Abstract
Objectives
Bowel and bladder symptoms are highly prevalent in patients with multiple sclerosis (MS). Bladder dysfunction (affecting 75% of these patients) is caused by disease in the spinal cord, whilst the pathophysiology of bowel dysfunction is unknown. Pathways regulating both the organs lie in close proximity to the spinal cord, and coexistence of their dysfunction might be the result of a common pathophysiology. If so, the prevalence of bladder symptoms should be greater in patients with MS and bowel symptoms. This hypothesis is tested in the study. We also evaluated how patient-reported symptoms quantify bowel dysfunction.
Patients and methods
The Neurogenic Bowel Dysfunction questionnaire and the presence of bladder symptoms were recorded in 71 patients with MS and bowel symptoms. Disability, a surrogate clinical measure of spinal cord disease, was assessed using the Expanded Disability Status Scale. Bowel and bladder symptoms were quantified by patient-reported frequency, expressed in time percentage (0, 25, 50, 75 or 100% of the time the symptom was perceived), and patient-reported severity on a visual analogue scale between 0 and 100.
Results
The prevalence of bladder symptoms was 85%, which is higher than that expected in an unselected population of patients with MS. Neurogenic Bowel Dysfunction score was significantly correlated with both patient-reported frequency (r=0.860, P<0.0001) and severity of bowel symptoms (r=0.659, P=<0.0001), as well as with the Expanded Disability Status Scale (r=0.526, P<0.0001).
Conclusion
Our findings suggest that gut dysfunction in patients with MS is secondary to spinal cord disease. Patient-reported bowel symptoms quantify bowel dysfunction well.
Keywords: bladder dysfunction, constipation, faecal incontinence, multiple sclerosis
Introduction
Bowel symptoms (constipation and/or faecal incontinence) affect up to two-thirds of patients with multiple sclerosis (MS) 1, causing social isolation 2 and general reduction of quality of life 3,4. Their origin is multifactorial and polypharmacy, disability, comorbidities and parity may have a causative role 5, but from the neurological standpoint MS can affect both extrinsic autonomic and voluntary control of the bowel. The effects on the end-organ include alterations of gut motility, anorectal sensation/coordination and anal sphincter control 6, but the neurological pathway that causes these disturbances is still unknown. This is mirrored in the lack of standards of treatment. MS symptoms relief, in the absence of a treatment for the primary neurological injury, is of paramount importance, but the area of bowel dysfunction remains the ‘Cinderella’ of MS research.
In contrast, bladder dysfunction, affecting around 75% of the patients with MS 7, has been well characterized. It is established that MS plaques in the spinal cord are central to cause urinary symptoms 8, and their treatment has been rationalized and standardized 9. Neurological pathways regulating pelvic organs are in close proximity within the spinal cord; thus, it is unsurprising that bowel and bladder symptoms often coexist in patients with MS 1,10,11. When this is the case, it could be that sclerotic plaques in the spinal cord simultaneously affect bladder and bowel function. However, in a study of MS patients with bladder dysfunction 12, the prevalence of bowel symptoms was only around 50%. This apparent discrepancy could be explained by the presence of the gut’s enteric nervous system, which would allow preservation of some bowel function in the presence of altered extrinsic hindgut modulation. This compensatory mechanism is not available to the bladder 13. Therefore, we hypothesized that if bowel and bladder dysfunction can be caused by the same MS-related neurological alteration, in a selected population of patients with MS with bowel symptoms, a higher prevalence of bladder symptoms should be observed than that in the general MS population. The aim of the study was to test this hypothesis. We also analysed how well bowel dysfunction is assessed by patient-reported bowel symptoms, and any correlations between patient-reported bowel and bladder symptoms.
Anatomical considerations
The neural control of defecation is not clearly defined, but the centre concerned with it probably lies in the pons, and is under conscious cortical modulation 14; defecation is also influenced by supraspinal centres 15.
It has been demonstrated that the spinal pathway for defecation, operating through sacral roots (S2–S4), lies in the lateral column of the cord, in close proximity to those pathways important for bladder control 15–17.
