Abstract
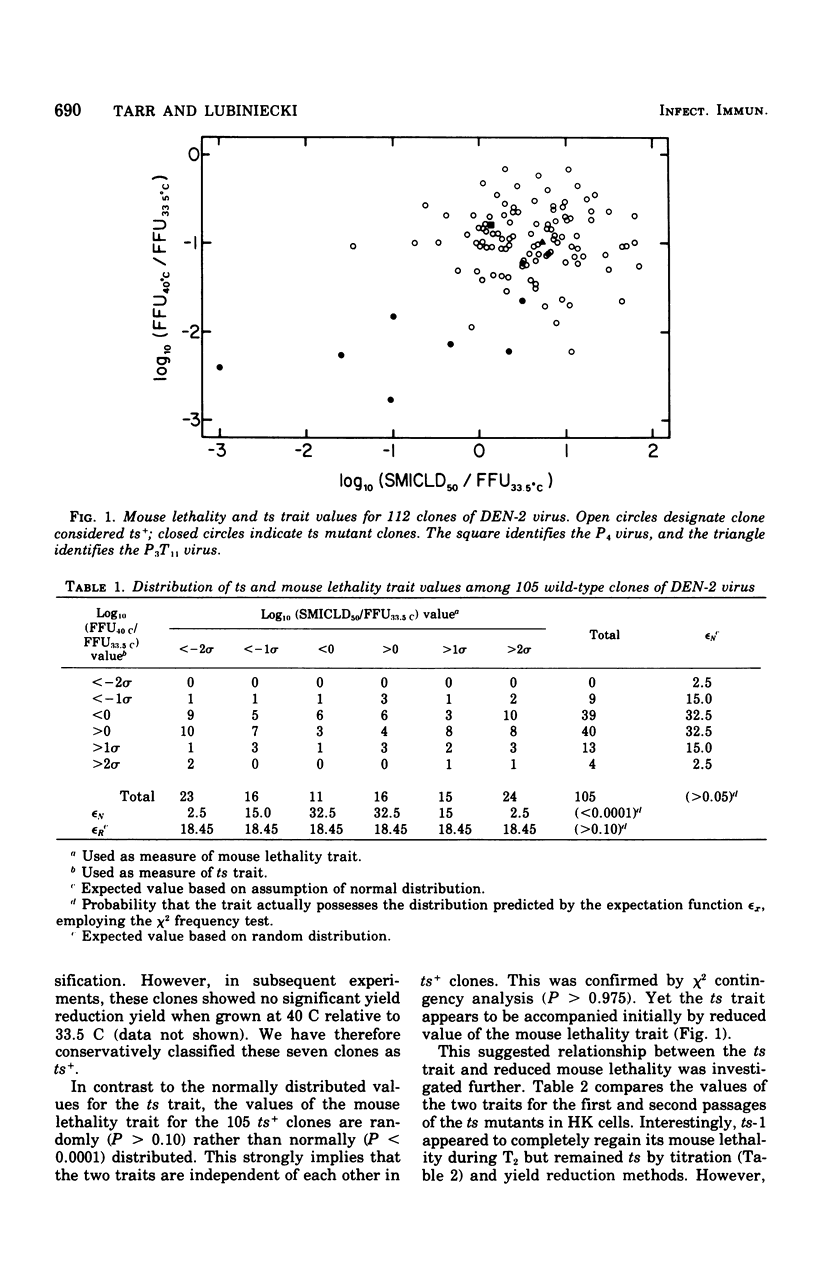
A series of temperature-sensitive (ts) mutants of dengue virus type 2 (DEN-2, TH-36 isolate) were induced by treatment with 5-azacytidine. These mutants and parental viruses were compared for the ts trait and/or attenuation in four systems: primary hamster kidney cells, suckling mice, golden Syrian hamsters, and rhesus monkeys. Seven clones judged to possess the ts trait in virto demonstrated a variety of patterns in vivo. On initial isolation, five of seven ts mutants exhibited reduced mouse lethality. The remaining two mutants possessed parental levels of mouse lethality. In hamsters, neither ts mutant nor parental viruses replicated very well, and then only when inoculated intracerebrally. Studies in rhesus monkeys indicated that all seven ts clones and parental viruses were capable of inducing abtibody responses; however, ts-1 and ts-2 failed to produce detectable viremia. After challenge with parental virus, all vaccinated monkeys demonstrated rapid secondary-type antibody response. Reversion from ts to ts(+) was confirmed to ts-1 in mice and ts-3 in monkeys, and was strongly suspected in several other instances.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R., Tershak D. R. Temperature-sensitive defect of type 2 poliovirus. Virology. 1974 Mar;58(1):209–218. doi: 10.1016/0042-6822(74)90155-x. [DOI] [PubMed] [Google Scholar]
- CHAPIN M., DUBES G. R. Cold-adapted genetic variants of polio viruses. Science. 1956 Sep 28;124(3222):586–587. doi: 10.1126/science.124.3222.586-a. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Koprowski H. Isolation of temperature-sensitive conditional lethal mutants of "fixed" rabies virus. J Virol. 1971 Mar;7(3):295–300. doi: 10.1128/jvi.7.3.295-300.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H. F., Wiktor T. J. Temperature-sensitivity characteristics distinguishing substrains of fixed rabies virus: lack of correlation with plague-size markers or virulence for mice. J Infect Dis. 1972 Jun;125(6):637–646. doi: 10.1093/infdis/125.6.637. [DOI] [PubMed] [Google Scholar]
- DORRANCE W. R., FRANKEL J. W., GORDON I., PATTERSON P. R., SCHLESINGER R. W., WINTER J. W. Clinical and serologic response of man to immunization with attenuated dengue and yellow fever viruses. J Immunol. 1956 Nov;77(5):352–364. [PubMed] [Google Scholar]
- DUBES G. R., WENNER H. A. Virulence of polioviruses in relation to variant characteristics distinguishable on cells in vitro. Virology. 1957 Oct;4(2):275–296. doi: 10.1016/0042-6822(57)90063-6. [DOI] [PubMed] [Google Scholar]
