SUMMARY
Objective
Deficits in executive function are increasingly noted in children with epilepsy and have been associated with poor academic and psychosocial outcomes. Impaired inhibitory control contributes to executive dysfunction in children with epilepsy; however, its neuroanatomic basis has not yet been investigated. We used functional Magnetic Resonance Imaging (fMRI) to probe the integrity of activation in brain regions underlying inhibitory control in children with epilepsy.
Methods
This cross-sectional study consisted of 34 children aged 8 to 17 years: 17 with well-controlled epilepsy and 17 age-and sex-matched controls. Participants performed the antisaccade (AS) task, representative of inhibitory control, during fMRI scanning. We compared AS performance during neutral and reward task conditions and evaluated task-related blood-oxygen level dependent (BOLD) activation.
Results
Children with epilepsy demonstrated impaired AS performance compared to controls during both neutral (non-reward) and reward trials, but exhibited significant task improvement during reward trials. Post-hoc analysis revealed that younger patients made more errors than older patients and all controls. fMRI results showed preserved activation in task-relevant regions in patients and controls, with the exception of increased activation in the left posterior cingulate gyrus in patients specifically with generalized epilepsy across neutral and reward trials.
Significance
Despite impaired inhibitory control, children with epilepsy accessed typical neural pathways as did their peers without epilepsy. Children with epilepsy showed improved behavioral performance in response to the reward condition, suggesting potential benefits of the use of incentives in cognitive remediation.
Keywords: Executive Function, Antisaccade, Pediatric
INTRODUCTION
Deficits in cognitive processes necessary for organization, inhibition, sustained attention, and planning and executing goal-directed tasks, often referred to as executive functions, are a known cognitive comorbidity in children with epilepsy.1–3 Executive function impairments can antedate seizures,4 persist during disease remission,5 predict lower quality of life,6 and relate to compromised cognitive development.2 These discrete cognitive skill impairments interfere with performance of complex tasks such as planning,7 and consequently, may pose a greater threat to a child’s overall function than seizures themselves.
In light of growing awareness of executive dysfunction in children with epilepsy, we aimed to provide a new approach to investigating its underlying neuroanatomic causes and conceptual models of cognitive remediation. Our methodology adds to prior investigations relating cortical structure to impaired behavioral task performance8 by studying the relationship between impaired executive function and associated cortical activity. The antisaccade (AS) task is representative of inhibitory control, a core component of executive function responsible for filtering out distractors while developing and executing a task-relevant plan of action.9 Accordingly, inhibitory control is crucial for accomplishing goal-directed behavior.10 It matures during mid- to late-adolescence11 and is thus potentially vulnerable to developmental disruptions at or before this time.
Successful completion of the AS task requires the recruitment of well-characterized cognitive and oculomotor control networks including the frontal, supplementary, and parietal eye fields, prefrontal cortex, striatum, thalamus, and cerebellum.12–14 AS task performance reliably predicts performance on traditional neuropsychological measures requiring intact inhibitory control in healthy children and adults.7 Additionally, impaired AS task performance has been described in children with attention deficit/hyperactivity disorder (ADHD)),15 in which executive dysfunction is commonly characterized as a core deficit.16 Children with epilepsy demonstrate impairments in sensorimotor and inhibitory functions necessary for the AS task.17 Our goal was to build upon these findings by investigating the activity of regions utilized during the AS task. To optimize AS performance, we chose a task modulated by reward18 to inform remediation of inhibitory control deficits.
Experimental Aims
Our first experimental objective was to probe the integrity of the functional network associated with inhibitory control in epilepsy patients compared to controls. We predicted that patients would make more AS task errors irrespective of reward contingency. In parallel with behavioral results supporting impaired inhibitory control,17 we expected patients to show decreased task-related functional activation in known cognitive and oculomotor control regions commonly utilized in AS tasks.12–14 Our second objective was to investigate the effect of reward-modulation on AS task performance in patients versus controls. Finally, given evidence of developmental vulnerabilities related to epilepsy occurring at younger ages,19 we performed an exploratory examination of age-related effects on behavioral and functional results.
METHODS
Participants
Participants were recruited and tested at the University of Pittsburgh Medical Center from January 2007 to March 2009. They ranged in age from eight to 17 years and met criteria for oculomotor task performance (e.g. no history of color blindness, amblyopia, strabismus, or other eye movement disorders) and MRI scanning (no contraindications such as metal implants or claustrophobia). In order to assure adequate task performance, participants were native English speakers with full-scale IQ’s ≥ 80, as determined by the Wechsler Abbreviated Scale of Intelligence (WASI).20 Participants provided written assent, and their parents/legal guardians provided informed consent. The study protocol was approved by the University of Pittsburgh Institutional Review Board and complied with the Declaration of Helsinki. Participants were paid for the behavioral ($50) and fMRI ($75) sessions.
We recruited seventeen children with a diagnosis of epilepsy according to ILAE classification21 from the Children’s Hospital of Pittsburgh Neurology Clinic. Patients had no history of MRI abnormalities on conventional 1.5 T clinical protocol and no neurological comorbidities such as Tourette’s syndrome or autism. Patients had several different epilepsy subtypes; classified as either generalized (n = 7) or focal (n = 10) (Table 1). Because of the high prevalence of psychiatric comorbidities in children with epilepsy, patients underwent screening using previously reported methods,17 with a summary of detected conditions in Table 1. We included six patients with comorbid ADHD for several reasons. First, ADHD occurs in greater than 30% of children with epilepsy,22, 23 therefore, a sample excluding patients with ADHD may not have adequately represented children with epilepsy with normal intelligence. In addition, inhibitory control deficits are characteristic in children with ADHD, although these impairments do differ among children with ADHD both with and without comorbid epilepsy.16, 24 Lastly, our previous work using an AS task revealed no additional executive function deficits among patients with epilepsy and ADHD beyond patients with epilepsy only.17 One patient with ADHD was on stimulant medication at the time of scanning. Patients continued their epilepsy medications during screening and data collection. Participants with epilepsy were not scanned if they experienced seizures within 24 hours of the session and were monitored using the scanner camera for clinical signs of seizure activity.
