. A heat-labile enterotoxin from strains of Eschericha coli enteropathogenic for pigs. J Infect Dis. 1969 Oct;120(4):419–426. doi: 10.1093/infdis/120.4.419. [DOI] [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- Heyworth B., Brown J. Jejunal microflora in malnourished Gambian children. Arch Dis Child. 1975 Jan;50(1):27–33. doi: 10.1136/adc.50.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T. M., Wu B. J. Biochemical properties of Escherichia coli low-molecular-weight, heat-stable enterotoxin. Infect Immun. 1974 Feb;9(2):342–347. doi: 10.1128/iai.9.2.342-347.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T. M., Wu B. J., Braemer A. C., Bidlack D. E. Properties of the enterotoxic component in Escherichia coli enteropathogenic for swine. Infect Immun. 1973 Feb;7(2):178–189. doi: 10.1128/iai.7.2.178-189.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F. Enterotoxigenic intestinal bacteria in tropical sprue. III. Preliminary characterization of Klebsiella pneumoniae enterotoxin. J Infect Dis. 1975 Aug;132(2):200–203. doi: 10.1093/infdis/132.2.200. [DOI] [PubMed] [Google Scholar]
- Klipstein F. A., Holdeman L. V., Corcino J. J., Moore W. E. Enterotoxigenic intestinal bacteria in tropical sprue. Ann Intern Med. 1973 Nov;79(5):632–641. doi: 10.7326/0003-4819-79-5-632. [DOI] [PubMed] [Google Scholar]
- Klipstein F. A., Horowitz I. R., Engert R. F., Schnenk E. A. Effect of Klebsiella pneumoniae enterotoxin on intestinal transport in the rat. J Clin Invest. 1975 Oct;56(4):799–807. doi: 10.1172/JCI108158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Schenk E. A. Enterotoxigenic intestinal bacteria in tropical sprue. II. Effect of the bacteria and their enterotoxins on intestinal structure. Gastroenterology. 1975 Apr;68(4 Pt 1):642–655. [PubMed] [Google Scholar]
- Kohler E. M. Enterotoxic activity of filtrates of escherichia coli in young pigs. Am J Vet Res. 1968 Dec;29(12):2263–2274. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lariviére S., Gyles C. L., Barnum D. A. Preliminary characterization of the heat-labile enterotoxin of Escherichia coli F11(P155). J Infect Dis. 1973 Sep;128(3):312–320. doi: 10.1093/infdis/128.3.312. [DOI] [PubMed] [Google Scholar]
- Levitt M. D. Volume and composition of human intestinal gas determined by means of an intestinal washout technic. N Engl J Med. 1971 Jun 24;284(25):1394–1398. doi: 10.1056/NEJM197106242842502. [DOI] [PubMed] [Google Scholar]
- Mitchell I. D., Tame M. J., Kenworthy R. Separation and purification of enterotoxins from a strain Escherichia coli pathogenic for pigs. J Med Microbiol. 1974 Nov;7(4):439–450. doi: 10.1099/00222615-7-4-439. [DOI] [PubMed] [Google Scholar]
- OLARTE J., FERGUSON W. W., HENDERSON N. D., TORREGROSA L. Klebsiella strains isolated from diarrheal infants. Human volunteer studies. Am J Dis Child. 1961 Jun;101:763–770. doi: 10.1001/archpedi.1961.04020070077011. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Wallace C. K. Stimulation of jejunal secretion by a crude Escherichia coli enterotixin. Gastroenterology. 1972 Sep;63(6):439–448. [PubMed] [Google Scholar]
- Powell D. W., Malawer S. J. Relationship between water and solute transport from isosmotic solutions by rat intestine in vivo. Am J Physiol. 1968 Jul;215(1):49–55. doi: 10.1152/ajplegacy.1968.215.1.49. [DOI] [PubMed] [Google Scholar]
- Rowe B., Taylor J., Bettelheim K. A. An investigation of traveller's diarrhoea. Lancet. 1970 Jan 3;1(7636):1–5. doi: 10.1016/s0140-6736(70)90520-9. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Gyles C. L. The relationship between two apparently different enterotoxins produced by enteropathogenic strains of Escherichia coli of porcine origin. J Med Microbiol. 1970 Aug;3(3):387–401. doi: 10.1099/00222615-3-3-387. [DOI] [PubMed] [Google Scholar]
- Smith P. B., Tomfohrde K. M., Rhoden D. L., Balows A. API system: a multitube micromethod for identification of Enterobacteriaceae. Appl Microbiol. 1972 Sep;24(3):449–452. doi: 10.1128/am.24.3.449-452.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil A. J., Ramchand S., Arias M. E. Nosocomial infection with Klebsiella type 25. N Engl J Med. 1966 Jul 7;275(1):17–22. doi: 10.1056/NEJM196607072750104. [DOI] [PubMed] [Google Scholar]


