Abstract
The endothelial lining of the vasculature performs a vital role in maintaining fluid barrier functions despite balancing nutrient and fluid content of tissues, repairing localized damage, coordinating responses of a plethora of factors, immune cells and platelets through a multitude of endothelial cell surface receptors. Viruses that nonlytically cause lethal hemorrhagic or edematous diseases engage receptors on vascular and lymphatic endothelial cells, altering normal cellular responses that control capillary leakage and fluid clearance functions with lethal consequences. Recent studies indicate that receptors directing dengue virus and hantavirus infection of the endothelium contribute to the dysregulation of normal endothelial cell signaling responses that control capillary permeability and immune responses that contribute to pathogenesis. Here we present recent studies of virally altered endothelial functions that provide new insight into targeting barrier functions of the endothelium as a potential therapeutic approach.
Introduction
The endothelium is a tissue that lines capillaries and regulates solute, gas, and fluid exchange between tissues and vascular compartments through a complex series of endothelial cell (EC) surface receptor interactions [1, 2]. The critical nature of the EC fluid barrier is evident from the redundant failsafe mechanisms in place to prevent a lethal vascular breach and a discrete lymphatic system designed to clear excess fluid from interstitial spaces [3]. Microvascular and lymphatic EC (MEC and LEC) surface receptors and the endothelial glycocalyx are keys to fluid management and vascular homeostasis. The endothelial glycocalyx is mainly composed of surface-anchored syndecans and glypicans carrying highly sulfated, linear glycosaminoglycan attachments such as heparan, chondroitin, and dermatan [4]. Interactions between the glycocaylx, cell surface integrins, (ie. αvβ3, αvβ1) adhesions molecules (ie. PECAM, ICAM, and VCAM), and inter-endothelial adherens junctions (VE-cadherin) form a meshwork of EC specific cell surface sensors that maintain EC barrier functions [4]. This task is complicated by the requirement for ECs to respond to a plethora of permeabilizing factors (ie. VEGF, TNFα, PAF), tissue conditions, damage responses, and immune cell extravasation that require junctional plasticity while maintaining a fluid barrier.
Although the endothelium normally prevents adhesion of leukocytes and platelets, pathogen activation of the endothelium directs localized immune cell adherence and extravasation without EC lysis or hemorrhage [4–12]. However, localized concentrations of cytokines, chemokines, clotting cascades, growth factors, and nitric oxide, whose concentrations are increased as a result of infection, may engage EC receptors and reduce barrier integrity [1, 2, 13–18]. Inflammatory mediators such as TNFα and LPS can also cause degradation or shedding of the EC glycocalyx [4]. TNFα induces EC activation, attracting mast cells and inducing responses of cytokines, proteases, and heparanases that degrade glycocalyx moieties and glycan receptors [4, 19].
Altered endothelial barrier functions are implicated as the cause of hemorrhagic disease following infection by a number of viruses, including dengue viruses, hantaviruses and arenaviruses, that nonlytically infect ECs [5–12, 20]. Changes in EC functions are likely to result from EC surface receptor and cytoplasmic signaling responses as well as EC interactions with immune cells. Dengue viruses engage EC surfaces through interactions with heparan sulfate moieties that direct viral entry [21]. Dengue virus infection of ECs results in changes in signaling pathways and cellular gene expression profiles, which in turn may influence EC fluid barrier functions both directly and through the induction and secretion of immune-enhancing chemokine and cytokine responses [21, 22]. Thus the means by which dengue attaches to and enters ECs is central to its ability to direct disease and fundamental to therapeutically resolving dengue-induced vascular permeability deficits.
Direct contact with EC surface receptors is also associated with changes in vascular permeability via signaling pathway responses resulting in the dissociation of VE-cadherin within adherens junctions (AJs) [23–26]. Under normal conditions VEGF directs the dissociation of AJs in order to repair vascular damage. However, VEGF is also 50,000x more potent than histamine in directing EC permeability, and high altitude induced pulmonary edema is the result of aberrant hypoxia-induced VEGF permeability [13, 14, 17, 26–29]. Hantaviruses bind and inactivate αvβ3 integrin conformers that normally form complexes with VEGF receptors, and thus hantaviruses similarly disengage the normal regulation of VEGF-induced permeability [7, 30–37].
The endothelium contains a vast array of receptors that play critical roles in the regulation of immune cell adherence, capillary permeability, platelet and complement activation, clotting, and vasodilation responses, all of which can be greatly altered by virus infection and contribute to hemorrhage or edema [10]. In addition, lymphatic tissues and lymphatic endothelial cells (LECs) are uniquely regulated by discrete cell surface receptors and emerging as a system critical to the regulation of edema, tolerance, and immunity [28, 38–40]. Although lymphatics are often forgotten, these vessels clear fluid from tissues and in lymph nodes LECs constitutively express MHCII and are sentinel antigen presenting cells that determine tolerance and clearance responses [38, 41]. Recent studies indicating that hantaviruses infect and dysregulate normal LEC functions further suggest roles for lymphatic EC receptor responses that alter pulmonary fluid clearance functions of hantavirus pulmonary syndrome (HPS) patients [39, 42, 43]. This review explores the ways in which dengue and hantaviruses contact and alter endothelial cell surface receptors and their corresponding signaling pathways, leading to hemorrhagic disease and vascular permeability.
