Abstract
Background
It is unknown how atrial fibrillation (AF) is actually initiated by triggers. Based on consistencies in atrial structure and function in individual patients between episodes of AF, we hypothesized that human AF initiates when triggers interact with deterministic properties of the atria and may engage organized mechanisms.
Methods and Results
In 31 AF patients we mapped AF initiation after spontaneous triggers or programmed stimulation. We used 64-pole basket catheters to measure regional dynamic conduction slowing and to create bi-atrial activation maps during transitions to AF. Sixty-two AF initiations were recorded (spontaneous, n=28; induced, n=34). Notably, AF did not initiate by disorganized mechanisms, but by either a dominant reentrant spiral wave (76%) or a repetitive focal driver. Both mechanisms were located 21±17mm from their triggers. AF-initiating spirals formed at the site showing the greatest rate-dependent slowing in each patient. Accordingly, in 10/12 patients with multiple observed AF episodes, AF initiated using spatially conserved mechanisms despite diverse triggers.
Conclusions
Human AF initiates from triggers by organized rather than disorganized mechanisms, either via spiral wave reentry at sites of dynamic conduction slowing, or via repetitive focal drivers. The finding that diverse triggers initiate AF at predictable, spatially conserved functional sites in each individual provides a novel deterministic paradigm for AF with therapeutic implications.
Keywords: atrial fibrillation, arrhythmia (mechanisms), cardiac electrophysiology, rotor, spiral waves, trigger
Introduction
Atrial fibrillation (AF) is the most prevalent sustained arrhythmia, associated with palpitations, stroke, and death1. While ablation is currently designed to prevent AF initiation, the actual mechanisms by which AF initiates from triggers are undefined1 because they are difficult to map and generally assumed complex2. Ablation therefore targets potential trigger sites rather than initiating mechanisms3. This contrasts with other supraventricular arrhythmias4 in which mapping and ablation target the initiating mechanism rather than the trigger sites5, 6.
Although AF has highly variable activation, multiple lines of evidence indicate that AF exhibits underlying spatial-temporal organization. This includes stable inter/intra-atrial frequency gradients7, consistent activation vectors 8, and initial evidence for stable rotors and focal drivers of ongoing AF9, 10.
We thus hypothesized that, despite its potential complexity, AF initiates from potentially diverse triggers by organized mechanisms that may involve dynamic conduction slowing. We tested this in AF patients by detailed mapping of the majority of both atria9 assessed systematically after triggers that initiated AF and ectopic beats that did not, relative to the regional rate-response of conduction.
Methods
Study Design and Enrollment
We enrolled consecutive subjects with symptomatic AF undergoing ablation1 at the Veterans Affairs and UC San Diego Medical Centers. The study was approved by our joint VA/UCSD Institutional Review Board, and all patients provided informed consent. Subjects were ≥21 years old with paroxysmal or persistent AF despite class I or III antiarrhythmics. The only exclusion was inability or refusal to provide consent. To study AF initiation, we included patients in sinus rhythm spontaneously or after cardioversion (31/57 screened patients).
Electrophysiology Study
Antiarrhythmic medications (Table 1) were discontinued for 5 half-lives (>60 days for amiodarone; median 202 days). Catheters were advanced to the right atrium (RA), coronary sinus, and transseptally to left atrium (LA). A 64-pole basket catheter (Constellation, Boston Scientific, Natick, MA) was advanced to map the LA. RA recordings were made using a second basket (simultaneously) in 17 patients (Figure 1a), or a quadrapolar RA catheter at the septum (to indicate RA initiation of AF if activation earlier than any LA electrode). Inter-electrode separation was 4–6mm along each spline, with inter-spline separation of 4–6mm proximally and distally, with worst-case average of ≈10mm for equatorial electrodes. This resolution was considered sufficient to identify changes in activation from a paced or sinus rhythm pattern to a reentrant wave or ectopic driver at AF onset. Heparin was infused to maintain activated clotting time >350 seconds.
Table 1.
Patient characteristics
| Characteristic | All Patients (n=31) | Paroxysmal AF Patients (n=21) | Persistent AF Patients (n=10) | P value |
|---|---|---|---|---|
| Age (years) | 62±9 | 61±10 | 63±9 | 0.16 |
| Male | 30 (97%) | 20 (95%) | 10 (100%) | |
| History of AF (months) | 33 (20–87) | 29 (19–53) | 65 (40–113) | 0.44 |
| Left atrial diameter (mm) | 42±7 | 40±5 | 48±6 | 0.01 |
| Left ventricular ejection fraction (%) | 57±9 | 61±8 | 50±7 | 0.01 |
| CHADS2 score | ||||
| --0 to 1 | 15 (48%) | 10 (48%) | 5 (50%) | 0.90 |
| --2 or more | 16 (52%) | 11 (52%) | 5 (50%) | 0.90 |
| NHYA functional class | ||||
| --I–II | 30 (97%) | 21 (100%) | 9 (90%) | 0.14 |
| --III–IV | 1 (1%) | 0 | 1 (10%) | 0.14 |
| Comorbidities | ||||
| --Hypertension (n) | 25 (81%) | 17 (81%) | 8 (80%) | 0.95 |
| --Diabetes mellitus (n) | 10 (32%) | 9 (43%) | 1 (10%) | 0.07 |
| --Prior stroke/TIA (n) | 6 (19%) | 3 (14%) | 3(30%) | 0.30 |
| --Coronary artery disease (n) | 11 (35%) | 7 (33%) | 4 (40%) | 0.72 |
| --Prior ablation | 5 (16%) | 1 (5%) | 4 (40%) | 0.01 |
| Previously failed >1 antiarrhythmic (n) | 9 (29%) | 5 (24%) | 4 (40%) | 0.35 |
| --Class I agent | 8 (26%) | 6 (29%) | 2 (20%) | 0.61 |
| --Sotalol | 13 (42%) | 8 (38%) | 5 (50%) | 0.53 |
| --Dofetilide | 5 (16%) | 3 (14%) | 2 (20%) | 0.69 |
| --Amiodarone | 10 (32%) | 6 (29%) | 4 (40%) | 0.52 |
| Concomitant drug therapy | ||||
| --ACEI/ARB | 17 (55%) | 11 (52%) | 6 (60%) | 0.69 |
| --Beta blocker | 17 (55%) | 10 (48%) | 7 (70%) | 0.24 |
| --Calcium channel blocker | 11 (35%) | 6 (29%) | 5 (50%) | 0.24 |
| --Statin | 19 (61%) | 15 (71%) | 4 (40%) | 0.09 |
Values are n (%), mean±SD or median (interquartile range).