Table 1.
Clinical characteristics of patients.
| Total | 8–12 years | 13–17 years | |
|---|---|---|---|
| Age of seizure onset in years (Mean(SD)) | 9.9 (3.5) | 7.3 (3.0) | 12.3 (1.7) |
| Epilepsy duration in years (Mean(SD)) | 2.3 (2.2) | 2.4 (2.8) | 2.2 (1.5) |
| Lifetime seizure total (n = 17) | |||
| < 5 | 7 | 2 | 5 |
| 5 – 10 | 5 | 3 | 2 |
| > 10 | 5 | 3 | 2 |
| Seizure type (n = 17) | |||
| Focal | 6 | 1 | 5 |
| BECTS | 4 | 4 | 0 |
| Generalized Tonic Clonic | 4 | 1 | 3 |
| Absence | 3 | 2 | 1 |
| AED (n = 17) | |||
| LTG | 7 | 3 | 4 |
| CBZ | 3 | 3 | 0 |
| OXCBZ | 2 | 2 | 0 |
| VPA | 2 | 0 | 2 |
| LEV | 2 | 0 | 2 |
| TPM | 1 | 0 | 1 |
| Psychiatric comorbidities (n = 8) | |||
| ADHD* | 6 | 4 | 2 |
| Depression | 2 | 0 | 2 |
BECTS = Benign epilepsy with centrotemporal spikes. AED = anti-epileptic drug. LTG = lamotrigine, CBZ = carbamazepine, OXCBZ = oxcarbazepine, VPA = valproic acid, LEV = levetiracetam, TPM = topiramate.
All patients with ADHD had the inattentive subtype.
Seventeen control participants were recruited from the community and matched to patients by age and sex after meeting the following inclusion criteria: (1) no personal or first-degree family history of neurological or psychiatric conditions, (2) no prior episodes of loss of consciousness greater than five minutes, (3) no psychotropic medication use, and (4) a history of school attendance and grades consistent with age.
Several controls and patients were excluded due to inclusion criteria violations or failed scanning. Selection is detailed in Figure S1 (Supporting Information). Characteristics of patients and controls included in the final analysis are summarized in Tables 1 and 2.
Table 2.
Demographic characteristics of controls and patients.
| Controls(n=17) | Patients (n=17) | |
|---|---|---|
| Age in years (Mean(SD)) | 12.7 (2.8) | 12.7 (3.0) |
| 8–12 years (n) | 7 | 8 |
| 13–17 years (n) | 10 | 9 |
| Sex (Male/Female(n)) | 9/8 | 9/8 |
| Maternal Income (Mean) | $25,001 – $50,000 | $25,001 – $50,000 |
| Paternal Income (Mean) | $50,001 – $75,000 | $50,001 – $75,000 |
| IQ ((Mean(SD))* | 110.9 (11.4) | 100.1 (10.1) |
| IQ (Range) | 95 – 131 | 81 – 121 |
| Edinburgh handedness (Right/Left(n)) | 18/0 | 18/0 |
= significant difference, t(1,32) = −2.9, p = 0.006.
Average parental incomes (determined by the Hollingshead SES scale25) did not differ between controls and patients. Patients had normal IQs ranging from 81 – 121 that were lower than controls (Table 2). The IQ scores in our patient cohort were higher than those in studies including patients with a range of intellectual abilities;26 Reilly et al reported that 55% of children with epilepsy had IQ scores lower than 85 in a population-based study.23 Yet the scores in our patient cohort were comparable to studies of “intellectually normal” children with epilepsy.1, 3, 5 There were no significant differences in seizure type, years of epilepsy duration, or distribution of seizure frequency between younger (aged 8–12 years) and older (aged 13–17 years) patients (Table 1).
Antisaccade Task
During the oculomotor task, participants were instructed to respond with an antisaccade (an eye movement to the mirror location) away from a suddenly appearing, peripheral visual stimulus in an unpredictable location.18 Each trial was composed of three 1.5 second phases beginning with a visual cue: either a ring of green dollar signs ($) indicating potential rewards for correct trial performance (reward condition), or a ring of blue pound signs (#) of equivalent size and luminance indicating that no reward was at stake (neutral condition). This cue was followed by the presentation of a red, central fixation cross. Lastly, during the response phase, the peripheral visual stimulus, a yellow sphere, appeared (Figure S2, Supporting Information). A white, central fixation cross delineated jittered intertrial intervals in equal proportions of 1.5, 3, or 4.5 seconds. Stimulus presentation was coded in E-Prime (Psychology Software Tools, Inc. Pittsburgh, PA). Stimuli were projected onto a flat screen behind the scanner bore, which participants viewed via a mirror on the head coil.
Trials were coded as “correct” when participants looked away from the peripheral stimulus and “error” when participants looked at the peripheral stimulus, even if they subsequently averted their gaze. To infer successful task performance within the scanner, patients and controls completed the AS task outside of the scanner within six months prior to the fMRI experiment. All participants performed the task appropriately outside the scanner. At the fMRI session, participants were told that there was potential to win “extra money” for correct responses during reward trials but that no money would be lost for incorrect responses. Performance on neutral trials would result in no monetary gains or losses. Participants were given five practice trials before the fMRI protocol began. The protocol consisted of four runs of 5 minutes 9 seconds each, with 14 reward trials and 14 neutral trials presented in random order during each run for a total of 56 reward trials and 56 neutral trials. Following task completion, all participants received the same amount of monetary compensation independent of performance as reviewed in the consent process.