Endothelial Cells and Dengue Virus
Dengue virus is a mosquito-borne flavivirus that causes a mild febrile illness, dengue fever (DF), and two highly lethal vascular permeability based diseases: dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) [44, 45]. DSS and DHF are edematous and hemorrhagic diseases, respectively, that occur in the absence of EC lysis [45]. The presence of dengue virus infected ECs in patients rationalizes their contribution to severe dengue disease [5, 46–49], and while their role in pathogenesis is still unknown, infected ECs can potentially alter barrier functions, permit immune cell targeting, elicit cytokine and chemokine responses, and contribute to viremia [50, 51]. Dengue virus reportedly infects a variety of cells including immune, dendritic, endothelial, and liver cells through attachment to cell surface receptors. The dengue virus envelope protein reportedly binds to Fc receptors, DC-SIGN, ICAM3, CD14, mannose receptor, HSP70/90, GRP78, laminin receptor, heparan sulfate proteoglycans (HSPGs), and the mannose receptor [52–62]. However, a consensus dengue virus receptor has not yet been defined.
Recent studies show that dengue virus infects ~80% of primary human ECs with viral antigen present by 24 hours after infection. Infection is rapidly productive, releasing ~105 FFUs/ml of dengue virus into the media 1 day post-infection [21]. Furthermore, heparin, heparan sulfate, heparinase and protease, but not antibodies to a number of other cell surface receptors, block dengue virus infection of primary human ECs. Dengue virus binds specifically to immobilized heparin and is competitively inhibited by the addition of excess heparin [21]. Thus, dengue virus productively infects human ECs via attachment to heparan sulfate-containing cell surface receptors. Indeed, carbohydrate moieties of cell surface glycoproteins, glycolipids, and proteoglycans serve as receptors for enveloped and non-enveloped viruses alike [63]. These negatively-charged carbohydrate receptors are also commonly responsible for specific tissue tropism, making them key targets for limiting viral spread [63].
Dengue nonstructural proteins may also enhance DV pathogenesis through a variety of cytoplasmic and cell surface receptor directed signaling mechanisms. In particular, the non-structural 1 (NS1) protein is expressed in cytosolic, secreted, and cell-surface expressed forms [64–66]. Secreted NS1 is highly abundant, highly antigenic, and shown to bind cellular heparan sulfate E present on liver and lung ECs [67]. Likewise, high quantities of adherent cross-reactive NS1 antibodies circulate in infected patient blood and are known to bind glycan surface receptors on platelets and ECs [68–70], triggering immune cell and complement activation [71, 72]. Furthermore, the secreted form of dengue NS1 also modulates classical complement activation by binding to the C4b binding protein, thereby inactivating C4b [72]. NS1 and NS1 antibodies form a potent combination capable of eliciting or regulating immune and complement responses through critical cell surface glycan interactions [73]. Interestingly, dengue virus infected ECs elicit immune enhancing cytokine and chemokine responses that may enhance immune responses and contribute to DSS and DHF diseases during secondary infections perhaps by targeting non-neutralizing dengue antigens in ECs. These interactions, and the intracellular signaling responses they trigger, may contribute to EC dysfunction and vascular leakage in dengue-infected patients [5, 68–70]. The differential role of dengue virus regulation of EC MHCII responses in primary and secondary dengue virus infections has yet to be considered but may also factor into pathogenic mechanisms during infection by a second dengue serotype. Consideration of barrier-stabilizing effectors that target the endothelium may also effectively reduce vascular leakage and associated inflammatory effects that contribute to dengue pathogenesis [74, 75].
Hantavirus Endothelial Cell Interactions
Diverse pathogenic hantaviruses that cause hemorrhagic fever with renal syndrome (HFRS) or hantavirus pulmonary syndrome (HPS) were found to commonly use αvβ3 integrins to enter primary human ECs or CHO cells expressing recombinant human αvβ3 receptors [34, 76–79]. Interestingly, nonpathogenic hantaviruses failed to use αvβ3 integrins and instead entered cells consistent with the use of β1 integrins, suggesting a fundamental difference in EC receptor usage that is tied to vascular permeability [34, 76]. αvβ3 integrin deficits cause vascular diseases and subsequent studies found that pathogenic hantaviruses bind inactive αvβ3 integrin conformers [34, 76, 78, 79]. αvβ3 integrins normally regulate permeabilizing responses of VEGF directed by VEGFR2 receptors and pathogenic hantavirus infections cause the hyperpermeability of ECs in response to VEGF days after infection [77, 80, 81]. These responses are mediated by increased VEGFR2 phosphorylation and increased internalization of VE-cadherin from AJs and suppressed by blocking VEGFR2-Src signaling pathways. The occurrence of these responses days after viral entry suggests that newly emergent cell-associated virus regulates αvβ3 responses (Figure 1). In fact pathogenic hantavirus accumulation on the EC surface was shown to occur through αvβ3 interactions and to recruit quiescent platelets to ECs [80]. These findings define EC receptors as targets of dysregulated VEGF-directed permeability responses and potential mechanisms by which hantaviruses inactivate platelets and contribute to thrombocytopenia. They also suggest quiescent platelet recruitment to infected ECs as a means of evading immune surveillance.
Figure 1.