The neural pathways involved in physiological bladder control operate through complex bulbospinal–bulbar pathways, in close proximity to the lateral pyramidal tracts, and are mediated peripherally through the sacral roots S2–S4 18,19. Cortical voluntary control of micturition is established by connection of the frontal cortex to the micturition centre in the pons 20.
Types of multiple sclerosis
MS is characterized by an autoimmune response that results in the disruption of the myelin sheath in the central nervous system (demyelination), and the subsequent gliosis leads to the widespread occurrence of plaques in the white matter of the central nervous system that affects signal transmission. The natural history of the disease is of a progressive accumulation of neurological symptoms leading to severe disability. On the basis of the rapidity of progression and of accumulation of disability, MS is classified as relapsing remitting (most common, where symptoms appear and fade away), secondary progressive (usually follows relapsing remitting, characterized by a sustained build up of disability, independent of any relapses) and primary progressive (where the disease is progressive from the start).
Patients and methods
The Ethics Committee of University College of London granted Ethical approval (REC reference number: 08/h07164/7), and patients who participated in the study signed a consent form. Entry criteria included a definite diagnosis of MS and normal bowel function before the onset of MS. Exclusion criteria included: concomitant primary bowel pathology, comorbidities (i.e. diabetes, thyroid dysfunction, coeliac disease, prostate hypertrophy, etc.) and sphincter injury. These were ruled out in all patients by means of negative investigation (colonoscopy, radiological or laboratory test) as appropriate. We recruited 71 consecutive patients with MS (55 women, aged 43±9, median disease duration 78±43 months) referred for bowel symptoms to a specialist neurogastroenterology clinic, in a tertiary referrals unit. None of the patients fulfilled any of the exclusion criteria.
Assessment of disability
Disability was measured with the Expanded Disability Status Scale (EDSS) 21, which is commonly used in patients with MS both in research and clinical practice. The EDSS scale ranges from 0 to 10 in 0.5 U increments that represent higher levels of disability and is principally based on ambulatory ability of the patient. For scores between 1 and 4.5 the patient is able to walk, and the score is mainly based on evaluation of eight functional systems: pyramidal, cerebellar, brain stem, sensory, bowel and bladder, visual function, cerebral and mental function, and lastly any other system. With an EDSS above 5, mobility is impaired, at 7, the patient is wheelchair-bound and for scores above 8, the patient is bed-bound. Ten is death because of MS.
Disability is thought to be dependent on spinal cord involvement in MS, a common site of demyelinating lesions 22. Although there is no correlation between the load of cord lesions on imaging and MS symptoms, it appears that spinal cord atrophy (signifying axonal loss) is a good radiological marker that correlates with MS symptoms 23,24. Furthermore, EDSS correlates with spinal cord atrophy 23–25 and is a reflection of diffusion of spinal cord disease 26.
Symptoms assessments
Neurogenic Bowel Dysfunction questionnaire
No bowel symptoms questionnaire has been specifically validated in MS. Considering that constipation and faecal incontinence are often coexisting and alternating, we aimed to use an instrument that would evaluate both, as well as their impact on quality of life.
We therefore used the Neurogenic Bowel Dysfunction (NBD) questionnaire, which has been designed and validated in patients with spinal cord injury 27. It includes questions about background parameters (n=8), faecal incontinence (n=10), constipation (n=10), obstructed defecation (n=8) and impact on quality of life (n=3). The NBD score weights each symptom of bowel dysfunction in relation to its impact on quality of life, and scores are categorized as follows: 0 to 6 very minor dysfunction, 7 to 9 minor dysfunction, 10 to 13 moderate dysfunction and 14 to 47 severe dysfunction.
Patient-reported symptoms of bowel and bladder dysfunction
Patient-reported outcome measures are increasingly used in medical studies 28–30 and were assessed within a structured interview, conducted in the outpatient clinic. The alternating and fluctuating pattern of bowel habit in patients with MS is similar to that of irritable bowel syndrome. In patients with this condition, the product of frequency and severity of symptoms has been employed to quantify bowel function 31,32. Therefore, each patient was asked what proportion of time of his or her life was affected by constipation and/or faecal incontinence, with five possible answers (0, 25, 50, 75 or 100% of the time). We then assessed severity by asking patients to use a visual analogue scale from 0 to 100, with 0 representing absence of symptoms, and 100 if the patient thought that bowel symptoms were the worst possible. These data were collected by a doctor experienced in assessing bowel symptoms (G.P.).
