Abstract
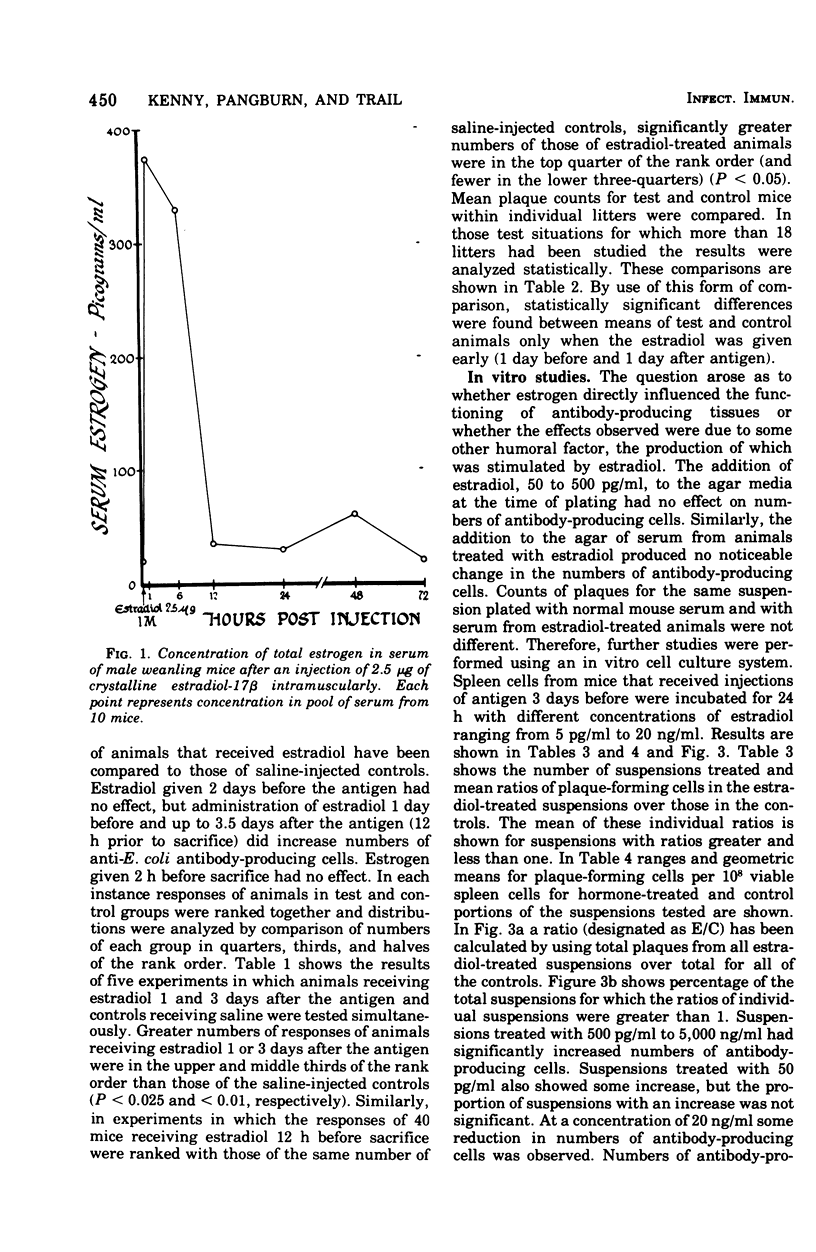
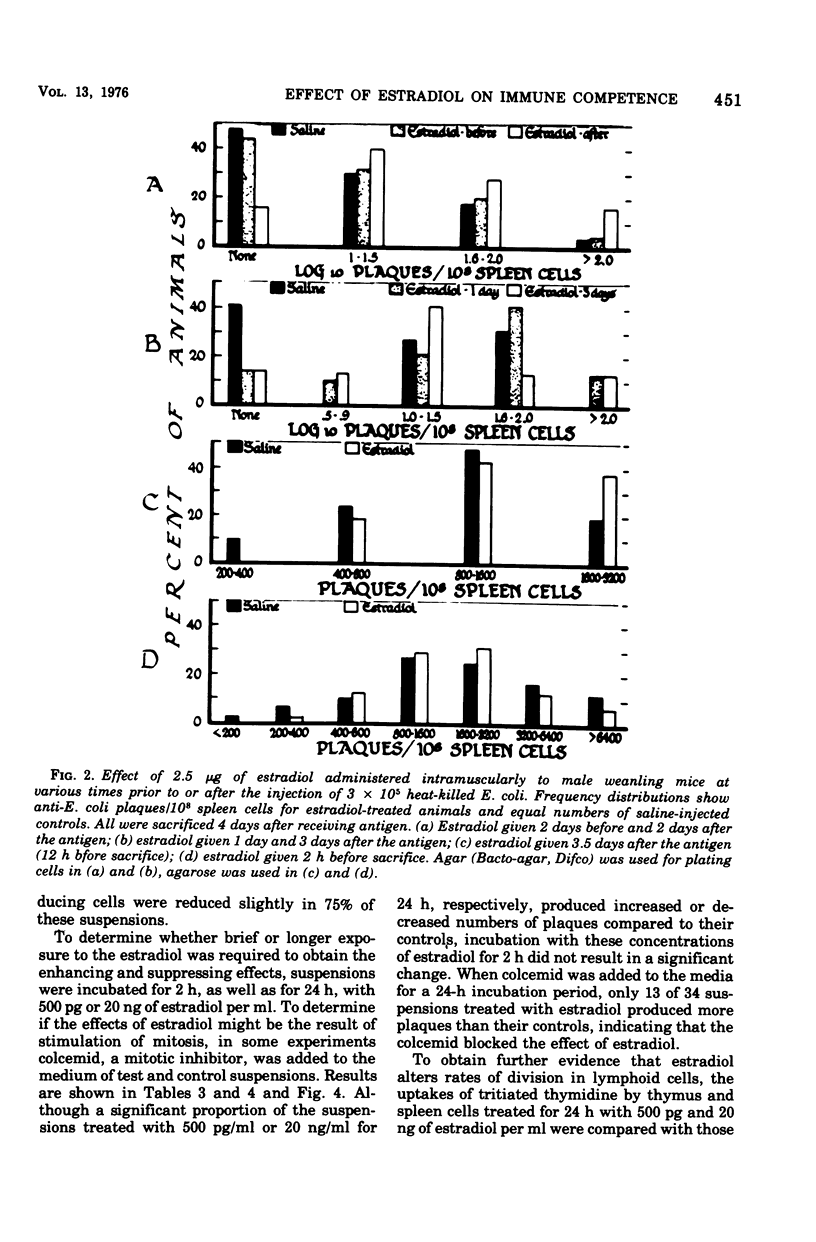
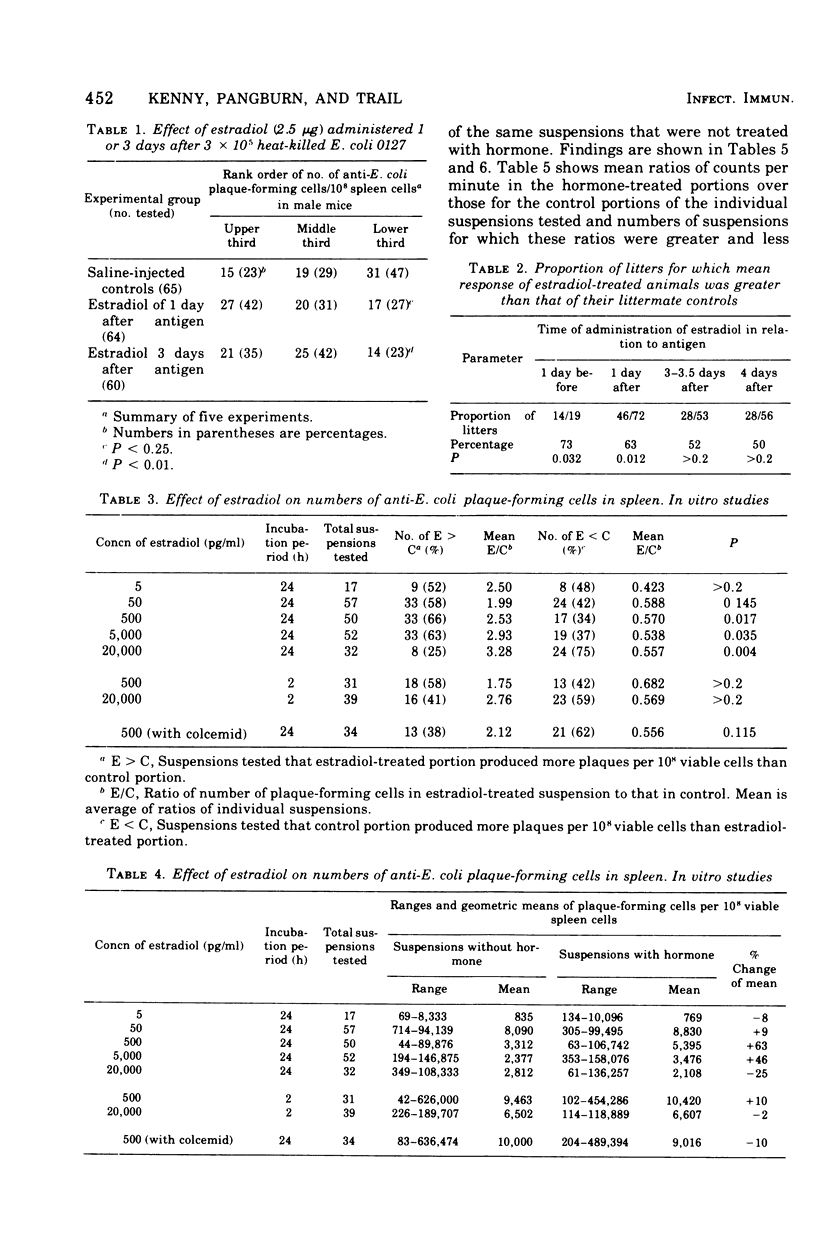
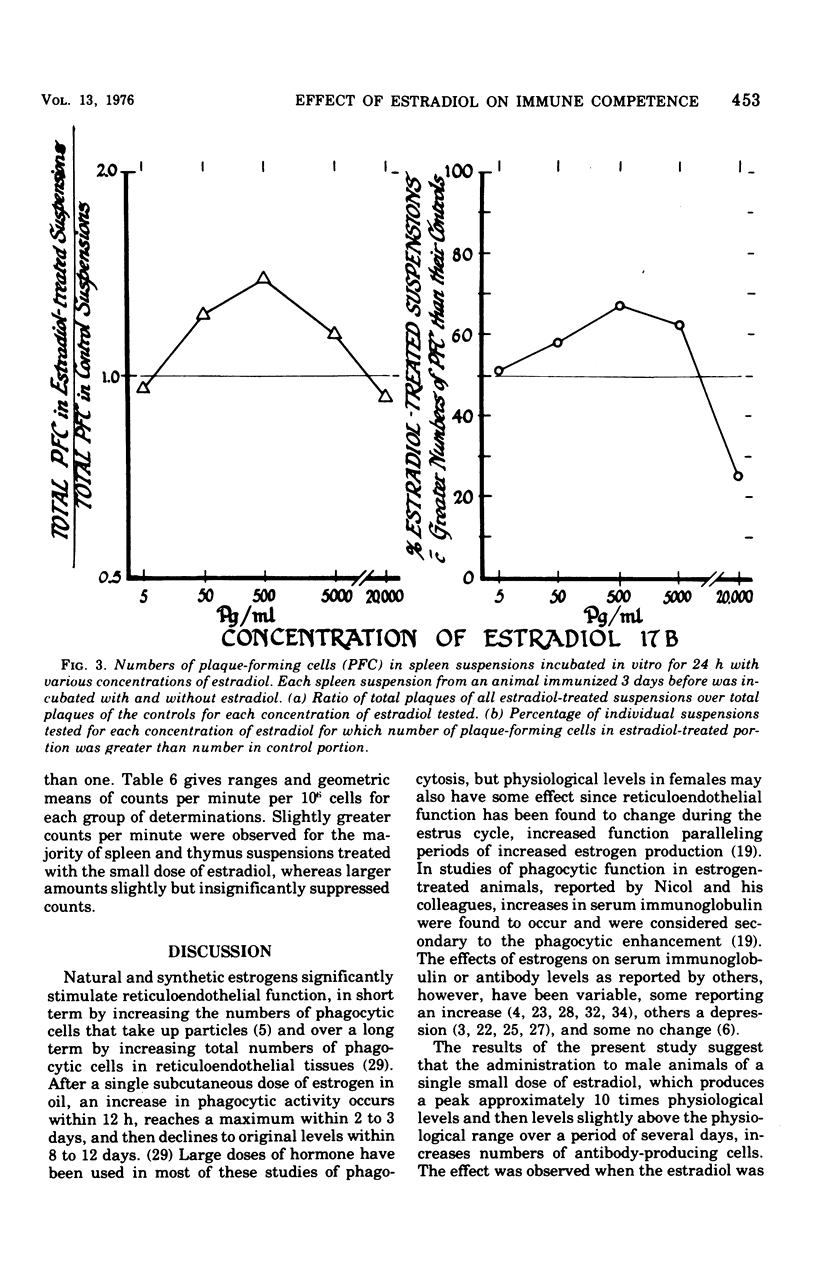
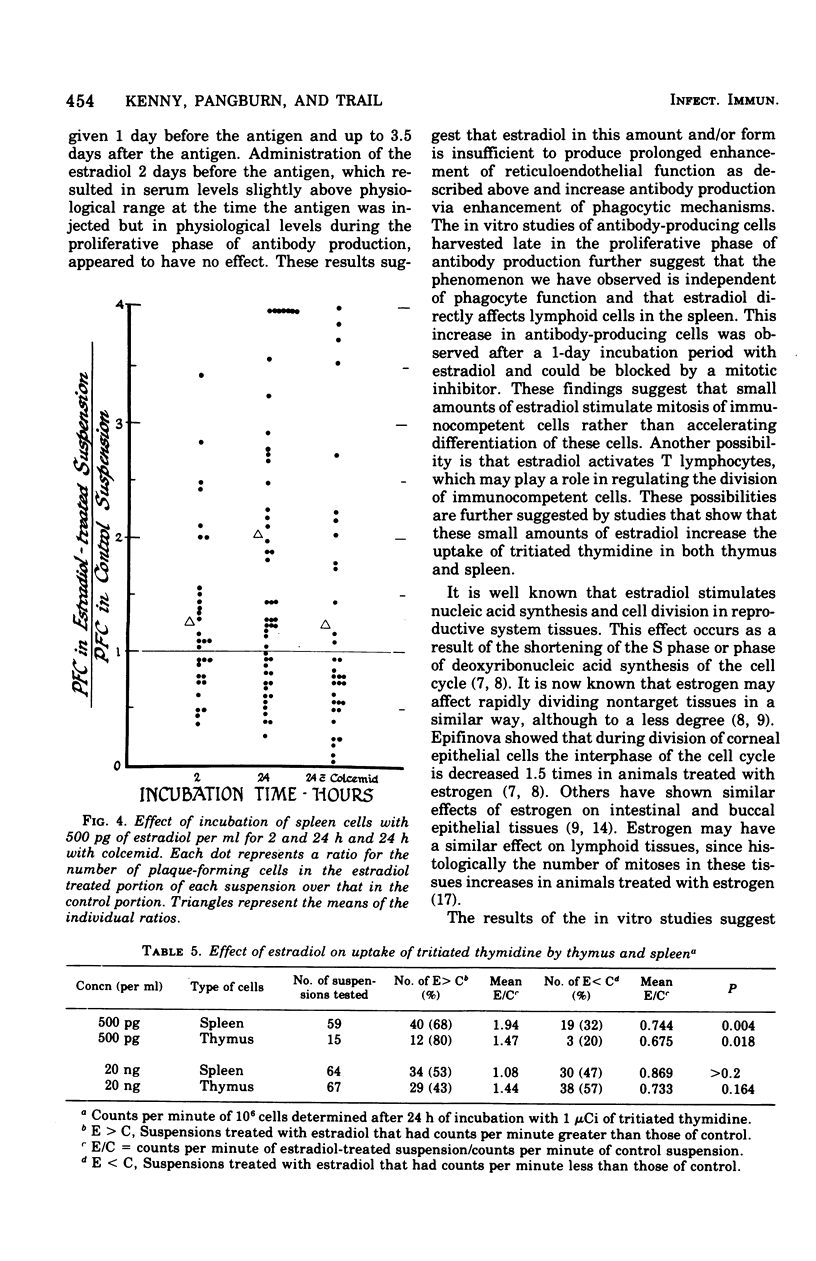
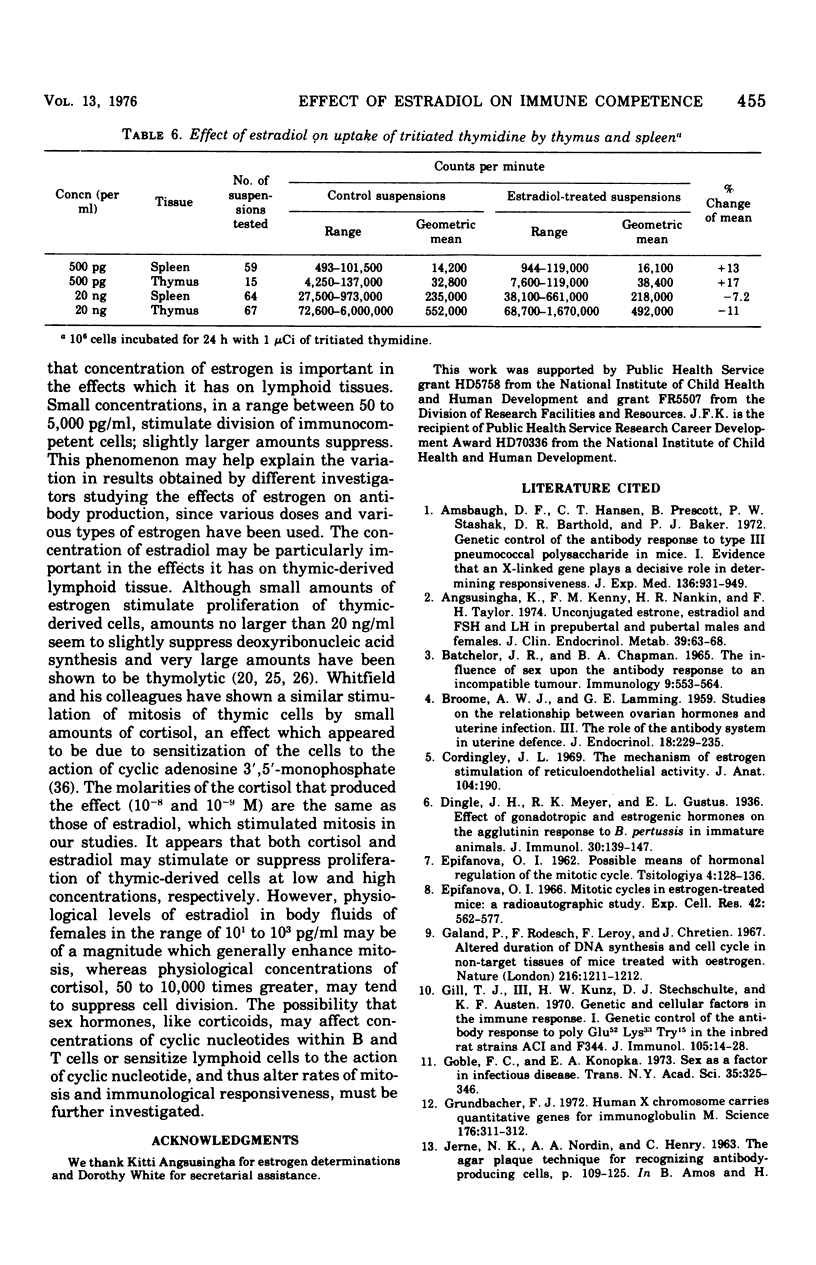
The administration of a single dose of 2.5 mug of microcrystalline estradiol-17 beta from 1 day before and up until 3.5 days after the administration of 3 X 10(5) heat-killed Escherichia coli significantly increased numbers of splenic anti-E. coli antibody-producing cells in male mice sacrificed 4 days after receiving anitgen. Administration early in the proliferative phase of antibody production, i.e., 1 day before or 1 day after the antigen, appeared to increase numbers of antibody-producing cells more than when it was administered at a later time. When given 2 days before the antigen or 2 h before sacrifice no effect was observed. Spleen cells harvested from male animals injected 3 days before with 5 X 10(6) heat-killed E. coli were incubated for 24 h in vitro with estradiol in concentrations ranging from 5 pg to 20 ng/ml. With concentrations of 500 pg to 5,000 