Eye Tracking
Eye movement latencies and directional responses were recorded in the MRI scanner using a long-range optics eye tracking system (Model 504LRO, Applied Science Laboratories, Bedford, MA). The direction, latency, and accuracy of eye movements were calculated off-line using ILAB software27 and an in-house MATLAB scoring suite (Mathworks, Inc.).
fMRI Acquisition and Preprocessing
MRI scanning was conducted using a 3-Tesla Siemens Allegra scanner with a standard head coil. To reduce head movement, participants undertook a mock scan with auditory motion feedback before the real scan, and pillows were used for head stabilization. Functional data were acquired using a whole-brain gradient-echo echo-planar imaging sequence sensitive to BOLD contrast (repetition time (TR) 1.5 s, echo time 25 ms, flip angle 70 degrees, matrix 64 × 64, field of view 20 × 20 cm, voxel size 3.125 × 3.125 mm). Twenty-nine 4-mm adjacent axial slices were collected, providing near whole-brain coverage to the mid-cerebellum. Structural images were acquired in the sagittal plane using a 3D magnetization prepared rapid acquisition gradient-echo (MP-RAGE) pulse sequence with 192 slices of 1 mm thickness. Structural and functional images were preprocessed using Analysis of Functional NeuroImages (AFNI)28 and FMRIB Software Library (FSL).29 A detailed description can be found in the Supporting Information.
fMRI Analyses
Group-wise analyses were conducted using AFNI. The model for individual participant deconvolution consisted of orthogonal regressors of interest for correctly performed reward and neutral AS trials. Nuisance regressors included six motion parameters and linear and non-linear trend terms. We modeled the hemodynamic response (HRF) using an incomplete gamma function approximating the BOLD response to a stimulus.
We first investigated whole-brain activation in controls versus all patients and in comparisons between patients with generalized versus focal epilepsies followed by voxel-wise, paired t-tests across the whole brain to identify clusters showing between-group activation differences (voxel threshold: p ≤ 0.001, corrected cluster threshold: p ≤ 0.05). A single voxel threshold required for a cluster level alpha value corrected for multiple comparisons was derived using a series of Monte Carlo simulation clustering procedures in AFNI 3dClustSim.
Region of Interest selection
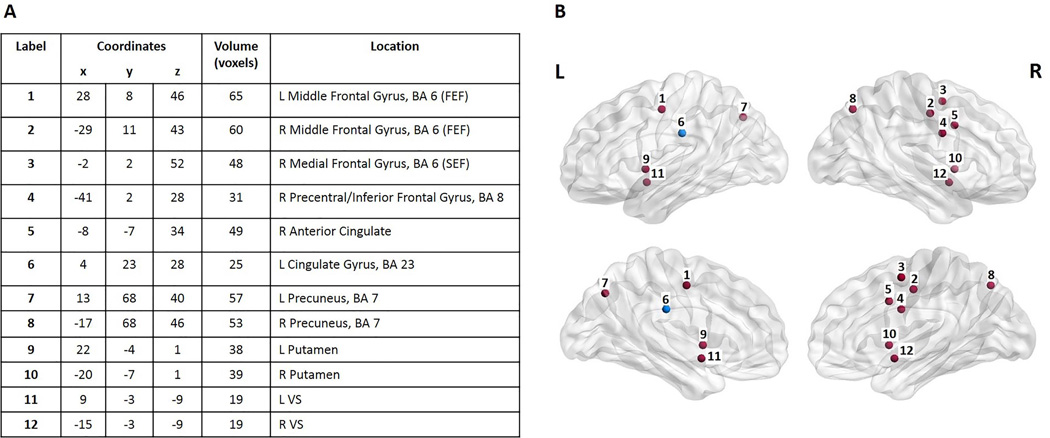
To analyze task-relevant areas, we defined regions of interest (ROIs) by task-related activation in an independent sub-cohort of controls aged 8–17 (not included in the present study) during a similar rewarded AS task.30 We selected 10 ROIs that demonstrated significant change from baseline across the experimental age range and conditions to delineate the basic circuitry activated in our study.31 These ROIs included regions implicated in oculomotor control, such as the putative frontal and supplementary eye fields (FEF and SEF), and reward processing regions, such as the anterior cingulate cortex (ACC) and putamen (Figure 1).30 Given their known engagement in reward processing, the bilateral ventral striatum (VS)18, 32 were added and defined anatomically according to the Talairach and Tourneaux atlas33 (for 12 total a priori ROIs). The mean magnitude estimate was extracted from each ROI for each participant.
Figure 1.
A) Region of interest (ROI) coordinates in Talaraich space. B) Spheres delineate the 12 regions of interest (ROIs) from 1A drawn using BrainNet Viewer. All ROIs are marked in red except for the Left Cingulate Gyrus (in blue), a region showing significant group differences. Spheres do not indicate the actual size or shape of the ROI. R = Right side. L = Left side.
Statistical Analyses
Task performance
Percent correct responses and response latencies (time to response) were entered into Repeated Measures (RM) ANOVA, testing the effects of incentive condition (within-subjects factor), and age group (described below) and clinical diagnosis (between-subjects factors). Interactions were examined for the following: condition and diagnosis, condition and age group, diagnosis and age group, and condition, diagnosis, and age group (three-way). The Benjamini-Hochberg (B-H) procedure was used to correct for multiple comparisons.34 To investigate possible developmental differences during active childhood disease, we performed specific analyses considering age as a dichotomized variable (younger: 8–12 years; older: 13–17 years). The age split was determined by prior developmental studies using similar partitions indicating significant shifts in cognitive skills underlying executive function11 and structural brain maturation.35 Lastly, we repeated the above RM ANOVA with three diagnostic groups (controls, focal epilepsy patients, and generalized epilepsy patients) to explore possible differences related to epilepsy subtypes.
fMRI activity
RM ANOVA for each ROI assessed the main effect of incentive condition as a within-subjects factor, and clinical diagnosis and age group as between-subjects factors. Interactions were again examined for the following: condition and diagnosis, condition and age group, diagnosis and age group, and condition, diagnosis, and age group (three-way) with B-H corrections. We repeated the above RM ANOVA using three diagnostic groups (controls, focal, and generalized).