The presence of bladder symptoms and on-going treatment (antimuscarinic agents, use of intermittent self-catheterization, permanent catheter) was ascertained from the patient’s history and clinical notes. Urgency was defined as a sudden compelling desire to pass urine that is difficult to defer 33; urge urinary incontinence was defined as incontinence accompanied by or immediately preceded by urgency. The patient was also asked about difficulty of initiating bladder voiding (hesitancy), interruption of flow, sense of incomplete bladder emptying and use of pads.
We aimed to quantify bladder and bowel dysfunction uniformly. Therefore, we asked the proportion of time a patient perceived bladder symptoms affected his or her life, with five possible answers (0, 25, 50, 75 or 100% of the time). Severity was assessed on a visual analogue scale from 0 to 100, similarly to bowel symptoms. These data were collected by a doctor experienced in assessing bladder symptoms (J.P.).
Study design and statistical analysis
Scores from questionnaires and outcome of outpatient interviews were prospectively collected. Prevalence of bladder dysfunction was established as the presence of at least one urinary symptom at least 25% of the time. Data were either ordinal or not normally distributed (according to Kolmogorov–Smirnov test); thus, they are expressed as median and interquartile ranges and nonparametric tests were used. Age and disease duration are presented as mean and SD. Correlations between our parameters (EDSS, MS type and duration, NBD and patient-reported bowel and bladder symptoms) were evaluated using Spearman’s rank test.
To evaluate how patient-reported symptoms quantified bowel dysfunction, we analysed correlations between NBD scores and patient-reported bowel symptoms with the Spearman’s rank test (r=correlation coefficient). The values of the NBD score for each of the four different categories of patient-reported frequency of bowel symptoms (25, 50, 75 or 100%) were compared with the Kruskal–Wallis test.
Statistical significance was two-sided, and declared for P values of 0.05 or less. Statistical analysis was performed using the statistical software package IBM SPSS statistics v21 for Mac (IBM, Armonk, New York, USA).
Results
Patients’ characteristics are reported in Table 1.
Table 1.
Patients’ baseline characteristics

Of the interviewed patients, 85% had some degree of urinary symptoms.
Correlation analysis
Correlation of EDSS, MS type and duration, NBD and patient-reported bowel symptoms is summarized in Table 2. Figure 1 shows the graphical correlation between EDSS and NBD.
Table 2.
Correlation analysis of bowel symptoms and patients characteristics
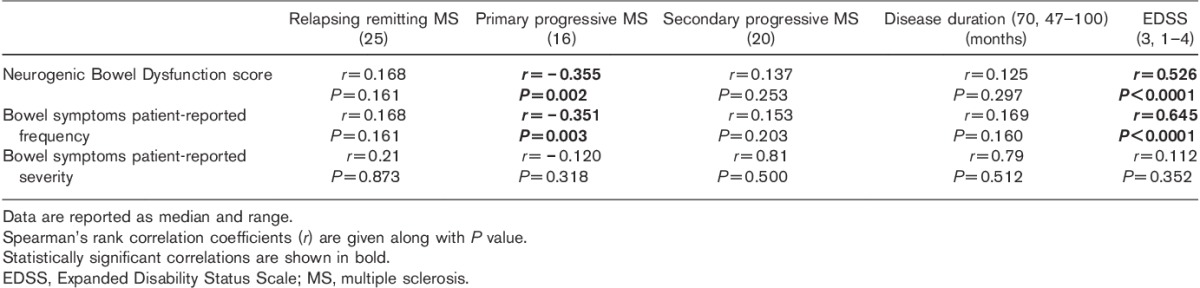
Fig. 1.
Correlation between Expanded Disability Status Scale and Neurogenic Bowel Dysfunction score (Spearman’s rank test).
Correlation between bowel and bladder patient-reported symptoms is summarized in Table 3 and Fig. 2.
Table 3.