pg/ml, significant increases in antibody-producing cells occurred, whereas at concentrations of 20 ng/ml some decrease was observed. The increase in antibody-producing cells was blocked by a mitotic inhibitor. Significant changes in numbers of antibody-producing cells were not observed after a 2-h incubation period. Uptake of titrated thymidine was increased in thymic and spleen cells incubated for 24 h with 500 pg of estradiol per ml; a concentration of 20 ng/ml slightly (but insignificantly) decreased uptake. Findings suggest that estradiol, in concentrations that approximate physiological serum levels in females, enhances mitosis of immunocompetent cells. This phenomenon may have bearing on the better immunological responsiveness of females than males.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsbaugh D. F., Hansen C. T., Prescott B., Stashak P. W., Barthold D. R., Baker P. J. Genetic control of the antibody response to type 3 pneumococcal polysaccharide in mice. I. Evidence that an X-linked gene plays a decisive role in determining responsiveness. J Exp Med. 1972 Oct 1;136(4):931–949. doi: 10.1084/jem.136.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angsusingha K., Kenny F. M., Nankin H. R., Taylor F. H. Unconjugated estrone, estradiol and FSH and LH in prepubertal and pubertal males and females. J Clin Endocrinol Metab. 1974 Jul;39(1):63–68. doi: 10.1210/jcem-39-1-63. [DOI] [PubMed] [Google Scholar]
- BROOME A. W., LAMMING G. E. Studies on the relationship between ovarian hormones and uterine infection. III. The role of the antibody system in uterine defence. J Endocrinol. 1959 May;18(3):229–235. doi: 10.1677/joe.0.0180229. [DOI] [PubMed] [Google Scholar]
- Batchelor J. R., Chapman B. A. The influence of sex upon the antibody response to an incompatible tumour. Immunology. 1965 Dec;9(6):553–564. [PMC free article] [PubMed] [Google Scholar]
- Cordingley J. L. The mechanism of oestrogen stimulation of reticuloendothelial activity. J Anat. 1969 Jan;104(Pt 1):190–190. [PubMed] [Google Scholar]
- EPIFANOVA O. I. [Possible routes of hormonal regulation of the mitotic cycle]. Tsitologiia. 1962 Mar-Apr;4:128–136. [PubMed] [Google Scholar]