- Ehrenkranz N. J., Ventura A. K., Cuadrado R. R., Pond W. L., Porter J. E. Pandemic dengue in Caribbean countries and the southern United States--past, present and potential problems. N Engl J Med. 1971 Dec 23;285(26):1460–1469. doi: 10.1056/NEJM197112232852606. [DOI] [PubMed] [Google Scholar]
- Fiszman M., Reynier M., Bucchini D., Girard M. Thermosensitive block of the Sabin strain of poliovirus type I. J Virol. 1972 Dec;10(6):1143–1151. doi: 10.1128/jvi.10.6.1143-1151.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghendon Y. Z., Marchenko A. T., Markushin S. G., Ghenkina D. B., Mikhejeva A. V., Rozina E. E. Correlation between TS phenotype and pathogenicity of some animal viruses. Arch Gesamte Virusforsch. 1973;42(2):154–159. doi: 10.1007/BF01270835. [DOI] [PubMed] [Google Scholar]
- Gould D. J., Mount G. A., Scanlon J. E., Sullivan M. F., Winter P. E. Dengue control on an island in the Gulf of Thailand. I. Results of an Aedes aegypti control program. Am J Trop Med Hyg. 1971 Sep;20(5):705–714. doi: 10.4269/ajtmh.1971.20.705. [DOI] [PubMed] [Google Scholar]
- HAMMON W. M., TIGERTT W. D., SATHER G. E., BERGE T. O., MEIKLEJOHN G. Epidemiologic studies of concurrent virgin epidemics of Japanese B encephalitis and of mumps on Guam, 1947-1948, with subsequent observations including dengue, through 1957. Am J Trop Med Hyg. 1958 Jul;7(4):441–467. doi: 10.4269/ajtmh.1958.7.441. [DOI] [PubMed] [Google Scholar]
- HOTTA S. Experimental studies on dengue. I. Isolation, identification and modification of the virus. J Infect Dis. 1952 Jan-Feb;90(1):1–9. doi: 10.1093/infdis/90.1.1. [DOI] [PubMed] [Google Scholar]
- Halstead S. B., Casals J., Shotwell H., Palumbo N. Studies on the immunization of monkeys against dengue. I. Protection derived from single and sequential virus infections. Am J Trop Med Hyg. 1973 May;22(3):365–374. doi: 10.4269/ajtmh.1973.22.365. [DOI] [PubMed] [Google Scholar]
- Halstead S. B., Shotwell H., Casals J. Studies on the pathogenesis of dengue infection in monkeys. I. Clinical laboratory responses to primary infection. J Infect Dis. 1973 Jul;128(1):7–14. doi: 10.1093/infdis/128.1.7. [DOI] [PubMed] [Google Scholar]
- Hammon W. M. Dengue hemorrhagic fever--do we know its cause? Am J Trop Med Hyg. 1973 Jan;22(1):82–91. doi: 10.4269/ajtmh.1973.22.82. [DOI] [PubMed] [Google Scholar]
- Hotta S., Fujita N., Shimazu Y., Yasui Y., Okubo Y. Research on dengue in tissue culture. 3. Immunogenic effect of a tissue-cultured (human-attenuated) type I virus strain. Kobe J Med Sci. 1966 Dec;12(4):199–205. [PubMed] [Google Scholar]
- Hozinski V. I., Seibil V. B., Pantelyeva N. S., Mazurova S. M., Novikova E. A. The rct40 and T50 markers and the characteristics of some variants of measles virus. Acta Virol. 1966 Jan;10(1):20–27. [PubMed] [Google Scholar]