RESULTS
Behavioral Results
Antisaccade accuracy
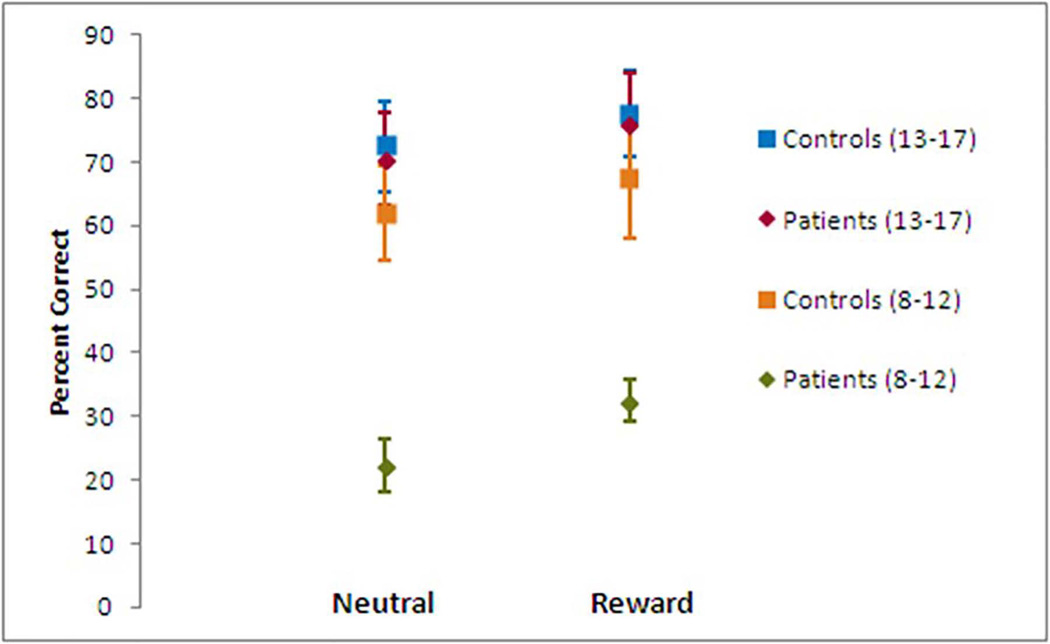
RM ANOVA analyzing percent correct AS responses showed a significant effect of clinical diagnosis surviving the B-H threshold, with controls achieving more correct responses than children with epilepsy (F (1,30) = 8.54, p = 0.007). We also found an effect of incentive (F (1,30) = 10.42 p = 0.003), with more correct responses in the reward condition across the two groups (Figure 2). There was no interaction between clinical diagnosis and incentive.
Figure 2.
Response accuracy shows effects of clinical diagnosis, incentive, and clinical diagnosis and age group (ages 8–12 years versus ages 13–17 years). Controls performed better than patients in both of the two incentive conditions. Both patients and controls performed better in the reward condition than the neutral condition. Task performance in younger patients lags significantly behind older patients and all controls. Error bars = SEM.
Younger participants (ages 8–12) made more errors than older participants (ages 13–17) (F (1,30) = 17.37, p < 0.001). There was also an interaction between clinical diagnosis and age group (F (1,30) = 7.09, p = 0.012). Post-hoc tests revealed that the difference in correct responses between controls and patients was primarily driven by younger patients (t(1,15) =-5.59, p < 0.001 and t(1,10.6) = −5.08, p < 0.001, for the neutral and reward conditions, respectively) (Figure 2). Younger and older controls did not show differences in either the neutral or reward condition. There were no interactions between incentive and age group or incentive, clinical diagnosis, and age group (three-way).
RM ANOVA analyzing percent correct AS responses between controls, generalized and focal epilepsies replicated the above results, additionally illustrating a significant effect of clinical diagnosis (F(2,28) = 5.15, p = 0.012). Post-hoc tests revealed a significant difference between patients with focal epilepsy and controls (patients with focal epilepsy made more errors across conditions, p = 0.009), but no differences between patients with generalized epilepsy and controls or patients with generalized and focal epilepsies (Figure S3, Supporting Information).
Offline analyses of eye movement data demonstrated that incorrect AS responses (erroneous prosaccades made toward the stimulus) were followed by corrective antisaccades to the proper location by patients and controls. There was no difference in the number of corrective antisaccades made by patients and controls during the experiment. Because participants made hasty errors that they subsequently corrected, it is clear that they understood the task instructions, and therefore responded incorrectly due to failed inhibitory control during these trials.18, 31
Antisaccade Latency
Both controls and patients in the neutral and reward conditions took longer to initiate correct responses (antisaccade latency) than incorrect responses (prosaccade latency) (F(1,30) = 36.89, p < 0.001; F(1,30) = 36.35, p < 0.001, respectively) (Table S1, Supporting Information). There were no differences between controls and patients for anti- and prosaccade latencies between either incentive conditions. Age group had an independent effect on anti- and prosaccade latencies in the reward condition only, with longer latencies in younger than older participants (F(1,30) = 6.43, p = 0.017). There were no interactions between clinical diagnosis and age group, incentive and age group, or a three-way interaction between incentive, clinical diagnosis, and age group. RM ANOVA analyzing latencies between controls, generalized and focal epilepsies replicated the above results, with no additional significant effects.