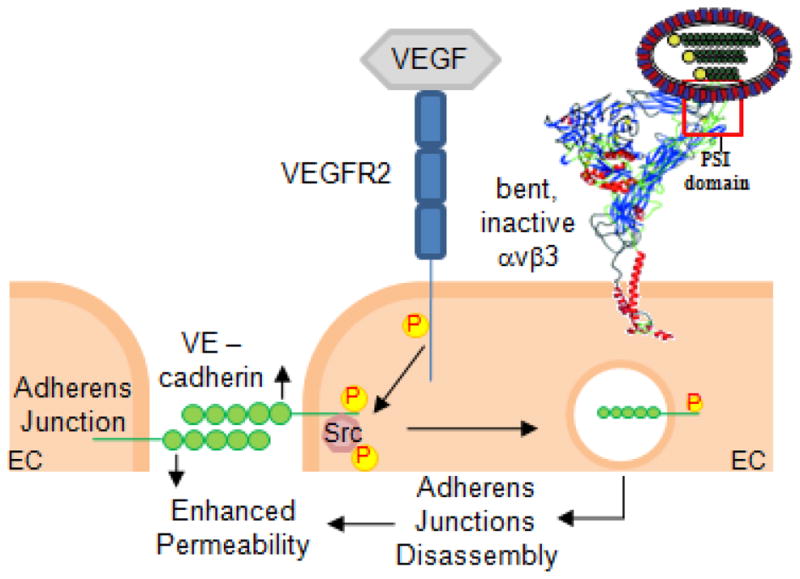
The figure depicts the inability to αvβ3 to regulate endothelial cell responses to hypoxia or VEGF at late stages of hantavirus infection resulting a localized increase in vascular permeability [7, 43, 79, 81, 86, 114]. αvβ3 integrins form an extracellular complex with VEGFR2 receptors that normally restrict the permeabilizing effects of VEGF [115–118]. The schematic indicates the effect of pathogenic hantavirus binding to inactive αvβ3 integrin conformers [79]. Consistent with hantavirus dysregulation of αvβ3-VEGFR2 responses, days after infection cell associated hantavirus coats the surface of endothelial cells [80, 119]. Hypoxia induced VEGF responses of HPS patients [120–123] are likely to enhance VEGFR2-Src signaling responses, which direct VE-cadherin internalization and dissociate adherens junctions (AJs) [7, 42, 43, 81, 86, 114]. VE-cadherin internalization decreases fluid barrier functions of the endothelium [97, 98] and may contribute to localized increases in vascular permeability [17, 29, 124] and edema during hantavirus infection [6, 18, 89, 121, 125]. Thus dysregulation of αvβ3 functions may contribute to the enhanced VEGF responsiveness and permeability of hantavirus infected lymphatic and microvascular endothelium.
HPS patients are acutely hypoxic and hypoxia is a known inducer of VEGF directed permeability and edema. Similar to patient responses, hantaviruses enhance the permeability of chemically- or O2 level-induced hypoxia in MECs and LECs. This constitutively activates a downstream mTOR-directed pathway that normally regulates hypoxic responses, VEGF signaling and cellular quiescence [42, 82–85]. Interestingly, hantavirus infected LECs are also hyperresponsive to VEGF and hypoxia and activate mTOR signaling responses that are inhibited by rapamycin as well as VEGF-C, which exclusively acts on LEC VEGFR3 receptors [42, 86, 87]. These findings link hantavirus pathogenesis to LEC receptor usage and further suggest a role for hantavirus infection of LECs as a determinant of fluid accumulation within HPS patients. Furthermore, these results suggest that VEGF permeability responses contribute to vascular permeability and clearance deficits and are potential targets for therapeutic intervention [88–93].
Therapeutic regulation of these responses appears to go hand in hand with the receptor and pathway specific regulation of VEGFR2-, Src-, and mTOR-directed responses that control EC barrier functions. Antibodies to VEGF suppress EC permeability and have the potential to antagonize VEGR2 signaling pathways as a means of reducing acute pulmonary edema in HPS [14, 27, 74, 81, 94–101]. In addition, well studied VEGFR2 and Src inhibitors, the mTOR inhibitor rapamycin, and other components which target intermediary steps in EC pathway activation are in clinical trials for treating human cancers but also have the potential to reduce the severity of viral EC permeability-based diseases [28, 81, 94–98, 101–103]. These include Ang-1, S1P, and the drugs pazopanib and dasatinib. Angiopoietin-1, an EC specific growth factor, binds Tie-2 receptors and blocks VEGFR2 directed permeability [100, 104–109] and S1P, a platelet derived lipid mediator, stabilizes vascular barrier functions through Edg-1 receptor signaling [74, 95, 96, 110–113]. The redundant regulation of EC barrier functions provides several mechanisms by which EC receptors may contribute to permeability deficits, but also provides a target rich environment for restoring MEC barrier and LEC fluid clearance functions during viral infection. Understanding these receptor and pathway specific mechanisms is likely to provide a means for resolving viral hemorrhagic and edematous diseases by therapeutically targeting EC responses.
Conclusions
These studies highlight important cell surface targets that have the potential to regulate virally induced vascular permeability and for which there currently are clinically available therapeutics. Targeting EC responses may be broadly applicable to counteracting the severity of additional viral infections that disrupt normal endothelial cell function.
Highlights (for review).
Viruses that nonlytically cause vascular leakage infect the vascular & lymphatic endothelium.
Dengue infected ECs elicit immune enhancing chemokines and direct immune cell targeting of ECs.
Hantaviruses increase vascular permeability of AJs in response to VEGF signaling in MECs and LECs.
The endothelium is a therapeutic target for resolving hemorrhagic and edematous disease.
Acknowledgments
We thank I. Gavrilovskaya, E. Gorbunova and Lewis Markoff for insightful discussions. This work was supported by grants AI75022, AI1092191 and AI097951, AI093792 and U54AI57158 (NBC-Lipkin) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* References which are of special interest for virally induced vascular permeability.
** Outstanding references with seminal findings that provide a basis for understanding microvascular and lymphatic vessel permeability and edema regulation, VEGF as a primal permeability factor and the endothelium as a potential target for regulating virally induced vascular leakage.