ACEI=angiotensin-converting enzyme inhibitor; AF=atrial fibrillation; ARB=angiotensin receptor blocker; NYHA=New York Heart Association; TIA=transient ischemic attack.
Figure 1.
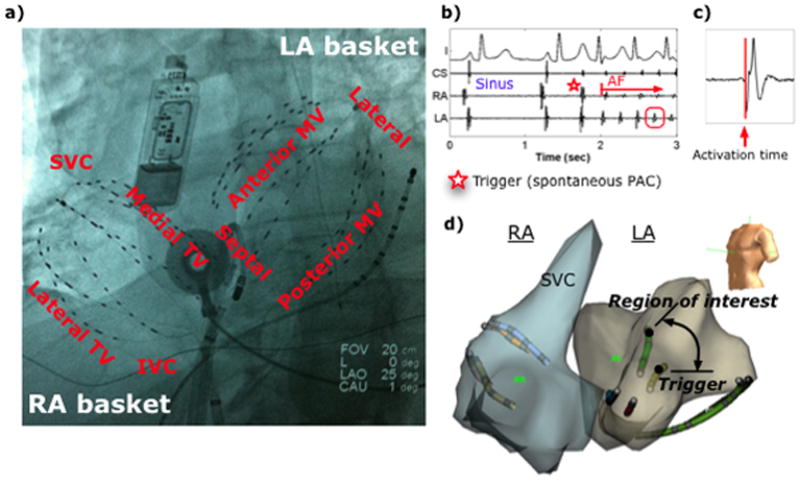
Catheter placement and recordings of AF initiation. (a) Fluoroscopy showing 64-pole basket catheter in each atrium, implanted ECG monitor (Reveal, Medtronic, MN), catheters in the coronary sinus and LA, and esophageal temperature probe. (b) ECG and intracardiac signals of spontaneous paroxysmal AF after a PAC trigger, with (c) activation time marking of electrogram. (d) NavX shells of both atria indicating the trigger and region of interest, with separation computed from respective (x,y,z) coordinates. (IVC=inferior vena cava, LA=left atrium, MV=mitral valve, RA=right atrium, SVC=superior vena cava, TV=tricuspid valve.)
Data Acquisition During AF
Electrograms were filtered at 0.05 Hz to 500 Hz and recorded at 1 kHz (Bard, Lowell, MA). To study AF initiation from diverse triggers, we analyzed spontaneous and pacing-induced initiations. In sinus rhythm, we waited ≤ 10 minutes to map spontaneous ectopy that did (Fig. 1b.) or did not (Fig 2; patient-specific controls) initiate AF. Patients not in sinus rhythm after ≤ 2 electrical cardioversions were excluded. AF induction was performed systematically first using single extrastimuli at shortening coupling intervals after an 8-beat drive (cycle length, CL500ms). Next, we performed burst pacing (CL500ms), reducing in 50ms steps to 300ms, then in 10ms steps to AF. Finally, if AF still had not initiated, we infused isoproterenol (≤ 10mcg/min). To mimic clinical triggers, we paced consistently from a pulmonary vein (left PVs n=17 (57%), right n=8 (27%)) or the high RA (n=4, 13%). Multiple AF initiations in each patient were compared. Patients then underwent clinical ablation.
Figure 2.