fMRI Results
Whole-brain analysis
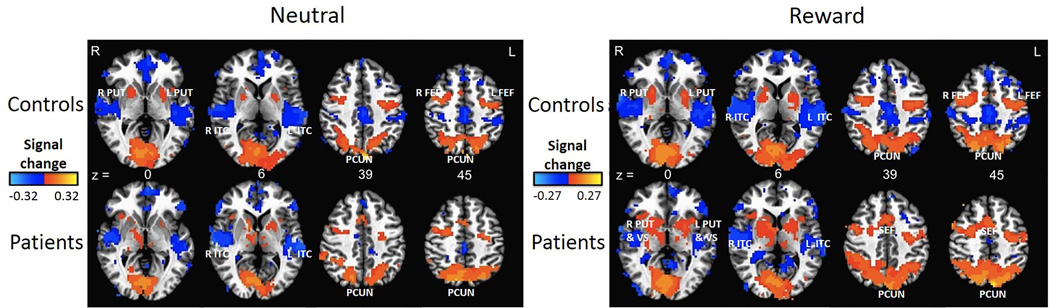
During correct AS task performance, the expected, relevant regions (including the bilateral frontal and supplementary eye fields, putamen, and precuneus) showed activation in controls and patients in the reward and neutral conditions (Figure 3). However, no differences in activation between controls and patients or in comparisons between patients with generalized and focal epilepsies survived our clustering thresholds.
Figure 3.
Whole brain activation maps (created using threshold of p = 0.01, t = 2.92). Red, orange, and yellow represent levels of increasing activation respectively, while dark blue and light blue represent levels of increasing deactivation, respectively. Patients and controls show similar task activation in the same regions. z = Talaraich coordinate. R = Right side. L = Left side. PUT = Putamen. ITC = Inferior Temporal Cortex. VS = Ventral Striatum. PCUN = Precuneus. FEF = Frontal Eye Field. SEF = Supplementary Eye Field.
ROI-based analyses and age group related effects
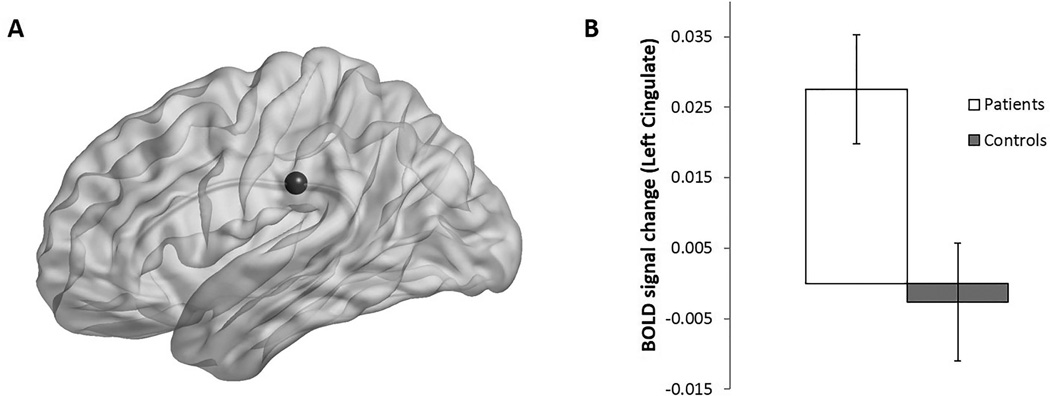
RM ANOVA with incentive as a within-subjects factor and clinical diagnosis and age group as between-subjects factors revealed differences in the left posterior cingulate gyrus (BA 23) between patients and controls during correct AS trials (F(1,30) = 5.26, p = 0.03). Here, patients displayed increased BOLD activation relative to controls (Figure 4). No ROIs showed an interaction between incentive and clinical diagnosis.
Figure 4.
A) A representative sphere marks the Left Cingulate Gyrus (ROI 6), drawn using BrainNet Viewer. B) Mean activation of the Left Cingulate Gyrus in patients and controls across both incentive conditions. Significant increase shown by patients. Error bars = SEM.
None of the ROIs showed an effect of age group. However, an interaction between incentive condition and age group was observed in the right medial frontal gyrus (F(1,30) = 4.36, p = 0.045). Younger participants showed increased activation during the reward condition compared to the neutral condition, while older participants showed equivalent activation during both incentive conditions. There were no interactions between clinical diagnosis and age group or incentive condition, clinical diagnosis, and age group (three-way).
RM ANOVA analyzing activation differences between controls, generalized and focal epilepsies replicated the above results, additionally illustrating a significant effect of clinical diagnosis in the left posterior cingulate gyrus (BA 23) (F(2,28) = 7.62, p = 0.002). Post-hoc tests revealed significantly increased activation in patients with generalized epilepsy as compared to both controls and patients with focal epilepsy (p = 0.004, p = 0.04, respectively) (Figure S4, Supporting Information).
DISCUSSION
This study investigated neural activation in children with epilepsy compared to controls during an antisaccade task evaluating inhibitory control and responsiveness to reward. Children with epilepsy exhibited impaired inhibitory control with significant improvements during the reward condition. Neuroimaging findings indicated intact neural circuitry during correct trials with the exception of increased task activation by generalized epilepsy patients in the left posterior cingulate gyrus. An exploratory investigation of age-related effects revealed that poor AS task performance in patients with epilepsy was almost exclusively driven by younger patients. However, we did not find corresponding differences in neural activation to support the same interaction between age and clinical diagnosis observed in task performance.
This study builds on a growing body of literature investigating the effects of neurological dysfunction on cognitive development in childhood epilepsy. Our results demonstrate impairments in inhibitory control among children with epilepsy that are consistent with previous findings, including age-related effects.17 These results inform future investigations to further delineate a functional and structural basis of cognitive impairment in epilepsy.