Correlation analysis of bowel and bladder symptoms
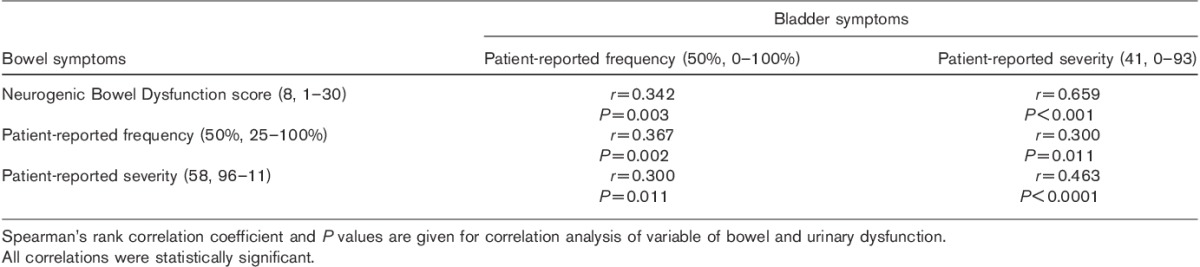
Fig. 2.
Correlation between patient-reported severity of bowel and bladder symptoms.
Evaluation of patient-reported symptoms
NBD scores were correlated with patient-reported frequency (r=0.860, P<0.0001) and severity (r=0.659, P<0.0001) of bowel symptoms.
There was significant difference between the values of NBD scores in the four categories of patient-reported frequency of bowel symptoms (Table 4 and Fig. 3).
Table 4.
Comparison of Neurogenic Bowel Dysfunction scores between the four categories of patient-reported frequency of bowel symptoms
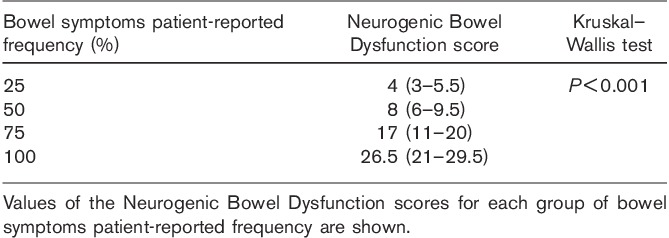
Fig. 3.
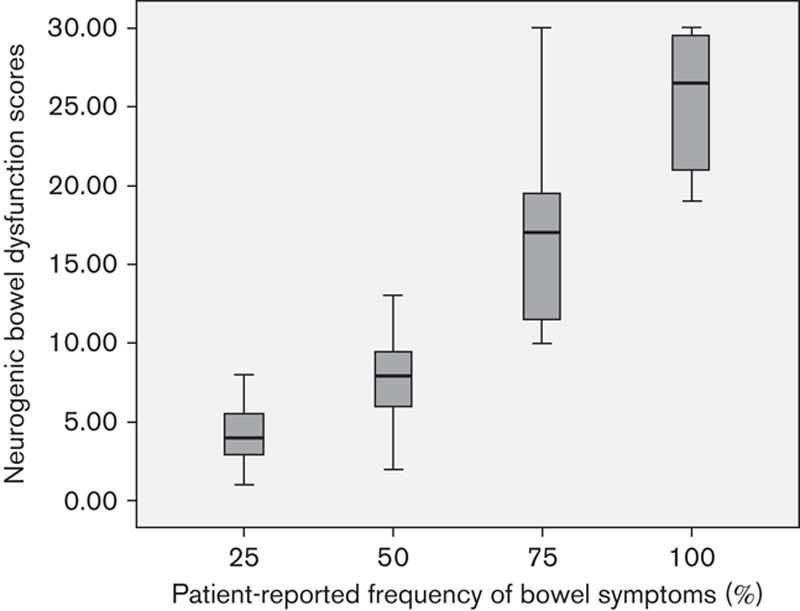
Comparison of Neurogenic Bowel Dysfunction scores between the four categories of patient-reported frequency of bowel symptoms. P value of Kruskal–Wallis test was less than 0.001 for all comparisons (Table 4).
Discussion
In our cohort, the prevalence of bladder dysfunction was 85%, which is higher than that expected in an unselected MS population, confirming our hypothesis and suggesting common pathophysiology of bowel and bladder dysfunction. Also, the NBD score was strongly correlated with the EDSS, which is a clinical indicator of spinal cord involvement in MS. These findings suggest that gut dysfunction in patients with MS is secondary to spinal cord involvement of the disease.
