Abstract
Basal levels of autophagy are elevated in most pancreatic ductal adenocarcinomas (PDAC). Suppressing autophagy pharmacologically using chloroquine (CQ) or genetically with RNAi to essential autophagy genes inhibits human pancreatic cancer growth in vitro and in vivo, which presents possible treatment opportunities for PDAC patients using the CQ-derivative hydroxychloroquine (HCQ). Indeed, such clinical trials are ongoing. However, autophagy is a complex cellular mechanism to maintain cell homeostasis under stress. Based on its biological role, a dual role of autophagy in tumorigenesis has been proposed: at tumor initiation, autophagy helps maintain genomic stability and prevent tumor initiation; while in advanced disease, autophagy degrades and recycles cellular components to meet the metabolic needs for rapid growth. This model was proven to be the case in mouse lung tumor models. However, in contrast to prior work in various PDAC model systems, loss of autophagy in PDAC mouse models with embryonic homozygous Trp53 deletion does not inhibit tumor growth and paradoxically increases progression. This raised concerns whether there may be a genotype-dependent reliance of PDAC on autophagy. In a recent study, our group used a Trp53 heterozygous mouse PDAC model and human PDX xenografts to address the question. Our results demonstrate that autophagy inhibition was effective against PDAC tumors irrespective of TP53/TRP53 status.
Keywords: PDAC, Trp53, TP53, Atg5, autophagy, chloroquine, pancreatic cancer
Pancreatic ductal adenocarcinoma is a highly lethal tumor, and novel therapeutic approaches are needed to improve patient outcomes. Over 90% of PDAC possess activating mutations in the KRAS oncogene and studies in mouse models have shown that it is critical for tumor initiation and can result in the generation of pancreatic intraepithelial neoplasias (PanIN), a benign precursor of PDAC. Additional mutations in tumor suppressors are required for efficient progression from PanIN to PDAC. One such tumor suppressor gene is TP53 which is mutated or lost in the majority of human PDAC. Such alterations typically occur through loss of heterozygosity (LOH) during tumorigenesis.
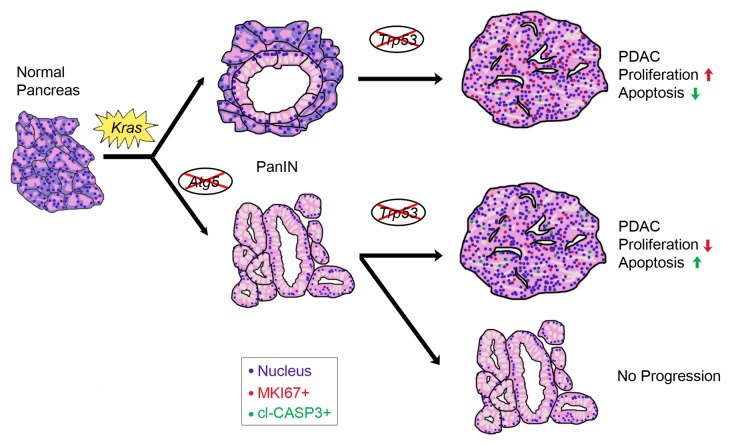
Elevation of basal autophagy level is a key characteristic of PDAC cells and is important to sustain growth in human tumor cell lines, xenografts, and mouse models, the majority of which have TP53/Trp53 alterations. Recently, a study showed that autophagy loss can accelerate tumor progression in a PDAC mouse model where both copies of Trp53 are simultaneously deleted during embryogenesis. We hypothesized that the differences between these and results from the previous work of our group and others were due to the nature of the model used. In particular, in contrast to human PDAC, tumors in this model do not undergo LOH of the Trp53 allele. To fully understand how autophagy affects tumorigenesis, we inhibited autophagy by breeding a conditional Atg5 null allele to a widely used PDAC mouse model with heterozygous Trp53 deletion and Kras mutation conditionally expressed in pancreas using a Pdx1-Cre allele. The second copy of Trp53 in this model is lost as an obligate step for tumor progression. Despite a small portion of mice dying prematurely from exocrine pancreas disruption, the autophagy-deficient cohort with Kras mutation and Trp53 heterozygous deletion exhibited longer survival times. Examination of pancreata at early and late timepoints showed that Atg5 null mice were more susceptible to early tumor initiation with a significantly increased number of PanINs, but were resistant to tumor progression, as invasive PDAC incidence was significantly diminished. Those tumors that did form in autophagy-incompetent pancreata showed increased DNA damage and apoptosis, with lower proliferation (Fig. 1).
Figure 1. Abrogation of autophagy affects PDAC tumorigenesis. Activation of Kras initiates transformation of normal pancreas to benign PanIN lesions. Atg5 deletion further promotes PanIN formation (more than 50 per mouse) compared with a wild-type mouse (2-10 PanINs per mouse). At later stages, Trp53 is lost via LOH resulting in PanIN transformation to invasive PDAC. PanINs in Atg5-deleted mice were impaired in their ability to progress to PDAC. Those that did develop PDAC had less proliferation and more apoptosis.
To specifically examine if Trp53 status affected response of tumor cells to acute autophagy inhibition, we performed studies using CQ treatment to block autophagosome degradation as well as RNAi to critical autophagy genes in PDAC cell lines derived from Trp53 heterozygous mice, Trp53 homozygous mice and Trp53R172H/+ mutated mice. These results were consistent with our previous findings that autophagy inhibition reduced both colony formation as well as oxidative phosphorylation in all PDAC cell lines independent of the original Trp53 genetic status.
Finally, we performed efficacy studies using a panel of PDAC patient-derived xenografts (PDXs) using the CQ-derivative hydroxychloroquine (HCQ) as this is currently being tested in multiple clinical trials. Responses were seen in nearly every PDX line (11 of which had mutated TP53). Interestingly, the only PDX line that did not respond to HCQ treatment was KRAS wild type and had minimal basal levels of autophagy. Assessment of the xenografts showed that HCQ effectively inhibited autophagy as demonstrated by increased LC3 puncta. Similar to the Atg5-deleted PDAC tumors, HCQ treated PDXs had elevated apoptosis and reduced proliferation.
Together our data shows that Trp53 status does not influence the response of PDAC to autophagy inhibition. While the concurrent homozygous deletion of Trp53 and Atg5 or Atg7 during embryogenesis may accelerate PDAC progression, this is a different situation than what is seen in human PDAC where TP53 is lost by LOH. In this scenario, autophagy appears to have a predominantly pro-tumorigenic role. Clinical trials in PDAC patients are currently assessing the efficacy of HCQ in combination with other therapies. Whether these will be successful will depend on a variety of factors, including the ability of HCQ to effectively inhibit autophagy in human patients. However, we think that our data do not support the exclusion of patients with TP53 mutations from these trials.
Disclosure of Potential Conflicts of Interest
ACK is a consultant for Forma Therapeutics.
Acknowledgments
ACK is supported by National Cancer Institute Grant R01CA157490, ACS Research Scholar Grant RSG-13-298-01-TBG, and the Lustgarten Foundation.