Inhibitory control
Disrupted executive function has been extensively described in pediatric patients with a variety of disease subtypes, including juvenile myoclonic epilepsy,36 rolandic epilepsy,37 absence seizures,38 and idiopathic generalized epilepsy with generalized tonic-clonic seizures.39 In patients with normal intelligence, recent research supports that executive function deficits do not differ by epilepsy subtype.2, 3, 17, 38 Therefore, a shared mechanism underlying executive function deficits in epilepsy patients with normal intelligence is sought in current research.
Our behavioral results indicate impairment in the proportion of correct trials in 8–12 year old children with epilepsy; yet there were no group differences by the ages of 13–17 years, suggesting a mechanism of delayed maturation that is compensated in adolescence. Surprisingly, there were few differences between patients and controls in activation underlying correct inhibitory trials. A recent study correlating morphometric and cognitive differences suggests that children with epilepsy rely on alternative (perhaps compensatory) regions for optimal performance.40 In contrast, patients in the current study recruited the same regions as controls indicating preserved access to typical neural pathways. The neural basis for observed behavioral differences may lie in the incorrect trials; or alternatively, the differences may not relate to specific activation patterns. Future investigations should include error-related activation during the AS task, and importantly, explore differences in state-related processes which may account for limitations in patients’ abilities to consistently maintain correct performance.41
Reward modulation: a potential model for cognitive remediation
In studies of typical development and in animal models of early life seizures, improved cognitive task performance corresponds with compensatory increases in task-relevant cortical activation.30, 42 In the present study, we expected children with epilepsy to activate compensatory regions associated with executive function in response to observed reward-mediated task improvements. Increased activation by patients was only evident in the left posterior cingulate gyrus, a reward-sensitive region not known to be recruited during inhibitory control.43 It is unlikely that the activation of the cingulate directly contributes to task performance, but rather, it may indirectly improve AS execution as a result of enhanced reward processing. This increased activation persisted across rewarded and unrewarded trials, suggesting that the general prospect of a reward provided significant motivation for patients. Recent developmental research in non-epilepsy adolescents suggests a role for the use of rewards to encourage them to adult levels of performance.44 Similarly, the results from the current study could inform cognitive and behavioral remediation using incentives (i.e. behavioral contingency management) in school or home settings.16
Epilepsy subtypes
Our exploratory subtype analyses implicate the posterior cingulate as a region relevant to generalized epilepsy, as generalized epilepsy patients displayed increased activation during correct task performance (compared to both controls and focal epilepsy patients). These results correspond to earlier studies showing fMRI deactivation related to generalized spike and wave discharges (GSWDs) and corresponding decreases in functional connectivity in patients with long-standing generalized epilepsy.45, 46 In the present study, the observed increased activation was likely due to mechanisms other than GSWDs. Future studies with adequate group sizes are needed to fully address the impact of epilepsy subtype on fMRI activation.
Age-related effects
The neural circuitry supporting inhibitory control develops during childhood, but is further strengthened and refined throughout early adolescence. The emergence of neurologic disease in late childhood and early adolescence can disrupt the integration of existing abilities into systems with more efficient and consistent performance.47 Our preliminary analysis of age-related effects showed that younger children with epilepsy performed significantly worse than both younger and older controls and older children with epilepsy. Disease duration was only several years for most patients, but the ages at seizure onset were varied, further supporting the concept that younger age of epilepsy onset confers a more significant impact on developing cognitive skills.19, 26 It is possible that the younger children in our study exhibited deficits resulting from epileptogenesis prior to or during a critical developmental period in which inhibitory control is optimized.
Study Strengths and Limitations
Several studies have correlated neuropsychological task scores with morphometric measurements to investigate the relationship between brain structure and cognitive function in children with epilepsy.8, 40 Our study contributes to the literature by using fMRI. fMRI studies of children with epilepsy often focus on pre-surgical mapping. These studies are limited in probing the neuroanatomic substrates of executive dysfunction, which is pervasive in both surgical and nonsurgical populations. We were therefore motivated to extend the potential of fMRI to search for neuroimaging biomarkers and characterize the progression of cognitive comorbidity.
It is often difficult to obtain reliable fMRI data from children, as they are less likely than adults to stay still while scanning and may therefore generate motion artifacts. A strength of our methodology was the addition of a simulation scanning session prior to data collection, ensuring that our sample would provide reliable results.
Recent investigations of the structure-executive function relationship in children with epilepsy used either aggregated task scores or parental ratings as measures, which may introduce sources of bias in reporting. Our study employed the AS task, on which children with epilepsy have demonstrated impairments.17 Furthermore, the AS task offers a well-defined neuroanatomic network sensitive to developmental stages and neuropsychiatric conditions.14, 17, 18 The interrogation of the network for a quantifiable and discrete function such as the AS task provides a starting point for investigating more complex functions underlying cognitive comorbidity and the effect of ADHD in epilepsy.24
Our sample was limited by its modest size and heterogeneity of epilepsy subtypes. It will be important for future studies to improve upon this limitation by investigating larger numbers of patients with specific subtypes. Despite these limitations, we enhanced the patient group by recruiting only children with well-controlled epilepsy with normal clinical neuroimaging, normal intelligence, and short disease durations. In light of these patient characteristics, our findings may not apply to more refractory pediatric patient populations.
There were several patient factors that we were unable to control for with our study design. For example, patients were treated with monotherapy, and yet, several different medications were used. The effects of specific anti-epileptic drugs (AEDs) on task performance and activation were not accounted for due to lack of statistical power. During scanning, we observed patients for clinical signs of seizure activity, which could potentially impact the hemodynamic response function.