In patients with primary progressive disease, there was an inverse relationship with bowel symptoms (i.e. the higher the EDSS the lower the level of symptoms). The lower level of disability of this subgroup might explain this; however, it might just indicate that patients with other MS types have more bowel symptoms.
Patient-reported frequency and severity of bowel and bladder symptoms were correlated.
With regards to bowel symptoms evaluation, simple questioning of patient-reported frequency and severity of bowel symptoms is as accurate as a validated questionnaire. Therefore, physicians, in the clinical setting, would be able to assess the impact of bowel dysfunction by determining the percent of time these symptoms are perceived. In contrast, the NBD score could be used to improve the quality of bowel studies in patients with MS.
The main strength of this study is the methodology used to evaluate our hypothesis, which is supported by anatomical considerations and supportive evidence. Also low level of disability in this cohort reduced the effect of confounders such as reduced mobility and polypharmacy.
There are also several limitations. A relevant one is the lack of a control group without bowel symptoms. Unfortunately, although bowel symptoms are so prevalent, it is very difficult to recruit such a reference population in a study.
It is well known that antimuscarinic drugs used for urological symptoms can cause constipation, and this could be a confounding factor affecting our findings. Still, in the study by Chia et al. 12, where patients were receiving pharmacological treatment for bladder dysfunction, no higher occurrence of bowel symptoms was observed.
Unfortunately we did not record drugs taken at the time. But antimuscarinics could have hypothetically masked symptoms of faecal incontinence, ultimately making patients overall more constipated. Still, patients with higher disability had high scores in both constipation and incontinence, suggesting that the confounder effect of drugs, if present, was minimal. In some of our male patients, the presence of undetected prostate hypertrophy might have also contributed to the presence of bladder symptoms.
Another limitation of this study is that bladder symptoms were not quantified with a standard questionnaire. The correlation of patient-reported bowel and bladder symptoms merits further evaluation, employing a validated urological questionnaire.
An element that merits further discussion is the low level of disability in our cohort. In fact for an EDSS of less than 5, functional systems other than the spinal cord are relevant. Overall, our findings suggest that, although the cause of bowel dysfunction in patients with MS is multifactorial, the spinal cord plays a central role from the neuropathophysiology standpoint. Reflex activity in the spinal cord has a crucial role in regulating bowel function, and this has been widely demonstrated in patients with spinal cord injury 34–36, with whom MS patients share many similarities. Physiological studies in MS showed that the conduction in the central motor pathways to the sphincteric sacral neurons is delayed 37. In addition, somatosensory evoked potentials from the spinal cord to the brain have been shown to be delayed in MS compared with controls, with normal potentials recorded at the lumbar spine 38.
The loss of cortical modulation of spinal reflex activity can result in autonomic dysfunction of colonic motility, resulting in a prevalence of the sympathetic tone and slow colonic transit (constipation) 38, or in the loss of inhibition of parasympathetic output, uncontrolled colonic contractions and diarrhoea 39. This could be the result of unopposed parasympathetic stimulation of the vagus nerve that supplies the colon up to the splenic flexure.
In addition to the motor effects, anorectal hyposensitivity is another common feature of MS and spinal cord injuries that can affect the anorectum 1.
Disability measured with EDSS (reflective of the spinal cord involvement in MS) has been found consistently, as in our study, to correlate with bowel symptoms 10,11. It could be that there is a specific pattern of neurological deficit resulting in lower limb dysfunction and bowel symptomatology, and/or that difficulty accessing the toilet contributes to bowel symptoms.