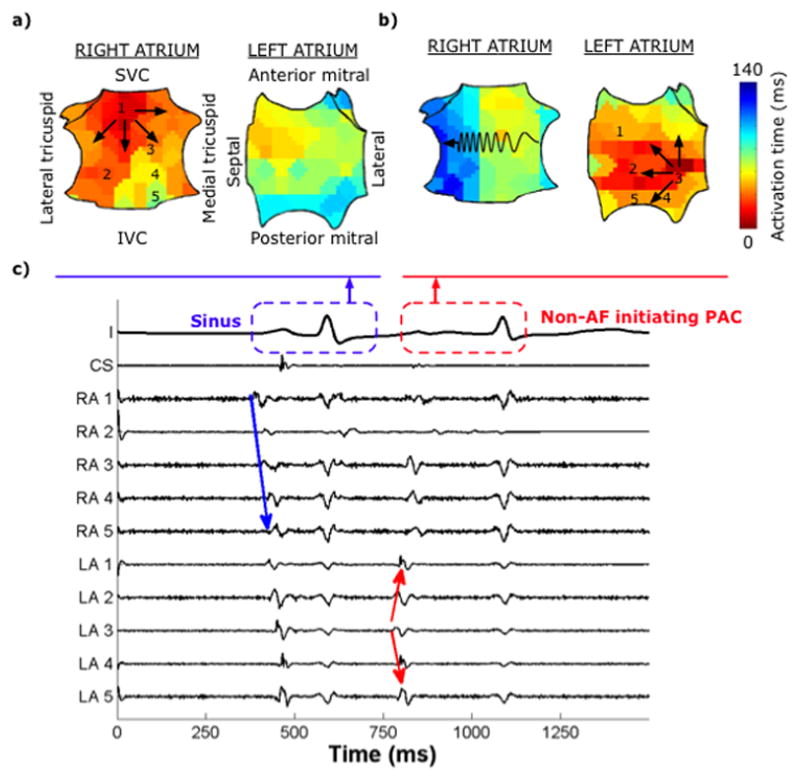
Bi-atrial spatial activation maps for a sinus beat and premature atrial complex (PAC) in a 74-year-old man with paroxysmal AF. (a) Sinus activity propagates centrifugally in the RA and conducts via Bachmann’s bundle to the LA. (b) Non-AF initiating PAC from the lateral LA shows non-centrifugal activation in the RA, with preferential septal-to-lateral slowing (zig-zag line). (c) Corresponding intracardiac recordings.
Spatial Mapping of Baseline Rhythm, Trigger, and AF Initiation
Bi-atrial activation times were analyzed at each electrode for baseline rhythm, the trigger beat, then AF initiation. The trigger beat was defined as the first cycle departing from baseline rhythm (Fig. 1b), while AF onset was identified by variations in cycle length and electrogram morphology, or (for induced AF) dissociation from pacing. Because intra-atrial propagation in AF quickly becomes non-1:1 with ambiguous propagation, we focused on the first 3 cycles of sustained AF. These electrograms are typically discrete, without fractionation, allowing construction of unambiguous isochronal maps. Activation time at each electrode was defined as the point of steepest slope of the first negative deflection in software11 using filtering12, then manually over-read blinded to the spatial map (Fig. 1c.)
Propagation maps for each cycle were constructed from activation times using custom software written in Matlab (Mathworks, Natick, MA.) Isochrones were constructed by drawing contour lines at early (red) to late (blue) activated regions. Figure 2 shows bi-atrial isochronal maps for a sinus beat and a LA premature atrial complex (PAC) oriented as in Figure 1a. A spiral wave9 was defined as reentry around a specific region, showing early-meeting-late activation. Focal activation was defined as centrifugal emanation from an origin. The trigger of spontaneous AF was the PAC site. For induced AF, the trigger was the site of pacing. In patients with multiple AF initiations, we studied whether AF initiation occurred at conserved sites, defined if their type (i.e. spiral wave vs. focal driver) and location (≤ 1 electrode distance) were conserved.
Quantification of Spatial Separation Between Triggers and AF-Initiating Mechanisms
Atrial geometric contours (NavX, St. Jude Medical, Minneapolis, MN) for each patient were used to compute distances between the trigger and site of each AF initiation. Separation of the (x,y,z) coordinates of each electrode (NavX, Fig. 1d) was measured as the average of the Euclidean (“ruler”) and shortest distance over a best-fit computed ellipsoid registered to each patient’s atrium.
Conduction Velocity Restitution
We studied rate-dependent conduction slowing (restitution) in both atria en route to AF in a subset of 22 patients during burst pacing. Conduction slowing was defined by activation time prolongation by ≥ 10ms (absolute) and ≥ 20% (relative)11 between fastest and slowest rates.
Statistical analysis
Continuous data are represented as mean±standard deviation (SD) or, if non-normally distributed, as median (interquartile range (IQR)). Comparisons were made with Student’s t tests if normally distributed, or with Mann-Whitney U- test otherwise. Paired continuous variables were compared using Wilcoxon Signed-Ranks Test. Categorical data are summarized with frequency counts and percentages. The Fisher exact test was applied to contingency tables. To account for multiple observations per subject, mixed model analysis is employed and continuous variables summarized using estimated means and standard errors. A probability of <0.05 was considered statistically significant.
Results
Patient Characteristics
Table I summarizes our study patients. We mapped 62 AF initiations (median 1 (IQR 1–2) per patient), comprising 28 spontaneous and 34 induced (27 burst pacing, 3 single extra-stimulus, 4 isoproterenol). Control data consisted of 50 spontaneous PACs in 12 patients which failed to initiate AF (median 5 (IQR 2–6) per patient).
Differences Between AF-Initiating and Non-Initiating Ectopy
Both AF-initiating ectopy (n=28) and non-AF initiating ectopy (n=50) arose bi-atrially (Table 2), with a non-significant trend towards LA predominance (p=0.07) (Table 2). Ectopic beats that initiated AF were more premature than non-AF-initiating ectopy (coupled 370±25 vs. 502±19ms; p<0.001; Table 2).
Table 2.