- Kelly R. K., Butel J. S. Demonstration of infectious DNA in transformed cells. II. Characterization of uptake of SV40-transformed mouse cell DNA by simian cells. Arch Virol. 1975;48(4):279–287. doi: 10.1007/BF01317426. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Arrobio J. O., Brandt C. D., Wright P., Hodes D., Chanock R. M., Parrott R. H. Safety and antigenicity of temperature sensitive (TS) mutant respiratory syncytial virus (RSV) in infants and children. Pediatrics. 1973 Jul;52(1):56–63. [PubMed] [Google Scholar]
- Kitayama T., Togo Y., Hornick R. B., Friedwald W. T. Low-Temperature-Adapted Influenza A2/AA/6/60 Virus Vaccine in Man. Infect Immun. 1973 Jan;7(1):119–122. doi: 10.1128/iai.7.1.119-122.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubiniecki A. S., Cypess R. H., Hammon W. M. Passive immunity for arbovirus infection. II. Quantitative aspects of naturally and artificially acquired protection in mice for Japanese (B) encephalitis virus. Am J Trop Med Hyg. 1973 Jul;22(4):535–542. doi: 10.4269/ajtmh.1973.22.535. [DOI] [PubMed] [Google Scholar]
- Lubiniecki A. S., Tarr G. C., Hammon W. M. Cloning of dengue virus type 2 by the direct fluorescent antibody technique. Proc Soc Exp Biol Med. 1973 Oct 1;144(1):70–72. doi: 10.3181/00379727-144-37529. [DOI] [PubMed] [Google Scholar]
- Morales A., Groot H., Russell P. K., McCown J. M. Recovery of dengue-2 virus from Aedes aegypti in Colombia. Am J Trop Med Hyg. 1973 Nov;22(6):785–787. doi: 10.4269/ajtmh.1973.22.785. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Chalhub E. G., Nusinoff S. R., Chanock R. M. Temperature-sensitive mutants of influenza virus. II. Attenuation of ts recombinants for man. J Infect Dis. 1972 Aug;126(2):170–178. doi: 10.1093/infdis/126.2.170. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Hodes D. S., Nusinoff S. R., Spring-Stewart S., Tierney E. L., Chanock R. M. Temperature-sensitive mutants of influenza virus. V. Evaluation in man of an additional ts recombinant virus with a 39 C shutoff temperature. J Infect Dis. 1974 Aug;130(2):144–149. doi: 10.1093/infdis/130.2.144. [DOI] [PubMed] [Google Scholar]
- Plotkin S. A., Farquhar J. D., Katz M., Buser F. Attenuation of RA 27-3 rubella virus in WI-38 human diploid cells. Am J Dis Child. 1969 Aug;118(2):178–185. doi: 10.1001/archpedi.1969.02100040180004. [DOI] [PubMed] [Google Scholar]
- Price W. H., Thind I. S., O'Leary W. The attenuation of the 26th mouse brain passage of New Guinea C strain of dengue 2 virus for use in the sequential immunization procedure against group B arboviruses. Am J Trop Med Hyg. 1973 Jan;22(1):92–99. doi: 10.4269/ajtmh.1973.22.92. [DOI] [PubMed] [Google Scholar]