- 1.Baumgartner-Parzer SM, Waldhausl WK. The endothelium as a metabolic and endocrine organ: its relation with insulin resistance. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S166–179. doi: 10.1055/s-2001-18579. [DOI] [PubMed] [Google Scholar]
- **2.Aird WC. Endothelium as an organ system. Crit Care Med. 2004;32(5 Suppl):S271–279. doi: 10.1097/01.ccm.0000129669.21649.40. [DOI] [PubMed] [Google Scholar]
- 3.Salmon AH, Satchell SC. Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J Pathol. 2012;226(4):562–574. doi: 10.1002/path.3964. [DOI] [PubMed] [Google Scholar]
- 4.Becker BF, Chappell D, Bruegger D, Annecke T, Jacob M. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res. 2010;87(2):300–310. doi: 10.1093/cvr/cvq137. [DOI] [PubMed] [Google Scholar]
- 5.Basu A, Chaturvedi UC. Vascular endothelium: the battlefield of dengue viruses. FEMS Immunol Med Microbiol. 2008;53(3):287–299. doi: 10.1111/j.1574-695X.2008.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duchin JS, Koster FT, Peters CJ, et al. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. The Hantavirus Study Group [see comments] N Engl J Med. 1994;330(14):949–955. doi: 10.1056/NEJM199404073301401. [DOI] [PubMed] [Google Scholar]
- *7.Gavrilovskaya IN, Gorbunova EE, Mackow NA, Mackow ER. Hantaviruses direct endothelial cell permeability by sensitizing cells to the vascular permeability factor VEGF, while angiopoietin 1 and sphingosine 1-phosphate inhibit hantavirus-directed permeability. J Virol. 2008;82(12):5797–5806. doi: 10.1128/JVI.02397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lahdevirta J. Clinical features of HFRS in Scandinavia as compared with East Asia. Scand J Infect Dis Suppl. 1982;36:93–95. [PubMed] [Google Scholar]
- 9.Mackow ER, Gavrilovskaya IN. Cellular receptors and hantavirus pathogenesis. Curr Top Microbiol Immunol. 2001;256:91–115. doi: 10.1007/978-3-642-56753-7_6. [DOI] [PubMed] [Google Scholar]
- 10.Mackow ER, Gavrilovskaya IN. Hantavirus regulation of endothelial cell functions. Thromb Haemost. 2009;102(6):1030–1041. doi: 10.1160/TH09-09-0640. [DOI] [PubMed] [Google Scholar]
- 11.Valbuena G, Walker DH. The endothelium as a target for infections. Annu Rev Pathol. 2006;1:171–198. doi: 10.1146/annurev.pathol.1.110304.100031. [DOI] [PubMed] [Google Scholar]
- 12.Zaki S, Greer P, Coffield L, et al. Hantavirus Pulmonary Syndrome: pathogenesis of an emerging infectious disease. Am J Pathol. 1995;146:552–579. [PMC free article] [PubMed] [Google Scholar]
- **13.Dvorak HF. Vascular permeability to plasma, plasma proteins, and cells: an update. Curr Opin Hematol. 2010;17(3):225–229. doi: 10.1097/MOH.0b013e3283386638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breen EC. VEGF in biological control. J Cell Biochem. 2007;102(6):1358–1367. doi: 10.1002/jcb.21579. [DOI] [PubMed] [Google Scholar]
- 15.Cines DB, Pollak ES, Buck CA, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91(10):3527–3561. [PubMed] [Google Scholar]
- 16.Coller BS, Shattil SJ. The GPIIb/IIIa (integrin alphaIIbbeta3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Blood. 2008;112(8):3011–3025. doi: 10.1182/blood-2008-06-077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *17.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146(5):1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 18.Berger MM, Hesse C, Dehnert C, et al. Hypoxia impairs systemic endothelial function in individuals prone to high-altitude pulmonary edema. Am J Respir Crit Care Med. 2005;172(6):763–767. doi: 10.1164/rccm.200504-654OC. [DOI] [PubMed] [Google Scholar]
- 19.Chappell D, Westphal M, Jacob M. The impact of the glycocalyx on microcirculatory oxygen distribution in critical illness. Curr Opin Anaesthesiol. 2009;22(2):155–162. doi: 10.1097/ACO.0b013e328328d1b6. [DOI] [PubMed] [Google Scholar]
- *20.Kunz S. The role of the vascular endothelium in arenavirus haemorrhagic fevers. Thromb Haemost. 2009;102(6):1024–1029. doi: 10.1160/TH09-06-0357. [DOI] [PubMed] [Google Scholar]
- 21.Dalrymple N, Mackow ER. Productive Dengue Virus Infection of Human Endothelial Cells is Directed By Heparan Sulfate-Containing Proteoglycan Receptors. J Virol. 2011;85:9478–9485. doi: 10.1128/JVI.05008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *22.Dalrymple NA, Mackow ER. Endothelial Cells Elicit Immune Enhancing Responses to Dengue Virus Infection. J Virol. 2012 doi: 10.1128/JVI.00213-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lampugnani MG, Dejana E. The control of endothelial cell functions by adherens junctions. Novartis Found Symp. 2007;283:4–13. doi: 10.1002/9780470319413.ch2. discussion 13–17, 238–241. [DOI] [PubMed] [Google Scholar]
- 24.Lampugnani MG, Dejana E. Adherens junctions in endothelial cells regulate vessel maintenance and angiogenesis. Thromb Res. 2007;120(Suppl 2):S1–6. doi: 10.1016/S0049-3848(07)70124-X. [DOI] [PubMed] [Google Scholar]
- 25.Gavard J. Breaking the VE-cadherin bonds. FEBS Lett. 2009;583(1):1–6. doi: 10.1016/j.febslet.2008.11.032. [DOI] [PubMed] [Google Scholar]
- **26.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121(Pt 13):2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 27.Moreira IS, Fernandes PA, Ramos MJ. Vascular endothelial growth factor (VEGF) inhibition--a critical review. Anticancer Agents Med Chem. 2007;7(2):223–245. doi: 10.2174/187152007780058687. [DOI] [PubMed] [Google Scholar]
