Abstract
Fungi are the known sources of irritation associated with atopic diseases (e.g., asthma, allergic rhinoconjunctivitis, and atopic eczema). To quantitatively estimate their presence in the indoor environment of atopic dermatitis-inflicted child patient's houses (ADCPHs), the high-efficiency particulate air (HEPA) filters installed inside the air cleaners of three different ADCPHs were investigated for the presence of mold. The air cleaner HEPA filters obtained from the three different ADCPHs were coded as HEPA-A, -B, and -C, respectively, and tested for the presence of mold. The colony forming units (CFUs) corresponding to the HEPA-A, -B, and -C filters were estimated to be 6.51 × 102 ± 1.50 × 102 CFU/cm2, 8.72 × 102 ± 1.69 × 102 CFU/cm2, and 9.71 × 102 ± 1.35 × 102 CFU/cm2, respectively. Aspergillus, Penicillium, Alternaria, Cladosporium, Trichoderma, and other fungal groups were detected in the 2,494 isolates. The distribution of these fungal groups differed among the three filters. Cladosporium was the major fungal group in filters HEPA-A and -C, whereas Penicillium was the major fungal group in the filter HEPA-B. Nine fungal species, including some of the known allergenic species, were identified in these isolates. Cladosporium cladosporioides was the most common mold among all the three filters. This is the first report on the presence of fungi in the air cleaner HEPA filters from ADCPHs in Korea.
Keywords: Atopic dermatitis, Fungi, High-efficiency particulate air filters, Indoor air
Atopic dermatitis (AD) is an inflammatory, chronically relapsing, and non-contagious pruritic skin disease. It is one of the most common allergic diseases in children. AD usually manifests itself during early infancy and childhood, but can persist up to adulthood or even after that [1]. Although AD has been correlated with hereditary factors, the rapidly growing incidence of AD in recent years cannot be explained by genetic factors alone. The mechanisms for the development of AD involve complex interactions between the susceptible genes and the environment. The role of indoor environment in the development of AD has been studied. Indoor air comprises carpet and building material, particles from cleaning solutions, tobacco smoke, particles generated from cooking and heating, as well as biological contaminants such as dust mites, animal allergens, and microorganisms [2]. Microbial contamination has become a major health issue. Although the indoor environment, where we spend long hours every day, is very important for our health, there have been few studies highlighting the relationship between household fungal contamination and airborne fungal contamination [3]. Molds are one of the most notorious bioaerosols that adversely impact the indoor air quality (IAQ) [4]. They have been known as allergens, pathogens, irritants, and toxigenic agents.
Airborne fungi are universally present and show highly variable occurrence. They are among the most common bioaerosols that humans inhale. Some fungal allergens are present in viable and nonviable conidia, hyphae, and fungal fragments [5]. The increased production of allergens by conidia during germination [6] and the exposure to smaller fungal fragments are largely responsible for the development of fungal allergy and the associated clinical outcomes [7]. The antigenic epitopes of several filamentous fungi such as Alternaria, Cladosporium, Aspergillus, and Penicillium have been characterized to varying degrees, and their role in the pathogenesis of allergic respiratory diseases have also been studied [8, 9]. As with other allergens, mold sensitization develops in genetically predisposed individuals in response to recurrent or chronic environmental exposure [10]. For this reason, many facilities use air cleaners to maintain good IAQ. Air cleaners are used inside offices and houses to maintain a comfortable indoor environment, but are often found to be contaminated with fungi. These air cleaners that disperse spores into the indoor environment with their strong air currents also cause fungal contamination and malodor. Similarly, fungal contamination has also been associated with the high-efficiency particulate air (HEPA) filters found inside air conditioners [11]. The recent increase in IAQ-related issues inside atopic dermatitis-inflicted child patient's houses (ADCPHs) has raised concerns. However, information pertaining to the associated fungi is not currently available in Korea. To generate information on the fungal contamination of ADCPHs, we have systematically examined the molds that are filtered by the air cleaner HEPA filters.