Characteristics of Ectopy and Initiating Mechanisms.
| Ectopy | Non-AF initiating (n=50 PACs) | AF initiating (n=28 PACs) | P value |
|---|---|---|---|
| Coupling interval (ms) | 502±19 | 370±25 | <0.001 |
| Left atrium | 24 (48%) | 19 (68%) | 0.23 |
| Right atrium | 23 (46%) | 7 (25%) | (LA vs. RA) |
| Septum | 3 (6%) | 2 (7%) | |
|
| |||
| AF Initiating Mechanism | Spiral Wave (n=45) | Focal Driver (n=16) | P value |
|
| |||
| Cycle length (ms) | 103±6 | 201±10 | <0.001 |
| Left atrial | 28 (62%) | 14 (88%) | 0.09 |
| Right atrial | 17 (38%) | 2 (13%) | (LA vs. RA) |
Values are n (%) or estimated means ± standard error of the estimate.
Identification and Classification of AF-Initiating Mechanisms
We found that AF initiation was not disorganized but exhibited 2 spatially organized mechanisms. The first comprised a reentrant spiral wave, demonstrating sequential activation (clockwise or counterclockwise; Fig. 3) seen in 76% (n=45) initiations. The second mechanism comprised a repetitive focal driver (Fig. 4) in 27% (n=16) initiations. In 2 AF initiations, both mechanisms were observed. Three AF initiations were excluded due to poor electrogram quality that reduced confidence in measurements.
Figure 3.
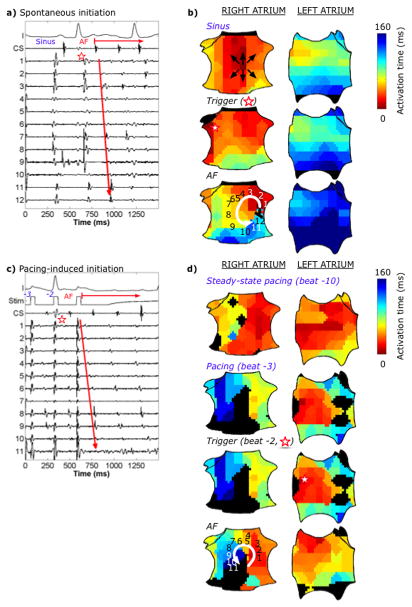
Multiple AF initiations via a spiral wave in an 81-year-old man with paroxysmal AF. (a) Spontaneous initiation: ECG and intracardiac recordings. Electrograms 1–12 represent sites 1–12 in panel (b). (b) Spatial activation maps show the corresponding sinus beat (top panel) followed by a spontaneous RA PAC (middle panel, red star, >22ms earlier than other sites) causing slowed conduction in the inferior RA. The first AF cycle (bottom panel) encounters tissue that activated late in the prior beat, causing block with formation of a counterclockwise spiral wave in the mid-septal RA that initiates AF. (c) Pacing-induced AF initiation, 10 minutes after the previous initiation, at burst pacing CL260ms near the right superior PV. Electrograms 1–11 represent sites 1–11 in panel (d). (d) Steady-state pacing (top panel, electrograms not shown) shows rapid activation that slowed with increasing rate in RA as shown in the final 2 paced beats prior to AF (second and third panels, respectively) leading to an AF-initiating spiral with similar location and CL to panels (a, b). Color maps in (d) represent true RA activation time referenced to pacing artifact. Despite different LA activity and quite different triggers, the same AF-initiating RA spiral wave is formed. Black areas represent diastole in the transition to established AF.
Figure 4.
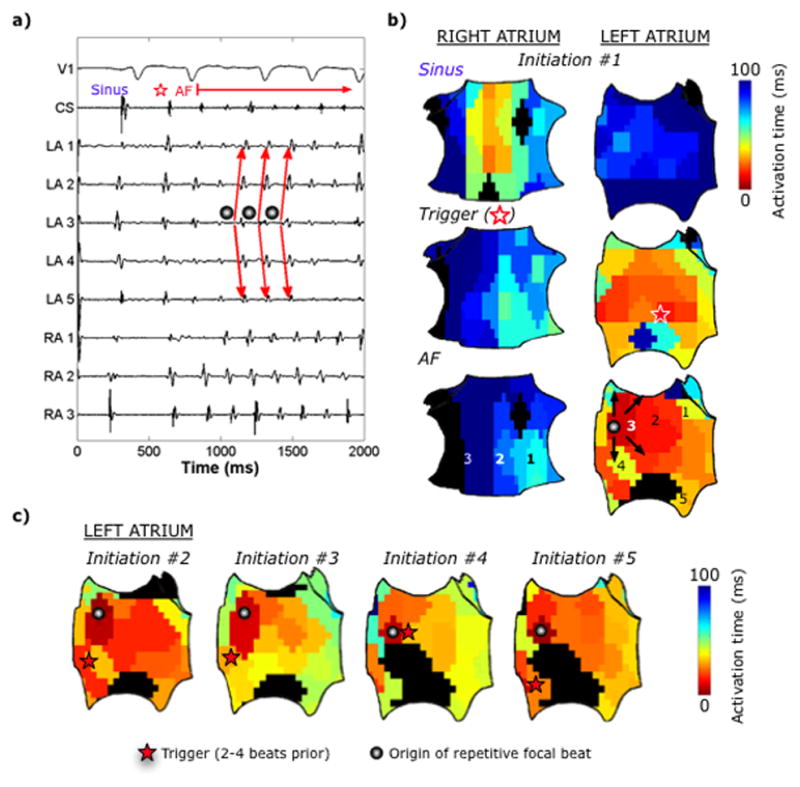
Multiple AF initiations via a repetitive focal driver in a 67-year-old man with persistent AF. (a) ECG and intracardiac recordings and (b) bi-atrial spatial activation map showing the last sinus beat (top panel), followed by a LA PAC trigger (middle), which leads to a focal AF driver on the anterior LA (CL150ms; bottom). (c) Left atrial activation map from 4 additional spontaneous initiations, each initiated by spatiotemporally similar focal drivers despite various preceding triggers.