- RHIM J. S., HAMMON W. M. Quantitative studies of Japanese B encephalitis virus in hamster kidney cell cultures. Proc Soc Exp Biol Med. 1963 Feb;112:419–424. doi: 10.3181/00379727-112-28063. [DOI] [PubMed] [Google Scholar]
- SABIN A. B. Research on dengue during World War II. Am J Trop Med Hyg. 1952 Jan;1(1):30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- Sabin A. B., Schlesinger R. W. PRODUCTION OF IMMUNITY TO DENGUE WITH VIRUS MODIFIED BY PROPAGATION IN MICE. Science. 1945 Jun 22;101(2634):640–642. doi: 10.1126/science.101.2634.640. [DOI] [PubMed] [Google Scholar]
- Scherer W. F., Breakenridge F. A., Dickerman R. W. Cross-protection studies and search for subclinical disease in new world monkeys infected sequentially with different immunologic types of Dengue viruses. Am J Epidemiol. 1972 Jan;95(1):67–79. doi: 10.1093/oxfordjournals.aje.a121372. [DOI] [PubMed] [Google Scholar]
- Schluter B., Bellomy B., Brown A. Pathogenesis of temperature-sensitive mutants of sindbis virus in the embryonated egg. I. Characterization and kinetics of viral multiplication. Infect Immun. 1974 Jan;9(1):68–75. doi: 10.1128/iai.9.1.68-75.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter B., Brown A. Pathogenesis of temperature-sensitive mutants of sindbis virus in the embryonated egg. II. Control of the infectious process. Infect Immun. 1974 Jan;9(1):76–80. doi: 10.1128/iai.9.1.76-80.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simizu B., Takayama N. Relationship between neurovirulence and temperature sensitivity of an attenuated western equine encephalitis virus. Arch Gesamte Virusforsch. 1971;35(2):242–250. doi: 10.1007/BF01249716. [DOI] [PubMed] [Google Scholar]
- Simizu B., Takayama N. Virulence of a temperature-sensitive mutant of western equine encephalitis virus. Arch Gesamte Virusforsch. 1972;38(4):328–337. doi: 10.1007/BF01262823. [DOI] [PubMed] [Google Scholar]
- Tarr G. C., Hammon W. M. Cross-protection between group B arboviruses: resistance in mice to Japanese B encephalitis and St. Louis encephalitis viruses induced by Dengue virus immunization. Infect Immun. 1974 May;9(5):909–915. doi: 10.1128/iai.9.5.909-915.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R. Pathogenicity and immunogenicity for mice of temperature-sensitive mutants of vesicular stomatitis virus. Infect Immun. 1974 Aug;10(2):309–315. doi: 10.1128/iai.10.2.309-315.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead R. H., Chaicumpa V., Olson L. C., Russell P. K. Sequential dengue virus infections in the white-handed gibbon (Hylobates lar). Am J Trop Med Hyg. 1970 Jan;19(1):94–102. doi: 10.4269/ajtmh.1970.19.94. [DOI] [PubMed] [Google Scholar]