A parallel phenomenon to bowel and bladder dysfunction, secondary to spinal cord disease, could be pelvic floor incoordination. In fact, the loss of cortical modulation of spinal reflexes due to MS plaques may result in autonomous functioning of the bladder, explaining bladder detrusor dyssynergia 40. This is the uncoordinated contraction of the detrusor muscle when the bladder attempts voiding. A similar and parallel mechanism might result in pelvic floor dyssynergia (uncoordinated contraction of the puborectalis and abdominal muscles on voiding the rectum), causing obstructed defecation and incomplete rectal emptying 41,42. A full rectum might in turn precipitate urgency and faecal incontinence in the presence of anal sphincter weakness and anorectal hyposensitivity or rectoanal incoordination 43, explaining coexistence of constipation and faecal incontinence in patients with MS. Pelvic floor dyssynergia might be also behavioural, and reversible with biofeedback 44. Successful biofeedback in non-neurological patients is associated with gut-specific changes in autonomic outflow to the large bowel, with spinal efferents playing a key role 45. Therefore, improvement of bowel symptoms with biofeedback might be attributed to the ability to recruit alternative neurological pathways through residual spinal cord function. This also suggests that neuromodulation in patients with residual spinal cord function could represent a targeted treatment option.
To improve our understanding of the natural history and aetiology of bowel dysfunction in MS, it would be helpful to correlate imaging and physiological studies in MS patients with bowel symptoms. Stratification of patients according to spinal cord disease, as clinically measured by the EDSS, could aid in the stratification of patients for targeted treatment.
Conclusion
Our findings and available evidence suggest that spinal cord involvement in MS is central to determination of bowel symptoms. This is highly relevant both to improve our understanding of bowel dysfunction in MS and in the development of further studies on the subject that should stratify patients on the basis of the clinical extent of spinal cord disease (EDSS). Physicians can easily assess lower limb function (a major determinant of EDSS), and a pragmatic treatment algorithm based on this parameter could be employed to improve bowel care in MS patients in the community. Residual spinal cord function could be a target of treatments such as neuromodulation, physiotherapy or biofeedback. The knowledge generated by studies on MS-related bowel dysfunction can also improve our understanding of the neural control of defecation and treatment of functional bowel disorders.
Finally, patient-reported frequency of bowel symptoms evaluates bowel dysfunction well, and so it is a valuable tool in the clinical setting. Equally, the Neurogenic Bowel Dysfunction score could be used to improve the quality of bowel studies in patients with MS.
Acknowledgements
Giuseppe Preziosi was supported by a Research Grant from the MS Society of Great Britain and Northern Ireland (grant #902-08). This includes costs for salary, equipment and consumables.
G. Preziosi and A. Emmanuel designed the study, obtained ethical approval, collected the data and drafted the manuscript. K. Thiruppathy, D.A. Raptis and J. Panicker contributed to the analysis of the data, their interpretation and critical review of the manuscript. J. Panicker also provided input as a specialist uro-neurologist. A. Raeburn contributed to the design of the study, in the application for ethical approval and in patients’ recruitment, and critically reviewed the manuscript. All authors approved the final version of the manuscript.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Nordenbo AM, Andersen JR, Andersen JT.Disturbances of ano-rectal function in multiple sclerosis.J Neurol 1996;243:445–451 [DOI] [PubMed] [Google Scholar]
- 2.Hornby A.The MS sufferer in the community.Nurs Times 1978;74Suppl130–131 [PubMed] [Google Scholar]