AF Initiating Mechanism 1: Dominant Reentrant Spiral Wave
Figures 3a–b illustrate isochronal maps of AF initiation by a spontaneous PAC in the lateral RA with conduction slowing in the inferior RA, leading to a spiral wave and AF. Figures 3c–d show a distinct AF initiation in this patient, 10 minutes later and from LA burst pacing. Notably these diverse triggers initiated two AF episodes by engaging a very similar, spatially-conserved spiral wave.
Overall, AF-initiating spiral waves formed in bi-atrial locations (Table 2), and occurred after spontaneous triggers that were coupled 380±12ms from baseline. Supplemental Figure 1 depicts additional examples of spiral wave AF initiations.
AF Initiating Mechanism 2: Repetitive Focal Driver
The remaining 27% of AF episodes were initiated by a repetitive focal driver. Figure 4 shows a spontaneous PAC from the inferior LA that triggered a focal driver (CL150ms) at the anterior LA that drove the atria into AF. Overall, repetitive focal drivers were bi-atrial (Table 2) and occurred after triggers coupled 345±21ms from baseline beats (p=0.17 vs spiral waves). Supplemental Figure 1 illustrates an additional AF focal driver. There was no statistically significant association between type of initiation mechanism and type of AF (p=0.21) or history of prior ablation (p=0.20).
Spatial Separation of Triggers from AF-Initiating Mechanisms
AF initiation sites were separated by 21±17mm from their preceding trigger (spiral waves, 23±3mm; focal drivers, 15±5mm; p=0.17), that did not differ for paroxysmal or persistent AF (21±3mm vs. 20±4; p=0.79). Indeed, in 12 AF initiations the mechanisms (spiral waves) were contralateral to its trigger.
There was no significant difference in the separation between an observed AF-initiating mechanism in each patient and PACs that did initiate AF and those that did not (18.0±13.4mm vs. 22.9±13.0mm; p=0.43), suggesting insufficient prematurity as the reason why AF did not initiate in these cases.
Role of Functional Conduction Slowing In AF Initiation
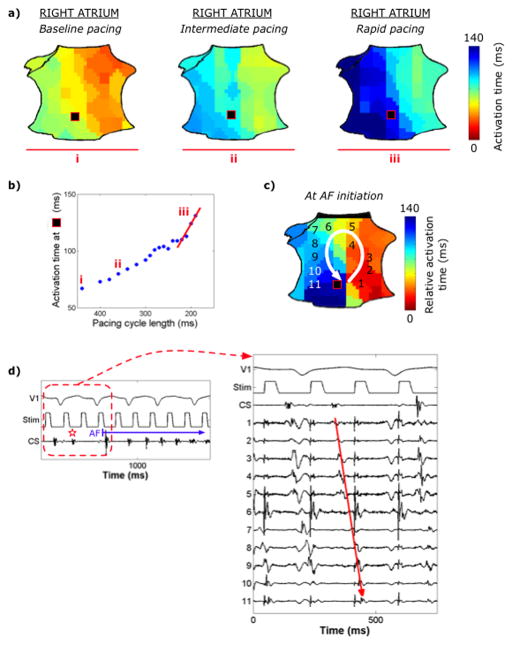
Figure 5 shows AF initiation that followed progressive RA conduction slowing with increasing rate, creating block then a spiral wave. Dynamic conduction slowing occurred at the site of AF initiation (by 113±74%, 52±34ms from baseline) in 86% of patients (19/22). In all but one patient, a spiral wave formed at this site. The 3 AF initiations without defined conduction slowing (25±21%, or 9±3ms, p=0.04) were initiated by a focal driver.
Figure 5.
AF initiation by a right atrial spiral wave at the site of dynamic conduction slowing. (a) RA activation map during LA pacing at baseline (CL450ms), intermediate (CL360ms), and rapid (CL200ms) rates, corresponding to sites (i, ii, iii) on the (b) conduction restitution curve, illustrating rate-dependent conduction slowing in the low posterior RA (black square). Critical delay in activation in the low RA at the fastest rate (pre-fibrillatory slowing, red line) causes block and (c) forms a counterclockwise spiral that initiates AF. (d) Corresponding ECG and intracardiac recordings.
We developed a predictive index for the site of spiral wave formation. Pre-fibrillatory slowing was defined as the slope of the restitution curve at shortest pacing CL (Fig. 5b), calculated for each electrode. The atrial site with the greatest rate-related slowing in conduction was identified. Four patients showed <20ms conduction prolongation between fastest and slowest rates, i.e. below our definition for slowing. The site of maximum pre-fibrillatory slowing predicted the site of AF initiation in 16 of the remaining 18 patients (89%).
Conservation of AF-Initiating Mechanisms Despite Varying Triggers
Twelve patients showed multiple AF initiations (43 initiations, median 3 (IQR 2–4) per patient). Spiral waves initiated 24/26 AF episodes (n=8 patients), and focal drivers initiated 9/10 episodes in 2 patients. Thus, AF-initiating mechanisms were conserved in 10 patients (83%; 95% CI 52% – 98%). In each patient, both the type and location of mechanisms were conserved despite diverse triggers.