- Epifanova O. I. Mitotic cycles in estrogen-treated mice: a radioautographic study. Exp Cell Res. 1966 Jun;42(3):562–577. doi: 10.1016/0014-4827(66)90269-2. [DOI] [PubMed] [Google Scholar]
- Galand P., Rodesch F., Leroy F., Chretien J. Altered duration of DNA synthesis and cell cycle in non-target tissues of mice treated with oestrogen. Nature. 1967 Dec 23;216(5121):1211–1212. doi: 10.1038/2161211a0. [DOI] [PubMed] [Google Scholar]
- Gill T. J., 3rd, Kunz H. W., Stechschulte D. J., Austen K. F. Genetic and cellular factors in the immune response. I. Genetic control of the antibody response to poly Glu52 Lys33 Tyr15 in the inbred rat strains ACI and F344. J Immunol. 1970 Jul;105(1):14–28. [PubMed] [Google Scholar]
- Grundbacher F. J. Human X chromosome carries quantitative genes for immunoglobulin M. Science. 1972 Apr 21;176(4032):311–312. doi: 10.1126/science.176.4032.311. [DOI] [PubMed] [Google Scholar]
- Jonek J., Konecki J., Jonek T. Effect of estrogens upon the mitotic cycle and DNA synthesis in buccal epithelial cells of castrated mice. Pol Med J. 1970;9(6):1532–1539. [PubMed] [Google Scholar]