- *28.Bahram F, Claesson-Welsh L. VEGF-mediated signal transduction in lymphatic endothelial cells. Pathophysiology. 2010;17(4):253–261. doi: 10.1016/j.pathophys.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Dvorak HF. Discovery of vascular permeability factor (VPF) Exp Cell Res. 2006;312(5):522–526. doi: 10.1016/j.yexcr.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Jin G, Miao H, Li JY, Usami S, Chien S. Integrins regulate VE-cadherin and catenins: dependence of this regulation on Src, but not on Ras. Proc Natl Acad Sci U S A. 2006;103(6):1774–1779. doi: 10.1073/pnas.0510774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMillan NA, Payne E, Frazer IH, Evander M. Expression of the alpha6 integrin confers papillomavirus binding upon receptor-negative B-cells. Virology. 1999;261(2):271–279. doi: 10.1006/viro.1999.9825. [DOI] [PubMed] [Google Scholar]
- 32.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73(2):309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 33.Berinstein A, Roivainen M, Hovi T, Mason PW, Baxt B. Antibodies to the vitronectin receptor (integrin alpha V beta 3) inhibit binding and infection of foot-and-mouth disease virus to cultured cells. J Virol. 1995;69(4):2664–2666. doi: 10.1128/jvi.69.4.2664-2666.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gavrilovskaya IN, Shepley M, Shaw R, Ginsberg MH, Mackow ER. beta3 Integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc Natl Acad Sci U S A. 1998;95(12):7074–7079. doi: 10.1073/pnas.95.12.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Huang DY, Huong SM, Huang ES. Integrin alphavbeta3 is a coreceptor for human cytomegalovirus. Nat Med. 2005;11(5):515–521. doi: 10.1038/nm1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feire AL, Koss H, Compton T. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc Natl Acad Sci U S A. 2004;101(43):15470–15475. doi: 10.1073/pnas.0406821101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chu JJ, Ng ML. Interaction of West Nile virus with alpha v beta 3 integrin mediates virus entry into cells. J Biol Chem. 2004;279(52):54533–54541. doi: 10.1074/jbc.M410208200. [DOI] [PubMed] [Google Scholar]
- 38.Tewalt EF, Cohen JN, Rouhani SJ, Engelhard VH. Lymphatic endothelial cells - key players in regulation of tolerance and immunity. Front Immunol. 2012;3:305. doi: 10.3389/fimmu.2012.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackow ER, Gorbunova EE, Dalrymple NA, Gavrilovskaya IN. Role of vascular and lymphatic endothelial cells in hantavirus pulmonary syndrome suggests targeted therapeutic approaches. Lymphat Res Biol. 2013;11(3):128–135. doi: 10.1089/lrb.2013.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schraufnagel DE. Lung lymphatic anatomy and correlates. Pathophysiology. 2010;17(4):337–343. doi: 10.1016/j.pathophys.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7(10):803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- *42.Gavrilovskaya IN, Gorbunova EE, Mackow ER. Hypoxia induces permeability and giant cell responses of Andes virus-infected pulmonary endothelial cells by activating the mTOR-S6K signaling pathway. J Virol. 2013;87(23):12999–13008. doi: 10.1128/JVI.02103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *43.Gorbunova EE, Gavrilovskaya IN, Mackow ER. Slit2-Robo4 receptor responses inhibit ANDV directed permeability of human lung microvascular endothelial cells. Antiviral Res. 2013;99(2):108–112. doi: 10.1016/j.antiviral.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guzman MG, Halstead SB, Artsob H, et al. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8(12 Suppl):S7–16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halstead SB. Pathophysiology. In: Halstead SB, editor. Dengue. Vol. 5. Imperial College Press; London: 2008. pp. 265–326. [Google Scholar]
- 46.Oishi K, Saito M, Mapua CA, Natividad FF. Dengue illness: clinical features and pathogenesis. J Infect Chemother. 2007;13(3):125–133. doi: 10.1007/s10156-007-0516-9. [DOI] [PubMed] [Google Scholar]
- 47.Noisakran S, Perng GC. Alternate hypothesis on the pathogenesis of dengue hemorrhagic fever (DHF)/dengue shock syndrome (DSS) in dengue virus infection. Exp Biol Med (Maywood) 2008;233(4):401–408. doi: 10.3181/0707-MR-198. [DOI] [PubMed] [Google Scholar]
- 48.Martina BE, Koraka P, Osterhaus AD. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev. 2009;22(4):564–581. doi: 10.1128/CMR.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McBride WJ, Bielefeldt-Ohmann H. Dengue viral infections; pathogenesis and epidemiology. Microbes Infect. 2000;2(9):1041–1050. doi: 10.1016/s1286-4579(00)01258-2. [DOI] [PubMed] [Google Scholar]
- 50.Krishnamurti C, Peat RA, Cutting MA, Rothwell SW. Platelet adhesion to dengue-2 virus-infected endothelial cells. Am J Trop Med Hyg. 2002;66(4):435–441. doi: 10.4269/ajtmh.2002.66.435. [DOI] [PubMed] [Google Scholar]
- 51.Azizan A, Sweat J, Espino C, Gemmer J, Stark L, Kazanis D. Differential proinflammatory and angiogenesis-specific cytokine production in human pulmonary endothelial cells, HPMEC-ST1.6R infected with dengue-2 and dengue-3 virus. J Virol Methods. 2006;138(1–2):211–217. doi: 10.1016/j.jviromet.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 52.Cabrera-Hernandez A, Duncan SR. Mammalian Dengue Virus Receptors. Dengue Bulletin. 2005;29 [Google Scholar]