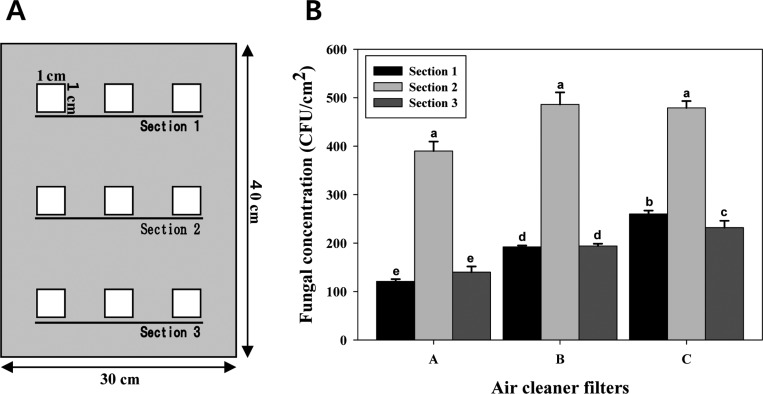
For isolating the fungi, we sampled three air cleaner HEPA filters from three ADCPHs located in Seoul and Youngin in Gyeonggi-do (Table 1). Air cleaner filters that had been used for 6 to 12 mon were sampled on January 16, 2012. During the sampling of the air cleaner filters, the air temperature and the relative humidity inside and outside the room where the air cleaner was placed (inside the ADCPH) were measured. The indoor humidity ranged from 38% to 42%, and the outdoor humidity ranged from 31% to 36% (Table 1). The indoor temperature ranged from 22℃ to 26℃, and the outdoor temperature ranged from 8℃ to 15℃ (Table 1). Both the temperature and humidity data showed that although it was winter, the indoor environmental condition was favorable to the growth of fungi inside the ADCPHs. Fungal sampling was performed at nine sites in three divided sections (top, middle, and bottom) of each HEPA filter with an area of 120 cm2 (Fig. 1A). For fungal isolation, a square patch (1 × 1 cm) was cut from the nine sampling sites of each HEPA filter. This patch was further fragmented into several pieces that were separately transferred to 50-mL sterile plastic tubes containing distilled water supplemented with 0.02% Tween 80. Each of the plastic tubes was vigorously vortexed for 1 min and centrifuged at 600 ×g. One hundred microliters of the supernatant from each of the plastic tubes was spread onto three potato dextrose agar (PDA) plates supplemented with ampicillin (100 µg/mL). These plates were incubated at 25℃ for 5~7 days. The number of colony forming units (CFUs) of the fungi was determined using a colony counter. Fungal CFUs were counted from the nine sites of each HEPA filter. A total of 2,494 colonies were obtained from the three filters. The mean ± standard deviation of fungal CFUs of the HEPA-A, -B and -C filters were equal to 6.51 × 102 ± 1.50 × 102 CFU/cm2, 8.72 × 102 ± 1.69 × 102 CFU/cm2, and 9.71 × 102 ± 1.35 × 102 CFU/cm2, respectively (Fig. 1B). No significant differences were observed in the fungal CFUs between sections 1 and 3 in the HEPA-A and -B filters. The number of fungal CFUs was slightly higher in section 3 than in section 1 in the HEPA-C filter, and the number of fungal CFUs for the middle section of each HEPA filter was consistently higher than that of the two other sections (p < 0.01) (Fig. 1). These results showed that the fungi inhabited the middle section of the three air cleaner HEPA filters more than the other two sections. Considering that more air passes through the middle section of air cleaner filters, we assumed that fungal spores would be filtered more by the middle section than by the other two sections. These results show that the population of the filtered fungi was not evenly distributed in the air cleaner. Commercial air cleaners are installed with HEPA filters of various sizes. Thus, it remains to be determined whether the fungi in the HEPA filters installed in the various air cleaners are distributed evenly.
Table 1.
Information on the air cleaner HEPA filter samples used in this study
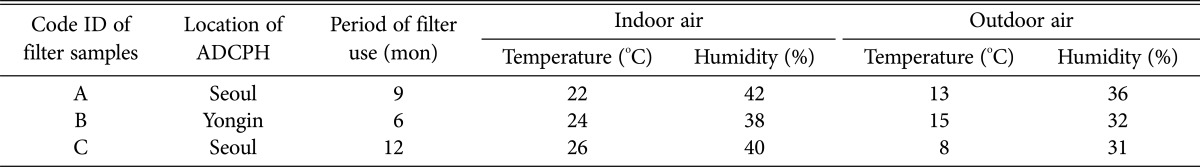
The indoor/outdoor temperature and humidity of the ADCPHs were measured in January 2012.
HEPA, high-efficiency particulate air; ADCPH, atopic dermatitis-inflicted child patient's house.
Fig. 1.
Sampling sites for fungal isolation (A) and fungal population (B) on the air cleaner high-efficiency particulate air (HEPA) filters obtained from the three atopic dermatitis-inflicted child patient's houses. Sections 1, 2, and 3 indicate the top, middle, and bottom areas of the HEPA filter used for fungal isolation. The mean separation was calculated using Duncan's multiple range test, with p < 0.05. The presence of identical letters above the bars implies no significant differences in the fungal population among the sampled sections of the HEPA filters. CFU, colony forming unit.