- 3.Norton C, Chelvanayagam S.Bowel problems and coping strategies in people with multiple sclerosis.Br J Nurs 2010;19:1–6 [DOI] [PubMed] [Google Scholar]
- 4.Nortvedt MW, Riise T, Frugard J, Mohn J, Bakke A, Skar AB, et al.Prevalence of bladder, bowel and sexual problems among multiple sclerosis patients two to five years after diagnosis.Mult Scler 2007;13:106–112 [DOI] [PubMed] [Google Scholar]
- 5.Swash M, Snooks SJ, Chalmers DH.Parity as a factor in incontinence in multiple sclerosis.Arch Neurol 1987;44:504–508 [DOI] [PubMed] [Google Scholar]
- 6.Wiesel PH, Norton C, Glickman S, Kamm MA.Pathophysiology and management of bowel dysfunction in multiple sclerosis.Eur J Gastroenterol Hepatol 2001;13:441–448 [DOI] [PubMed] [Google Scholar]
- 7.DasGupta R, Fowler CJ.Bladder, bowel and sexual dysfunction in multiple sclerosis: management strategies.Drugs 2003;63:153–166 [DOI] [PubMed] [Google Scholar]
- 8.Betts CD, D’Mellow MT, Fowler CJ.Urinary symptoms and the neurological features of bladder dysfunction in multiple sclerosis.J Neurol Neurosurg Psychiatry 1993;56:245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilman S, Low PA, Quinn N, Albanese A, Ben-Shlomo Y, Fowler CJ, et al.Consensus statement on the diagnosis of multiple system atrophy.J Auton Nerv Syst 1998;74:189–192 [PubMed] [Google Scholar]
- 10.Hinds JP, Eidelman BH, Wald A.Prevalence of bowel dysfunction in multiple sclerosis. A population survey.Gastroenterology 1990;98:1538–1542 [DOI] [PubMed] [Google Scholar]
- 11.Munteis E, Andreu M, Tellez MJ, Mon D, Ois A, Roquer J.Anorectal dysfunction in multiple sclerosis.Mult Scler 2006;12:215–218 [DOI] [PubMed] [Google Scholar]
- 12.Chia YW, Fowler CJ, Kamm MA, Henry MM, Lemieux MC, Swash M.Prevalence of bowel dysfunction in patients with multiple sclerosis and bladder dysfunction.J Neurol 1995;242:105–108 [DOI] [PubMed] [Google Scholar]
- 13.Craggs MD, Balasubramaniam AV, Chung EA, Emmanuel AV.Aberrant reflexes and function of the pelvic organs following spinal cord injury in man.Auton Neurosci 2006;126-127:355–370 [DOI] [PubMed] [Google Scholar]
- 14.Weber J, Denis P, Mihout B, Muller JM, Blanquart F, Galmiche JP, et al.Effect of brain-stem lesion on colonic and anorectal motility. Study of three patients.Dig Dis Sci 1985;30:419–425 [DOI] [PubMed] [Google Scholar]
- 15.Nathan PW, Smith MC.Spinal pathways subserving defaecation and sensation from the lower bowel.J Neurol Neurosurg Psychiatry 1953;16:245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan PW, Smith MC.The centrifugal pathway for micturition within the spinal cord.J Neurol Neurosurg Psychiatry 1958;21:177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nathan PW, Smith MC.The centripetal pathway from the bladder and urethra within the spinal cord.J Neurol Neurosurg Psychiatry 1951;14:262–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley WE, Timm GW, Scott FB.Innervation of the detrusor muscle and urethra.Urol Clin North Am 1974;1:3–27 [PubMed] [Google Scholar]
- 19.De Groat WC.Neural control of the urinary bladder and large bowel.Integrative function of the autonomous nervous system 1979.North Holland:Elsevier;50–67 [Google Scholar]
- 20.McLellan FC.The neurologic bladder 1939.Springfield, Illinois:Charles C. Thomas;57–116 [Google Scholar]
- 21.Kurtzke JF.Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS).Neurology 1983;33:1444–1452 [DOI] [PubMed] [Google Scholar]
- 22.Oppenheimer DR.The cervical cord in multiple sclerosis.Neuropathol Appl Neurobiol 1978;4:151–162 [DOI] [PubMed] [Google Scholar]
- 23.Kidd D, Thorpe JW, Thompson AJ, Kendall BE, Moseley IF, MacManus DG, et al.Spinal cord MRI using multi-array coils and fast spin echo. II. Findings in multiple sclerosis.Neurology 1993;43:2632–2637 [DOI] [PubMed] [Google Scholar]
- 24.Kidd D, Thorpe JW, Kendall BE, Barker GJ, Miller DH, McDonald WI, et al.MRI dynamics of brain and spinal cord in progressive multiple sclerosis.J Neurol Neurosurg Psychiatry 1996;60:15–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Losseff NA, Webb SL, O’Riordan JI, Page R, Wang L, Barker GJ, et al.Spinal cord atrophy and disability in multiple sclerosis. A new reproducible and sensitive MRI method with potential to monitor disease progression.Brain 1996;119Pt 3701–708 [DOI] [PubMed] [Google Scholar]