- 53.Kaufmann B, Rossmann MG. Molecular mechanisms involved in the early steps of flavivirus cell entry. Microbes Infect. 2011;13(1):1–9. doi: 10.1016/j.micinf.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Y, Maguire T, Marks RM. Demonstration of binding of dengue virus envelope protein to target cells. J Virol. 1996;70(12):8765–8772. doi: 10.1128/jvi.70.12.8765-8772.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller JL, de Wet BJ, Martinez-Pomares L, et al. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog. 2008;4(2):e17. doi: 10.1371/journal.ppat.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mondotte JA, Lozach PY, Amara A, Gamarnik AV. Essential role of dengue virus envelope protein N glycosylation at asparagine-67 during viral propagation. J Virol. 2007;81(13):7136–7148. doi: 10.1128/JVI.00116-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tassaneetrithep B, Burgess TH, Granelli-Piperno A, et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med. 2003;197(7):823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Navarro-Sanchez E, Altmeyer R, Amara A, et al. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 2003;4(7):723–728. doi: 10.1038/sj.embor.embor866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hilgard P, Stockert R. Heparan sulfate proteoglycans initiate dengue virus infection of hepatocytes. Hepatology. 2000;32(5):1069–1077. doi: 10.1053/jhep.2000.18713. [DOI] [PubMed] [Google Scholar]
- 60.Germi R, Crance JM, Garin D, et al. Heparan sulfate-mediated binding of infectious dengue virus type 2 and yellow fever virus. Virology. 2002;292(1):162–168. doi: 10.1006/viro.2001.1232. [DOI] [PubMed] [Google Scholar]
- 61.Chen Y, Maguire T, Hileman RE, et al. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med. 1997;3(8):866–871. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 62.Lin YL, Lei HY, Lin YS, Yeh TM, Chen SH, Liu HS. Heparin inhibits dengue-2 virus infection of five human liver cell lines. Antiviral Res. 2002;56(1):93–96. doi: 10.1016/s0166-3542(02)00095-5. [DOI] [PubMed] [Google Scholar]
- 63.Olofsson S, Bergstrom T. Glycoconjugate glycans as viral receptors. Ann Med. 2005;37(3):154–172. doi: 10.1080/07853890510007340. [DOI] [PubMed] [Google Scholar]
- 64.Alcon-LePoder S, Drouet MT, Roux P, et al. The secreted form of dengue virus nonstructural protein NS1 is endocytosed by hepatocytes and accumulates in late endosomes: implications for viral infectivity. J Virol. 2005;79(17):11403–11411. doi: 10.1128/JVI.79.17.11403-11411.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacobs MG, Robinson PJ, Bletchly C, Mackenzie JM, Young PR. Dengue virus nonstructural protein 1 is expressed in a glycosyl-phosphatidylinositol-linked form that is capable of signal transduction. FASEB J. 2000;14(11):1603–1610. doi: 10.1096/fj.14.11.1603. [DOI] [PubMed] [Google Scholar]
- 66.Noisakran S, Dechtawewat T, Avirutnan P, et al. Association of dengue virus NS1 protein with lipid rafts. J Gen Virol. 2008;89(Pt 10):2492–2500. doi: 10.1099/vir.0.83620-0. [DOI] [PubMed] [Google Scholar]
- 67.Avirutnan P, Zhang L, Punyadee N, et al. Secreted NS1 of dengue virus attaches to the surface of cells via interactions with heparan sulfate and chondroitin sulfate E. PLoS Pathog. 2007;3(11):e183. doi: 10.1371/journal.ppat.0030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin CF, Lei HY, Shiau AL, et al. Endothelial cell apoptosis induced by antibodies against dengue virus nonstructural protein 1 via production of nitric oxide. J Immunol. 2002;169(2):657–664. doi: 10.4049/jimmunol.169.2.657. [DOI] [PubMed] [Google Scholar]
- 69.Lin CF, Lei HY, Shiau AL, et al. Antibodies from dengue patient sera cross-react with endothelial cells and induce damage. J Med Virol. 2003;69(1):82–90. doi: 10.1002/jmv.10261. [DOI] [PubMed] [Google Scholar]
- 70.Lin YS, Lin CF, Lei HY, et al. Antibody-mediated endothelial cell damage via nitric oxide. Curr Pharm Des. 2004;10(2):213–221. doi: 10.2174/1381612043453469. [DOI] [PubMed] [Google Scholar]
- 71.Avirutnan P, Fuchs A, Hauhart RE, et al. Antagonism of the complement component C4 by flavivirus nonstructural protein NS1. J Exp Med. 2010;207(4):793–806. doi: 10.1084/jem.20092545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Avirutnan P, Hauhart RE, Somnuke P, Blom AM, Diamond MS, Atkinson JP. Binding of flavivirus nonstructural protein NS1 to C4b binding protein modulates complement activation. J Immunol. 2011;187(1):424–433. doi: 10.4049/jimmunol.1100750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin CF, Chiu SC, Hsiao YL, et al. Expression of cytokine, chemokine, and adhesion molecules during endothelial cell activation induced by antibodies against dengue virus nonstructural protein 1. J Immunol. 2005;174(1):395–403. doi: 10.4049/jimmunol.174.1.395. [DOI] [PubMed] [Google Scholar]
- 74.Teijaro JR, Walsh KB, Cahalan S, et al. Endothelial Cells Are Central Orchestrators of Cytokine Amplification during Influenza Virus Infection. Cell. 2011;146(6):980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pawitan JA. Dengue virus infection: predictors for severe dengue. Acta Med Indones. 2011;43(2):129–135. [PubMed] [Google Scholar]
- 76.Gavrilovskaya IN, Brown EJ, Ginsberg MH, Mackow ER. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by beta3 integrins. J Virol. 1999;73(5):3951–3959. doi: 10.1128/jvi.73.5.3951-3959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gavrilovskaya IN, Peresleni T, Geimonen E, Mackow ER. Pathogenic hantaviruses selectively inhibit beta3 integrin directed endothelial cell migration. Arch Virol. 2002;147(10):1913–1931. doi: 10.1007/s00705-002-0852-0. [DOI] [PubMed] [Google Scholar]