Figure 3 illustrates 2 distinct AF initiations in the same patient; one after a spontaneous RA PAC (Fig. 3a) and the second after PV pacing (Fig. 3b). In both cases, conduction slowing formed a spiral wave with conserved locations and cycle lengths that initiated AF. Figure 4 shows 5 conserved focal AF drivers (CL153±4ms) at the LA roof despite preceding LA PAC triggers at diverse locations. Figure 6 summarizes trigger and AF-mechanism locations for all AF initiations in patients with multiple AF initiations.
Figure 6.
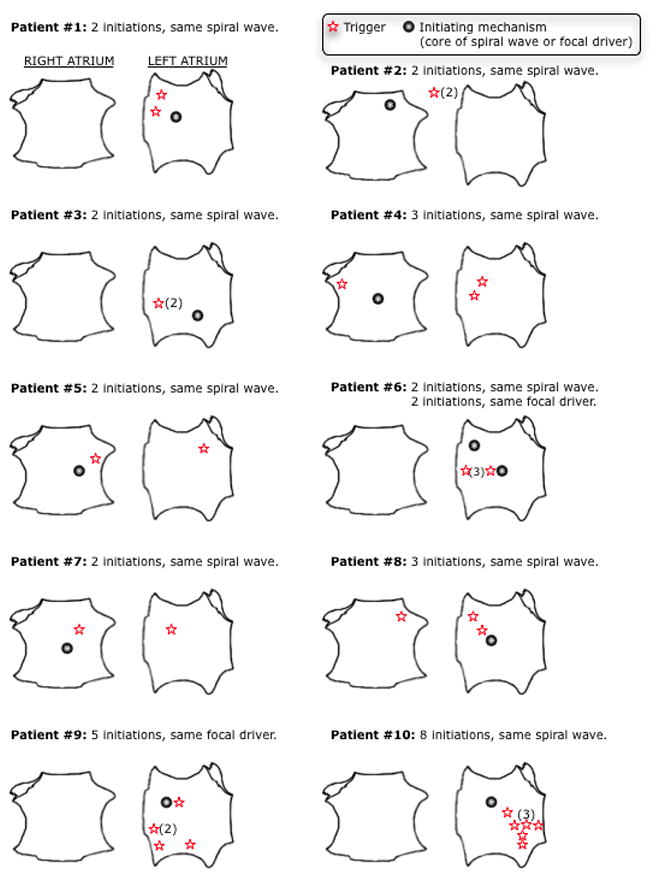
Conserved initiation of AF in 10 patients with multiple initiations. Right and left atria displayed for each patient with the location of the conserved initiating mechanism and triggers for each individual initiation. Section headings display the number of conserved initiations engaging the common initiating mechanism. Numbers in parentheses indicate multiple triggers from that location. Triggers depicted outside both atria represent an inter-atrial septum origin
Discussion
This study shows that AF is not initiated by complex mechanisms, but by an orderly process of conduction slowing causing block and formation of an organized spiral wave or focal driver with subsequent fibrillatory conduction. Organized AF-initiating mechanisms were separated spatially from their trigger and, unexpectedly, were conserved in each patient for multiple AF initiations from diverse trigger sites and types. Notably, sites of AF initiations in each patient could be predicted in sinus rhythm from the sites of maximum trigger-induced conduction slowing (pre-fibrillatory slowing). These data influence our conceptualization of AF initiation and may have therapeutic implications for improving ablation to prevent AF onset.
AF Initiating Mechanisms Differ from Triggers
Prior reports have mapped spontaneous ectopic triggers for AF3, 5, 13, and demonstrated their spatial diversity in both atria and prematurity in rate using both contact and non-contact mapping. However, few studies have defined whether PACs, rapid tachycardias, or other triggers5, 13, 14 initiate AF via solitary or multiple reentrant or focal circuits, or in relation to conduction slowing that may represent the increasingly studied atrial properties of fibrosis/scar15.
AF initiation by spiral reentry arose at sites of maximum pre-fibrillatory slowing detected in pacing11, that were not evident in sinus rhythm (i.e. not anatomical). Spatially, the separation of AF initiating sites from triggers may be explained by the distance needed for conduction block and the formation of a reentrant circuit. We focused on regions of maximum change in conduction between fastest and slowest rates (i.e. restitution), rather than conduction time per se. While regions upstream from the AF initiation site may also show conduction delay, the greatest rate-dependent change (“pre-fibrillatory slowing”) likely reflected conduction block/reentry at the site of AF onset. The fact that AF initiation by focal drivers also followed premature beats suggests that they may represent microreentry or triggered activity. Studies are needed to define if regional restitution represents zones bordering fibrosis15.
Temporally, non-AF initiating PACs were less premature than AF-initiating PACs, and caused less conduction restitution. Future work should define the spatial-temporal “zone of vulnerability” for ectopy, wherein those falling within a range of prematurity and potentially spatial proximity may be more or less successful triggers for AF initiation.
Consistency of Initiating Mechanisms For Diverse Triggers
We demonstrate, for the first time, that multiple AF episodes from very different triggers may initiate AF in individual patients by a conserved and deterministic mechanism. This again reinforces the concept that AF is not “chaotic”. Future studies should examine a greater number of spontaneous and induced initiations, and study if regions exhibiting the steepest rate-related conduction represent specific fiber angles16, fibrosis, or scar15, or are predicated on spatial distributions of dynamic gradients of conduction or repolarization17.