- Kaliss N. Jerne plaque assay for antibody-forming spleen cells: some technical modifications. Transplantation. 1971 Aug;12(2):146–147. doi: 10.1097/00007890-197108000-00009. [DOI] [PubMed] [Google Scholar]
- Michaels R. M., Rogers K. D. A sex difference in immunologic responsiveness. Pediatrics. 1971 Jan;47(1):120–123. [PubMed] [Google Scholar]
- NICOL T., BILBEY D. L., CHARLES L. M., CORDINGLEY J. L., VERNON-ROBERTS B. OESTROGEN: THE NATURAL STIMULANT OF BODY DEFENCE. J Endocrinol. 1964 Oct;30:277–291. doi: 10.1677/joe.0.0300277. [DOI] [PubMed] [Google Scholar]
- Reilly R. W., Thompson J. S., Bielski R. K., Severson C. D. Estradiol-induced wasting syndrome in neonatal mice. J Immunol. 1967 Feb;98(2):321–330. [PubMed] [Google Scholar]
- Rhodes K., Markham R. L., Maxwell P. M., Monk-Jones M. E. Immunoglobulins and the X-chromosome. Br Med J. 1969 Aug 23;3(5668):439–441. doi: 10.1136/bmj.3.5668.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERN K., DAVIDSOHN I. Effect of estrogen and cortisone on immune hemoantibodies in mice of inbred strains. J Immunol. 1955 Jun;74(6):479–484. [PubMed] [Google Scholar]