- 78.Matthys VS, Gorbunova EE, Gavrilovskaya IN, Mackow ER. Andes virus recognition of human and Syrian hamster beta3 integrins is determined by an L33P substitution in the PSI domain. J Virol. 2009;84(1):352–360. doi: 10.1128/JVI.01013-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *79.Raymond T, Gorbunova E, Gavrilovskaya IN, Mackow ER. Pathogenic hantaviruses bind plexin-semaphorin-integrin domains present at the apex of inactive, bent alphavbeta3 integrin conformers. Proc Natl Acad Sci U S A. 2005;102(4):1163–1168. doi: 10.1073/pnas.0406743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gavrilovskaya I, Gorbunova EE, Mackow ER. Pathogenic Hantaviruses Direct the Adherence of Quiescent Platelets to Infected Endothelial Cells. Journal of Virology. 2010 doi: 10.1128/JVI.02405-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gorbunova EE, Gavrilovskaya IN, Pepini T, Mackow ER. VEGFR2 and Src Kinase Inhibitors Suppress ANDV Induced Endothelial Cell Permeability. J Virol. 2011;85(5):2296–2303. doi: 10.1128/JVI.02319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim DD, Kleinman DM, Kanetaka T, et al. Rapamycin inhibits VEGF-induced microvascular hyperpermeability in vivo. Microcirculation. 2010;17(2):128–136. doi: 10.1111/j.1549-8719.2009.00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xue Q, Nagy JA, Manseau EJ, Phung TL, Dvorak HF, Benjamin LE. Rapamycin inhibition of the Akt/mTOR pathway blocks select stages of VEGF-A164-driven angiogenesis, in part by blocking S6Kinase. Arterioscler Thromb Vasc Biol. 2009;29(8):1172–1178. doi: 10.1161/ATVBAHA.109.185918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Land SC, Tee AR. Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J Biol Chem. 2007;282(28):20534–20543. doi: 10.1074/jbc.M611782200. [DOI] [PubMed] [Google Scholar]
- 85.El-Hashemite N, Walker V, Zhang H, Kwiatkowski DJ. Loss of Tsc1 or Tsc2 induces vascular endothelial growth factor production through mammalian target of rapamycin. Cancer Res. 2003;63(17):5173–5177. [PubMed] [Google Scholar]
- *86.Gavrilovskaya IN, Gorbunova EE, Mackow ER. Andes virus infection of lymphatic endothelial cells causes giant cell and enhanced permeability responses that are rapamycin and vascular endothelial growth factor C sensitive. J Virol. 2012;86(16):8765–8772. doi: 10.1128/JVI.00817-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gavrilovskaya I, Gorbunova E, Matthys V, Dalrymple N, Mackow E. The Role of the Endothelium in HPS Pathogenesis and Potential Therapeutic Approaches. Adv Virol. 2012;2012:467059. doi: 10.1155/2012/467059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Christou H, Yoshida A, Arthur V, Morita T, Kourembanas S. Increased vascular endothelial growth factor production in the lungs of rats with hypoxia-induced pulmonary hypertension. Am J Respir Cell Mol Biol. 1998;18(6):768–776. doi: 10.1165/ajrcmb.18.6.2980. [DOI] [PubMed] [Google Scholar]
- **89.Dehler M, Zessin E, Bartsch P, Mairbaurl H. Hypoxia causes permeability oedema in the constant-pressure perfused rat lung. Eur Respir J. 2006;27(3):600–606. doi: 10.1183/09031936.06.00061505. [DOI] [PubMed] [Google Scholar]
- 90.Hanaoka M, Droma Y, Naramoto A, Honda T, Kobayashi T, Kubo K. Vascular endothelial growth factor in patients with high-altitude pulmonary edema. J Appl Physiol (1985) 2003;94(5):1836–1840. doi: 10.1152/japplphysiol.00575.2002. [DOI] [PubMed] [Google Scholar]
- 91.Hopkins SR, Garg J, Bolar DS, Balouch J, Levin DL. Pulmonary blood flow heterogeneity during hypoxia and high-altitude pulmonary edema. Am J Respir Crit Care Med. 2005;171(1):83–87. doi: 10.1164/rccm.200406-707OC. [DOI] [PubMed] [Google Scholar]
- 92.Mukhopadhyay D, Tsiokas L, Zhou XM, Foster D, Brugge JS, Sukhatme VP. Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature. 1995;375(6532):577–581. doi: 10.1038/375577a0. [DOI] [PubMed] [Google Scholar]
- 93.Scherrer U, Rexhaj E, Jayet PY, Allemann Y, Sartori C. New insights in the pathogenesis of high-altitude pulmonary edema. Prog Cardiovasc Dis. 2010;52(6):485–492. doi: 10.1016/j.pcad.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 94.Peng X, Hassoun PM, Sammani S, et al. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169(11):1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 95.Sanchez T, Estrada-Hernandez T, Paik JH, et al. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J Biol Chem. 2003;278(47):47281–47290. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- 96.Schmid G, Guba M, Ischenko I, et al. The immunosuppressant FTY720 inhibits tumor angiogenesis via the sphingosine 1-phosphate receptor 1. J Cell Biochem. 2007;101(1):259–270. doi: 10.1002/jcb.21181. [DOI] [PubMed] [Google Scholar]
- **97.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8(11):1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 98.Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell. 2008;14(1):25–36. doi: 10.1016/j.devcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 99.Acevedo LM, Barillas S, Weis SM, Gothert JR, Cheresh DA. Semaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factor. Blood. 2008 doi: 10.1182/blood-2007-08-110205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jho D, Mehta D, Ahmmed G, et al. Angiopoietin-1 opposes VEGF-induced increase in endothelial permeability by inhibiting TRPC1-dependent Ca2 influx. Circ Res. 2005;96(12):1282–1290. doi: 10.1161/01.RES.0000171894.03801.03. [DOI] [PubMed] [Google Scholar]