Clinical Implications
Our results provide a novel potential mechanism for the greater success of wide area circumferential ablation (~2cm from PV triggers) over PV ostial ablation18, by ablating regions where AF actually initiates from PV or atrial triggers. These results suggest that ablation at sites of conduction restitution may complement trigger isolation. Importantly, preventive strategies could be based on these results. For instance, spiral wave AF initiation could be prevented by pre-emptive pacing to prevent reentry, or drug approaches to ameliorate conduction restitution. Patients with focal AF initiators may be treated by strategies to prevent triggered activity. These results complement our recent data that human AF may be perpetuated by localized sources, supported by the acute and chronic elimination of AF by targeted ablation (i.e. FIRM)9, 10, 19, 20. Ongoing studies are examining how the single AF-initiating mechanism in the present study evolves into the concurrent AF-sustaining sources identified by phase-mapping9.
Study limitations
Our 128 mapping electrodes covered the vast majority of the endocardial surfaces of both atria and likely provided sufficient resolution to identify changes in propagation at AF onset using each patient as their own control. Nevertheless, higher resolution would be preferred. Distance estimates from (x,y,z) coordinates likely included errors from cardiorespiratory motion; however, this is likely considerably less than the separation of trigger to AF-initiating site (21±17mm). We did not dissect precise location data that may be difficult to define, for instance, given difficulties in assigning PV ostial locations in funnel-shaped antrums, but instead defined the functional relationship between any trigger and AF initiation. However, studies should reference trigger and AF initiation sites to anatomical sites of pathophysiology such as scar, fibrosis21 or fiber angles 16. Pacing from multiple locations in the same patient would shed light on the interaction of triggers with regional anisotropy, but was not performed because the protocol was already lengthy. Activation time was used as a surrogate for conduction velocity that is accurate unless marked changes in propagation direction occur, which was not observed. We did not ablate initiating mechanisms because the lengthy analysis was performed offline, but ongoing studies of prospective ablation are planned. Finally, we deliberately included both induced and spontaneous AF to study if initiations were conserved between them. Several recent reports show that both forms of AF show conserved frequency and rate gradients22 or spatial propagation23 in the same patient.
Conclusions
AF initiation is not a disorganized process, but operates by the dynamic formation of a spiral wave at sites of conduction slowing, or a focal driver. These mechanisms are often spatially conserved for a given patient despite triggers with diverse spatial locations and prematurities, and are spatially separated from their trigger. These data open the possibility of new strategies to prevent AF onset by modulating these patient-specific AF-initiating mechanisms.
Supplementary Material
Acknowledgments
We thank John Miller, MD for his helpful comments on the manuscript. We thank Judith Hildreth, RN, Cherie Jaynes, RN, Elizabeth Greer, RN, Stephanie Yoakum, NP, Donna Cooper, RN, Anthony Moyeda, CVT, and Kenneth Hopper, CVT for their great assistance in the clinical study. We thank Kathleen Mills, BA for data coordination. Dr. Schricker placed 1st in the Young Investigator Awards Competition of the 2013 Heart Rhythm Society Scientific Sessions with this study.
Funding Sources: This work was supported in part by grants from the American College of Cardiology and Merck Foundation to A.A.S., the American Heart Association to D.E.K., the Doris Duke Charitable Foundation, and the NIH (HL70529, HL83359) to S.M.N.
Footnotes
Conflict of Interest Disclosures: Drs. Narayan and Rappel are authors of intellectual property owned by the University of California Regents and licensed to Topera Inc. Topera does not sponsor any research, including that presented here. Drs. Narayan and Rappel hold equity in Topera. Dr. Narayan reports having received honoraria from Medtronic, St. Jude Medical, and Biotronik. Drs. Schricker, Lalani, and Krummen report no conflicts.
References
- 1.Calkins CH. 2012 hrs/ehra/ecas expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Heart Rhythm. 2012;9:632–696. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 2.Allessie MA, Konings K, Kirchhof CJ, Wijffels M. Electrophysiologic mechanisms of perpetuation of atrial fibrillation. Am J Cardiol. 1996;77:10A–23A. doi: 10.1016/s0002-9149(97)89114-x. [DOI] [PubMed] [Google Scholar]
- 3.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 4.Jackman WM, Wang XZ, Friday KJ, Roman CA, Moulton KP, Beckman KJ, McClelland JH, Twidale N, Hazlitt HA, Prior MI, Margolis PD, Calame JD, Overholt ED, Lazzara R. Catheter ablation of accessory atrioventricular pathways (wolff-parkinson-white syndrome) by radiofrequency current. N Engl J Med. 1991;324:1605–1611. doi: 10.1056/NEJM199106063242301. [DOI] [PubMed] [Google Scholar]