The age range of patients in our study offers a unique perspective regarding epilepsy’s effects across development. Unfortunately, we did not have sufficient participants to provide insight into specific developmental periods. It is possible that cortical regions which would have shown compensatory activation differed between the younger and older children in the current study, as Padmanabhan et al showed.30 This result was likely not elucidated due to lack of statistical power for fMRI analysis by separate age groups. Further work would therefore investigate the age-related behavioral differences we found with greater numbers of children with epilepsy in discrete age groups. Additionally, as the present study was cross-sectional, future longitudinal studies would better inform our knowledge of cognitive comorbidity during development, epilepsy progression and remission.
Conclusion
Our study contributes the first investigation of functional activation underlying impaired executive function in children with epilepsy. During correct AS trials, task-related activation was relatively consistent between patients and controls, suggesting preserved integrity of the networks supporting inhibitory control. Our results indicate subtle differences between epilepsy subtypes and an increased vulnerability in younger children with epilepsy. The underlying mechanisms are likely multi-factorial and warrant further study. The implications of the preserved integrity of neural networks, even despite identified disease, underscore the complex nature of cognitive comorbidity and suggest a period of plasticity for either the augmentation or stagnation of neural functions, which may be more vulnerable at younger ages.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the NIH (NINDS K23 NS052234), which provided funding for study design, data collection, and data analysis. The funder was not involved in manuscript preparation or publication decisions. RLT wrote the first draft of this manuscript. We give special thanks to Meg Meachim and Alina Vaisleib, who tested participants and assisted in data scoring and Kevin Ellsworth, David Montez, and Will Foran who assisted with data analysis. No authors received any honorarium, grant, or other form of payment for producing this manuscript.
RLT receives research support from the NIMH (2R25 MH054318 [research fellow]) and has received research support from the Child Neurology Foundation Swaiman Medical Scholarship Award (2011).
BL receives research support from the NIH (NIMH RO1 MH067924 [PI], MH080243 [PI], R21 HD074850 [PI], 1 U01 AA021690 [co-investigator]) and the Pennsylvania Department of Health (PA-HEAL [PI]).
WDG receives (grant) support from NIH (NINDS, NIMH, NICHD), NSF, PCORI, American Epilepsy Society, Epilepsy Foundation, CURE, and Infantile Epilepsy Research Foundation (funded by Lundbeck). He sits on the editorial board of Epilepsia and Epilepsy Research, and holds stock with his spouse from Johnson and Johnson, GlaxoSmithKline, Eli Lilly, Pfizer, Siemens, General Electric. His salary is ultimately based on medical fees related to patient care of patients with epilepsy.
MRA has received research support from the NIH (NINDS K23 NS052234 [PI]) and (R01 MH080243 [co-investigator]).
Footnotes
DISCLOSURE OF CONFLICTS OF INTEREST
KV and AP report no disclosures.
REFERENCES
- 1.Fastenau PS, Johnson CS, Perkins SM, et al. Neuropsychological status at seizure onset in children: risk factors for early cognitive deficits. Neurology. 2009;73:526–534. doi: 10.1212/WNL.0b013e3181b23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hermann BP, Jones JE, Sheth R, et al. Growing up with epilepsy: a two-year investigation of cognitive development in children with new onset epilepsy. Epilepsia. 2008;49:1847–1858. doi: 10.1111/j.1528-1167.2008.01735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oostrom KJ, van Teeseling H, Smeets-Schouten A, et al. Three to four years after diagnosis: cognition and behaviour in children with 'epilepsy only'. A prospective, controlled study. Brain. 2005;128:1546–1555. doi: 10.1093/brain/awh494. [DOI] [PubMed] [Google Scholar]
- 4.Hesdorffer DC, Ludvigsson P, Olafsson E, et al. ADHD as a risk factor for incident unprovoked seizures and epilepsy in children. Arch Gen Psychiatry. 2004;61:731–736. doi: 10.1001/archpsyc.61.7.731. [DOI] [PubMed] [Google Scholar]
- 5.Berg AT, Langfitt JT, Testa FM, et al. Residual cognitive effects of uncomplicated idiopathic and cryptogenic epilepsy. Epilepsy Behav. 2008;13:614–619. doi: 10.1016/j.yebeh.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Sherman EM, Slick DJ, Eyrl KL. Executive dysfunction is a significant predictor of poor quality of life in children with epilepsy. Epilepsia. 2006;47:1936–1942. doi: 10.1111/j.1528-1167.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- 7.Asato MR, Sweeney JA, Luna B. Cognitive processes in the development of TOL performance. Neuropsychologia. 2006;44:2259–2269. doi: 10.1016/j.neuropsychologia.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Hermann BP, Jones J, Sheth R, et al. Cognitive and magnetic resonance volumetric abnormalities in new-onset pediatric epilepsy. Semin Pediatr Neurol. 2007;14:173–180. doi: 10.1016/j.spen.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjorklund DF, Harnischfeger K. The evolution of inhibition mechanisms and their role in human cognition and behavior. In: Dempster FN, Brainerd CJ, editors. Interference & inhibition in cognition. San Diego: Academic Press; 1995. pp. 141–173. [Google Scholar]
- 10.Fuster JM. The Prefrontal Cortex. 3rd ed. New York: Raven; 1997. [Google Scholar]