- Sljivić V. S., Warr G. W. Activity of the reticuloendothelial system and the antibody response. II. Effect of stilboestrol on the immune response to sheep erythrocytes in the mouse. Br J Exp Pathol. 1973 Feb;54(1):69–78. [PMC free article] [PubMed] [Google Scholar]
- Terres G., Morrison S. L., Habicht G. S. A quantitative difference in the immune response between male and female mice. Proc Soc Exp Biol Med. 1968 Mar;127(3):664–667. doi: 10.3181/00379727-127-32768. [DOI] [PubMed] [Google Scholar]
- Thompson J. S., Crawford M. K., Reilly R. W., Severson C. D. The effect of estrogenic hormones on immune responses in normal and irradiated mice. J Immunol. 1967 Feb;98(2):331–335. [PubMed] [Google Scholar]
- Thompson J. S., Severson C. D., Reilly R. W. Autoradiographic study of the effect of estradiol and irradiation on nucleic acid metabolism of the thymus and lymph node of mice. Radiat Res. 1969 Oct;40(1):46–62. [PubMed] [Google Scholar]
- Toivanen P. Effect of estrogens on the humoral antibody response in guinea pigs. Ann Med Exp Biol Fenn. 1967;45(2):152–156. [PubMed] [Google Scholar]
- Vernon-Roberts B. The effects of steroid hormones on macrophage activity. Int Rev Cytol. 1969;25:131–159. doi: 10.1016/s0074-7696(08)60202-8. [DOI] [PubMed] [Google Scholar]