- 101.Gavard J, Hou X, Qu Y, et al. A role for a CXCR2/phosphatidylinositol 3-kinase gamma signaling axis in acute and chronic vascular permeability. Mol Cell Biol. 2009;29(9):2469–2480. doi: 10.1128/MCB.01304-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115(1):84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 103.Porkka K, Khoury HJ, Paquette RL, Matloub Y, Sinha R, Cortes JE. Dasatinib 100 mg once daily minimizes the occurrence of pleural effusion in patients with chronic myeloid leukemia in chronic phase and efficacy is unaffected in patients who develop pleural effusion. Cancer. 2010;116(2):377–386. doi: 10.1002/cncr.24734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jain RK, Munn LL. Leaky vessels? Call Ang1! Nat Med. 2000;6(2):131–132. doi: 10.1038/72212. [DOI] [PubMed] [Google Scholar]
- 105.Kim I, Oh JL, Ryu YS, et al. Angiopoietin-1 negatively regulates expression and activity of tissue factor in endothelial cells. Faseb J. 2002;16(1):126–128. doi: 10.1096/fj.01-0556fje. [DOI] [PubMed] [Google Scholar]
- 106.Pizurki L, Zhou Z, Glynos K, Roussos C, Papapetropoulos A. Angiopoietin-1 inhibits endothelial permeability, neutrophil adherence and IL-8 production. Br J Pharmacol. 2003;139(2):329–336. doi: 10.1038/sj.bjp.0705259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Satchell SC, Anderson KL, Mathieson PW. Angiopoietin 1 and vascular endothelial growth factor modulate human glomerular endothelial cell barrier properties. J Am Soc Nephrol. 2004;15(3):566–574. doi: 10.1097/01.asn.0000115397.22519.03. [DOI] [PubMed] [Google Scholar]
- 108.Thurston G, Rudge JS, Ioffe E, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6(4):460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 109.Thurston G, Suri C, Smith K, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286(5449):2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 110.Igarashi J, Erwin PA, Dantas AP, Chen H, Michel T. VEGF induces S1P1 receptors in endothelial cells: Implications for cross-talk between sphingolipid and growth factor receptors. Proc Natl Acad Sci U S A. 2003;100(19):10664–10669. doi: 10.1073/pnas.1934494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McVerry BJ, Garcia JG. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell Signal. 2005;17(2):131–139. doi: 10.1016/j.cellsig.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 112.Takuwa Y, Takuwa N, Sugimoto N. The Edg family G protein-coupled receptors for lysophospholipids: their signaling properties and biological activities. J Biochem (Tokyo) 2002;131(6):767–771. doi: 10.1093/oxfordjournals.jbchem.a003163. [DOI] [PubMed] [Google Scholar]
- 113.Wang L, Dudek SM. Regulation of vascular permeability by sphingosine 1-phosphate. Microvasc Res. 2009;77(1):39–45. doi: 10.1016/j.mvr.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gorbunova E, Gavrilovskaya IN, Mackow ER. Pathogenic hantaviruses Andes virus and Hantaan virus induce adherens junction disassembly by directing vascular endothelial cadherin internalization in human endothelial cells. J Virol. 2010;84(14):7405–7411. doi: 10.1128/JVI.00576-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Borges E, Jan Y, Ruoslahti E. Platelet-derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta 3 integrin through its extracellular domain. J Biol Chem. 2000;275(51):39867–39873. doi: 10.1074/jbc.M007040200. [DOI] [PubMed] [Google Scholar]
- 116.Reynolds LE, Wyder L, Lively JC, et al. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8(1):27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- **117.Robinson SD, Reynolds LE, Wyder L, Hicklin DJ, Hodivala-Dilke KM. Beta3-integrin regulates vascular endothelial growth factor-A-dependent permeability. Arterioscler Thromb Vasc Biol. 2004;24(11):2108–2114. doi: 10.1161/01.ATV.0000143857.27408.de. [DOI] [PubMed] [Google Scholar]
- 118.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 119.Goldsmith CS, Elliott LH, Peters CJ, Zaki SR. Ultrastructural characteristics of Sin Nombre virus, causative agent of hantavirus pulmonary syndrome. Arch Virol. 1995;140(12):2107–2122. doi: 10.1007/BF01323234. [DOI] [PubMed] [Google Scholar]
- 120.Pham I, Uchida T, Planes C, et al. Hypoxia upregulates VEGF expression in alveolar epithelial cells in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2002;283(5):L1133–1142. doi: 10.1152/ajplung.00464.2001. [DOI] [PubMed] [Google Scholar]
- 121.Gavrilovskaya I, Gorbunova E, Koster F, Mackow E. Elevated VEGF Levels in Pulmonary Edema Fluid and PBMCs from Patients with Acute Hantavirus Pulmonary Syndrome. Adv Virol. 2012;2012:674360. doi: 10.1155/2012/674360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Koster F, Mackow E. Pathogenesis of the Hantavirus Pulmonary Syndrome. Future Virology. 2012;7(1):41–51. [Google Scholar]
- 123.Safronetz D, Prescott J, Feldmann F, et al. Pathophysiology of hantavirus pulmonary syndrome in rhesus macaques. Proc Natl Acad Sci U S A. 2014;111(19):7114–7119. doi: 10.1073/pnas.1401998111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dvorak HF, Sioussat TM, Brown LF, et al. Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels. J Exp Med. 1991;174(5):1275–1278. doi: 10.1084/jem.174.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bustamante EA, Levy H, Simpson SQ. Pleural fluid characteristics in hantavirus pulmonary syndrome. Chest. 1997;112(4):1133–1136. doi: 10.1378/chest.112.4.1133. [DOI] [PubMed] [Google Scholar]