- 11.Luna B, Garver KE, Urban TA, et al. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- 12.Brown MRG, Goltz HC, Vilis T, et al. Inhibition and generation of saccades: Rapid event-related fMRI of prosaccades, antisaccades, and nogo trials. NeuroImage. 2006;33:644–659. doi: 10.1016/j.neuroimage.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Luna B, Sweeney JA. Cognitive functional magnetic resonance imaging at very-high-field: eye movement control. Top Magn Reson Imaging. 1999;10:3–15. doi: 10.1097/00002142-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda T, Matsuura M, Ohkubo T, et al. Functional MRI mapping of brain activation during visually guided saccades and antisaccades: cortical and subcortical networks. Psychiatry Res. 2004;131:147–155. doi: 10.1016/j.pscychresns.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Mahone EM, Mostofsky SH, Lasker AG, et al. Oculomotor anomalies in attention-deficit/hyperactivity disorder: evidence for deficits in response preparation and inhibition. J Am Acad Child Adolesc Psychiatry. 2009;48:749–756. doi: 10.1097/CHI.0b013e3181a565f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macallister WS, Vasserman M, Rosenthal J, et al. Attention and Executive Functions in Children With Epilepsy: What, Why, and What to Do. Appl Neuropsychol Child. 2014 doi: 10.1080/21622965.2013.839605. [DOI] [PubMed] [Google Scholar]
- 17.Asato MR, Nawarawong N, Hermann B, et al. Deficits in oculomotor performance in pediatric epilepsy. Epilepsia. 2011;52:377–385. doi: 10.1111/j.1528-1167.2010.02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geier CF, Terwilliger R, Teslovich T, et al. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berg AT, Zelko FA, Levy SR, et al. Age at onset of epilepsy, pharmacoresistance, and cognitive outcomes: a prospective cohort study. Neurology. 2012;79:1384–1391. doi: 10.1212/WNL.0b013e31826c1b55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D W. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, Tex: Psychological Corp; 1999. [Google Scholar]
- 21.Engel J. A Proposed Diagnostic Scheme for People with Epileptic Seizures and with Epilepsy: Report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- 22.Hermann B, Jones J, Dabbs K, et al. The frequency, complications and aetiology of ADHD in new onset paediatric epilepsy. Brain. 2007;130:3135–3148. doi: 10.1093/brain/awm227. [DOI] [PubMed] [Google Scholar]
- 23.Reilly C, Atkinson P, Das KB, et al. Neurobehavioral Comorbidities in Children With Active Epilepsy: A Population-Based Study. Pediatrics. 2014 doi: 10.1542/peds.2013-3787. [DOI] [PubMed] [Google Scholar]
- 24.MacAllister WS, Vasserman M, Vekaria P, et al. Neuropsychological endophenotypes in ADHD with and without epilepsy. Appl Neuropsychol Child. 2012;1:121–128. doi: 10.1080/21622965.2012.709421. [DOI] [PubMed] [Google Scholar]
- 25.Hollingshead AA. Four-factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]
- 26.Sherman EM, Brooks BL, Fay-McClymont TB, et al. Detecting epilepsy-related cognitive problems in clinically referred children with epilepsy: is the WISC-IV a useful tool? Epilepsia. 2012;53:1060–1066. doi: 10.1111/j.1528-1167.2012.03493.x. [DOI] [PubMed] [Google Scholar]
- 27.Gitelman DR. ILAB: a program for postexperimental eye movement analysis. Behav Res Methods Instrum Comput. 2002;34:605–612. doi: 10.3758/bf03195488. [DOI] [PubMed] [Google Scholar]
- 28.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 29.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 30.Padmanabhan A, Geier CF, Ordaz SJ, et al. Developmental changes in brain function underlying the influence of reward processing on inhibitory control. Dev Cogn Neurosci. 2011;1:517–529. doi: 10.1016/j.dcn.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjork JM, Knutson B, Fong GW, et al. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical; 1988. [Google Scholar]
- 34.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 35.Asato MR, Terwilliger R, Woo J, et al. White matter development in adolescence: a DTI study. Cereb Cortex. 2010;20:2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pulsipher DT, Seidenberg M, Guidotti L, et al. Thalamofrontal circuitry and executive dysfunction in recent-onset juvenile myoclonic epilepsy. Epilepsia. 2009;50:1210–1219. doi: 10.1111/j.1528-1167.2008.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neri ML, Guimaraes CA, Oliveira EP, et al. Neuropsychological assessment of children with rolandic epilepsy: executive functions. Epilepsy Behav. 2012;24:403–407. doi: 10.1016/j.yebeh.2012.04.131. [DOI] [PubMed] [Google Scholar]
- 38.D'Agati E, Cerminara C, Casarelli L, et al. Attention and executive functions profile in childhood absence epilepsy. Brain Dev. 2012;34:812–817. doi: 10.1016/j.braindev.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Gelziniene G, Jurkeviciene G, Marmiene V, et al. Executive functions in adolescents with idiopathic generalized epilepsy. Medicina (Kaunas) 2011;47:313–319. [PubMed] [Google Scholar]
- 40.Tosun D, Siddarth P, Toga AW, et al. Effects of childhood absence epilepsy on associations between regional cortical morphometry and aging and cognitive abilities. Hum Brain Mapp. 2011;32:580–591. doi: 10.1002/hbm.21045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Velanova K, Wheeler ME, Luna B. The Maturation of Task Set-Related Activation Supports Late Developmental Improvements in Inhibitory Control. J Neurosci. 2009;29:12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kleen JK, Wu EX, Holmes GL, et al. Enhanced oscillatory activity in the hippocampal-prefrontal network is related to short-term memory function after early-life seizures. J Neurosci. 2011;31:15397–15406. doi: 10.1523/JNEUROSCI.2196-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu X, Hairston J, Schrier M, et al. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geier CF, Luna B. Developmental Effects of Incentives on Response Inhibition. Child Dev. 2012;83:1262–1274. doi: 10.1111/j.1467-8624.2012.01771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Archer JS, Abbott DF, Waites AB, et al. fMRI "deactivation" of the posterior cingulate during generalized spike and wave. Neuroimage. 2003;20:1915–1922. doi: 10.1016/s1053-8119(03)00294-5. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z, Liao W, Chen H, et al. Altered functional-structural coupling of large-scale brain networks in idiopathic generalized epilepsy. Brain. 2011;134:2912–2928. doi: 10.1093/brain/awr223. [DOI] [PubMed] [Google Scholar]
- 47.Luna B, Sweeney JA. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Ann N Y Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.