- 26.Lycklama á Nijeholt GJ, Castelijns JA, Lazeron RH, van Waesberghe JH, Polman CH, Uitdehaag BM, et al.Magnetization transfer ratio of the spinal cord in multiple sclerosis: relationship to atrophy and neurologic disability.J Neuroimaging 2000;10:67–72 [DOI] [PubMed] [Google Scholar]
- 27.Krogh K, Christensen P, Sabroe S, Laurberg S.Neurogenic bowel dysfunction score.Spinal Cord 2006;44:625–631 [DOI] [PubMed] [Google Scholar]
- 28.Ang D, Talley NJ, Simren M, Janssen P, Boeckxstaens G, Tack J.Review article: endpoints used in functional dyspepsia drug therapy trials.Aliment Pharmacol Ther 2011;33:634–649 [DOI] [PubMed] [Google Scholar]
- 29.Rahimi K, Malhotra A, Banning AP, Jenkinson C.Outcome selection and role of patient reported outcomes in contemporary cardiovascular trials: systematic review.BMJ 2010;341:c5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paterson C.Measuring outcomes in primary care: a patient generated measure, MYMOP, compared with the SF-36 health survey.BMJ 1996;312:1016–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talley NJ, Phillips SF, Melton J, 3rd, Wiltgen C, Zinsmeister AR.A patient questionnaire to identify bowel disease.Ann Intern Med 1989;111:671–674 [DOI] [PubMed] [Google Scholar]
- 32.Talley NJ, Phillips SF, Wiltgen CM, Zinsmeister AR, Melton LJ, 3rd.Assessment of functional gastrointestinal disease: the bowel disease questionnaire.Mayo Clin Proc 1990;65:1456–1479 [DOI] [PubMed] [Google Scholar]
- 33.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al.The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society.Neurourol Urodyn 2002;21:167–178 [DOI] [PubMed] [Google Scholar]
- 34.Krogh K, Christensen P.Neurogenic colorectal and pelvic floor dysfunction.Best Pract Res Clin Gastroenterol 2009;23:531–543 [DOI] [PubMed] [Google Scholar]
- 35.Gore RM, Mintzer RA, Calenoff L.Gastrointestinal complications of spinal cord injury.Spine (Phila Pa 1976) 1981;6:538–544 [DOI] [PubMed] [Google Scholar]
- 36.Glickman S, Kamm MA.Bowel dysfunction in spinal-cord-injury patients.Lancet 1996;347:1651–1653 [DOI] [PubMed] [Google Scholar]
- 37.Snooks SJ, Swash M.Motor conduction velocity in the human spinal cord: slowed conduction in multiple sclerosis and radiation myelopathy.J Neurol Neurosurg Psychiatry 1985;48:1135–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haldeman S, Glick M, Bhatia NN, Bradley WE, Johnson B.Colonometry, cystometry, and evoked potentials in multiple sclerosis.Arch Neurol 1982;39:698–701 [DOI] [PubMed] [Google Scholar]
- 39.Glick ME, Meshkinpour H, Haldeman S, Bhatia NN, Bradley WE.Colonic dysfunction in multiple sclerosis.Gastroenterology 1982;83:1002–1007 [PubMed] [Google Scholar]
- 40.Kalsi V, Fowler CJ.Therapy Insight: bladder dysfunction associated with multiple sclerosis.Nat Clin Pract Urol 2005;2:492–501 [DOI] [PubMed] [Google Scholar]
- 41.Gill KP, Chia YW, Henry MM, Shorvon PJ.Defecography in multiple sclerosis patients with severe constipation.Radiology 1994;191:553–556 [DOI] [PubMed] [Google Scholar]
- 42.Mathers SE, Ingram DA, Swash M.Electrophysiology of motor pathways for sphincter control in multiple sclerosis.J Neurol Neurosurg Psychiatry 1990;53:955–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waldron DJ, Horgan PG, Patel FR, Maguire R, Given HF.Multiple sclerosis: assessment of colonic and anorectal function in the presence of faecal incontinence.Int J Colorectal Dis 1993;8:220–224 [DOI] [PubMed] [Google Scholar]
- 44.Wiesel PH, Norton C, Roy AJ, Storrie JB, Bowers J, Kamm MA.Gut focused behavioural treatment (biofeedback) for constipation and faecal incontinence in multiple sclerosis.J Neurol Neurosurg Psychiatry 2000;69:240–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emmanuel AV, Kamm MA.Response to a behavioural treatment, biofeedback, in constipated patients is associated with improved gut transit and autonomic innervation.Gut 2001;49:214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]