- 5.Schmitt C, Ndrepepa G, Weber S, Schmieder S, Weyerbrock S, Schneider M, Karch MR, Deisenhofer I, Schreieck J, Zrenner B, Schomig A. Biatrial multisite mapping of atrial premature complexes triggering onset of atrial fibrillation. Am J Cardiol. 2002;89:1381–1387. doi: 10.1016/s0002-9149(02)02350-0. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen JC, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, Pehrson S, Englund A, Hartikainen J, Mortensen LS, Hansen PS. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. New Engl J Med. 2012;367:1587–1595. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 7.Lazar S, Dixit S, Marchlinski FE, Callans DJ, Gerstenfeld EP. Presence of left-to-right atrial frequency gradient in paroxysmal but not persistent atrial fibrillation in humans. Circulation. 2004;110:3181–3186. doi: 10.1161/01.CIR.0000147279.91094.5E. [DOI] [PubMed] [Google Scholar]
- 8.Gerstenfeld EP, Sahakian AV, Swiryn S. Evidence for transient linking of atrial excitation during atrial fibrillation in humans. Circulation. 1992;86:375–382. doi: 10.1161/01.cir.86.2.375. [DOI] [PubMed] [Google Scholar]
- 9.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel W-J, Miller J. Treatment of atrial fibrillation by the ablation of localized sources: The conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation: Confirm trial. J Am Coll Cardiol. 2012;60:628–636. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shivkumar K, Ellenbogen KA, Hummel JD, Miller JM, Steinberg JS. Acute termination of human atrial fibrillation by identification and catheter ablation of localized rotors and sources: First multicenter experience of focal impulse and rotor modulation (firm) ablation. J Cardiovasc Electrophysiol. 2012;23:1277–1285. doi: 10.1111/jce.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lalani G, Schricker A, Gibson M, Rostamanian A, Krummen DE, Narayan SM. Atrial conduction slows immediately before the onset of human atrial fibrillation: A bi-atrial contact mapping study of transitions to atrial fibrillation. J Am Coll Cardiol. 2012;59:595–606. doi: 10.1016/j.jacc.2011.10.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haws CW, Lux RL. Correlation between in vivo transmembrane action potential durations and activation-recovery intervals from electrograms. Effects of interventions that alter repolarization time. Circulation. 1990;81:281–288. doi: 10.1161/01.cir.81.1.281. [DOI] [PubMed] [Google Scholar]
- 13.Chen SA, Hsieh MH, Tai CT, Tsai CF, Prakash VS, Yu WC, Hsu TL, Ding YA, Chang MS. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: Electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. 1999;100:1879–1886. doi: 10.1161/01.cir.100.18.1879. [DOI] [PubMed] [Google Scholar]
- 14.Hindricks G, Kottkamp H. Simultaneous noncontact mapping of left atrium in patients with paroxysmal atrial fibrillation. Circulation. 2001;104:297–303. doi: 10.1161/01.cir.104.3.297. [DOI] [PubMed] [Google Scholar]
- 15.Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, Deneke T, Duytschaever M, Neumann T, Mansour M, Mahnkopf C, Herweg B, Daoud E, Wissner E, Bansmann P, Brachmann J. Association of atrial tissue fibrosis identified by delayed enhancement mri and atrial fibrillation catheter ablation: The decaaf study. JAMA. 2014;311:498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 16.Klos M, Calvo D, Yamazaki M, Zlochiver S, Mironov S, Cabrera JA, Sanchez-Quintana D, Jalife J, Berenfeld O, Kalifa J. Atrial septopulmonary bundle of the posterior left atrium provides a substrate for atrial fibrillation initiation in a model of vagally mediated pulmonary vein tachycardia of the structurally normal heart. Circ Arrhythm Electrophysiol. 2008;1:175–183. doi: 10.1161/CIRCEP.107.760447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaquero M, Calvo D, Jalife J. Cardiac fibrillation: From ion channels to rotors in the human heart. Heart Rhythm. 2008;5:872–879. doi: 10.1016/j.hrthm.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arentz T, Weber R, Bürkle G, Herrera C, Blum T, Stockinger J, Minners J, Neumann F, Kalusche D. Small or large isolation areas around the pulmonary veins for the treatment of atrial fibrillation? Results from a prospective randomized study. Circulation. 2007;115:3057–3063. doi: 10.1161/CIRCULATIONAHA.107.690578. [DOI] [PubMed] [Google Scholar]
- 19.Haissaguerre M, Hocini M, Shah AJ, Derval N, Sacher F, Jais P, Dubois R. Noninvasive panoramic mapping of human atrial fibrillation mechanisms: A feasibility report. J Cardiovasc Electrophysiol. 2013;24:711–717. doi: 10.1111/jce.12075. [DOI] [PubMed] [Google Scholar]
- 20.Miller JM, Kowal RC, Swarup V, Daubert JP, Daoud EG, Day JD, Ellenbogen KA, Hummel JD, Baykaner T, Krummen DE, Narayan SM, Reddy VY, Shivkumar K, Steinberg JS, Wheelan KR. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: Multicenter firm registry. J Cardiovasc Electrophysiol. 2014;25:921–929. doi: 10.1111/jce.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGann CJ, Kholmovski EG, Oakes RS, Blauer JJ, Daccarett M, Segerson N, Airey KJ, Akoum N, Fish E, Badger TJ, DiBella EV, Parker D, MacLeod RS, Marrouche NF. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J Am Coll Cardiol. 2008;52:1263–1271. doi: 10.1016/j.jacc.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 22.Calvo D, Atienza F, Jalife J, Martinez-Alzamora N, Bravo L, Almendral J, Gonzalez-Torrecilla E, Arenal A, Bermejo J, Fernandez-Aviles F, Berenfeld O. High-rate pacing-induced atrial fibrillation effectively reveals properties of spontaneously occurring paroxysmal atrial fibrillation in humans. Europace. 2012;14:1560–1566. doi: 10.1093/europace/eus180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narayan SM, Krummen DE, Rappel WJ. Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. J Am Coll Cardiol. 2012;23:447–454. doi: 10.1111/j.1540-8167.2012.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.