- Vischer T. L. Culture of mouse lymphoid cells in serum-free medium. J Immunol Methods. 1972 Jan;1(2):199–202. doi: 10.1016/0022-1759(72)90046-4. [DOI] [PubMed] [Google Scholar]
- WASHBURN T. C., MEDEARIS D. N., Jr, CHILDS B. SEX DIFFERENCES IN SUSCEPTIBILITY TO INFECTIONS. Pediatrics. 1965 Jan;35:57–64. [PubMed] [Google Scholar]
- WHEATER D. W., HURST E. W. The effect of sex on bacterial infections in mice and on the chemotherapy of one of them. J Pathol Bacteriol. 1961 Jul;82:117–130. doi: 10.1002/path.1700820115. [DOI] [PubMed] [Google Scholar]
- WILLOUGHBY D. S., WATSON D. W. HOST-PARASITE RELATIONSHIPS AMONG GROUP A STREPTOCOCCI. II. INFLUENCE OF SEX ON THE SUSCEPTIBILITY OF INBRED MICE TOWARD STREPTOCOCCAL INFECTION. J Bacteriol. 1964 Jun;87:1457–1461. doi: 10.1128/jb.87.6.1457-1461.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Rixon R. H. Cyclic AMP-mediated stimulation of thymocyte proliferation by low concentrations of cortisol. Proc Soc Exp Biol Med. 1970 Sep;134(4):1170–1174. doi: 10.3181/00379727-134-34967. [DOI] [PubMed] [Google Scholar]