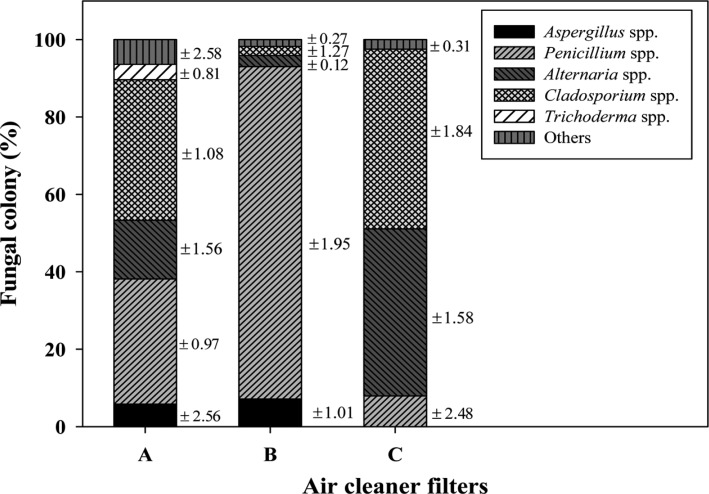
The 2,494 fungal colonies obtained were divided into six groups based on the morphological characteristics of the colonies, including their color, appearance, and diameters, as well as on the microscopic observations of microstructures (e.g., the presence of conidiophores and conidial shapes) using a phase-contrast microscope (Axioskop 40; Carl Zeiss, Jena, Germany) and a dissecting microscope (SZ2-ILST; Olympus, Tokyo, Japan). The six groups were divided into Alternaria [12], Aspergillus [13], Cladosporium [14], Penicillium [15], Trichoderma [16, 17, 18], and other genera comprising several fungal species. The distribution of these six fungal groups (Aspergillus spp., Penicillium spp., Alternaria spp., Cladosporium spp., and Trichoderma spp.) on the three HEPA filters was calculated based on the macroscopic and microscopic characters. The distribution of each group differed among the three filters (Fig. 2). Cladosporium (36 ± 1.08% and 46 ± 1.84%) was the major fungal group on the HEPA-A and -C filters, whereas Penicillium (85 ± 1.95%) was the major fungal group on the HEPA-B filter. Alternaria, Cladosporium, and Penicillium were commonly present on all three HEPA filters. Trichoderma was present only on the HEPA-A filter. Aspergillus was detected on the HEPA-A and -B filters, but not on the HEPA-C filter. These results clearly show that the fungal distribution on the HEPA filter varies depending on the environment. It implies that the distribution of the fungi in the indoor air also differs among the three ADCPHs. Therefore, we suggest that investigating the air cleaner filters should be an alternate approach to understanding the IAQ of a house with AD patients.
Fig. 2.
Comparison among the five major varieties of mold present in the three air cleaner filters used inside the three atopic dermatitis-inflicted child patient's houses. The mean number of colony forming units of each mold is indicated above each column. The SD values corresponding to the percentage of fungal colonies is indicated on the right side of each column.
Currently, sparse information is available on the fungal species detected on HEPA filters installed in air cleaners. During this study, we identified the most dominant fungal colony types present on each of the HEPA filters. Twenty-two dominant fungal colonies belonging to each group from each of the HEPA filters were transferred for pure culture on PDA supplemented with ampicillin (100 µg/mL). The pure-cultured fungal isolates were identified at the species level by observing the microstructure and by sequencing the internal transcribed spacer (ITS) region and the translation elongation factor 1 alpha (tef-1α) gene. The genomic DNA preparation and the polymerase chain reaction (PCR) analysis were performed as described previously [19]. The ITS region was PCR-amplified with the ITS1 (5'-TCCGTAGGTGAACCTGCG-3') and ITS4 (5'-TCCTCCGCTTATTGATATGC-3') primer pair [20]. For the Alternaria, Cladosporium, Fusarium, and Trichoderma isolates, the tef-1α gene was PCR-amplified with the TEF728 (5'-CATCGAGAAGTTCGAGAAGG-3') and TEF1 (5'-GCCATCCTTGGAGATACC AGC-3') primer set [21, 22]. DNA sequencing was performed at Macrogen (Seoul, Korea). A BLAST search of the GenBank database (http://www.ncbi.nlm.nih.gov/genbank/) using the determined nucleotide sequences revealed that these sequences showed a 99% to 100% sequence identity with those of the known fungal species (Table 2). Nine kinds of fungal species were identified from some of the dominant isolates of the major groups obtained from the three HEPA filters in the ADCPHs (Table 2, Fig. 3). The ITS rDNA and tef-1α gene sequences of all the isolates determined in this study were deposited in the GenBank DNA database (Table 2). All the species identified in this study were deposited in the Dankook University Culture Collection (DUCC), Cheonan, Korea. Alternaria alternata, Aspergillus cf. niger, Cladosporium cladosporioides, Fusarium pseudonygamai, Rhizopus oryzae, and Trichoderma viride were detected in HEPA filter-A, whereas A. alternata, A. cf. niger, C. cladosporioides, Penicillium cf. chrysogenum, and Rhizopus arrhizus were detected in HEPA filter-B. C. cladosporioides, Fusarium proliferatum, P. cf. chrysogenum, and R. oryzae were detected in HEPA filter-C. C. cladosporioides was commonly detected in all three HEPA filters mentioned above. Fungi belonging to one of the identified species, A. cf. niger, have been known to produce β-xylosidase, an occupational allergen [23], whereas those belonging to A. alternata are the common irritant species found in asthma patients. Epidemiological studies from a variety of locations worldwide indicate that Alternaria sensitivity is closely related to the development of asthma [24]. In addition, up to 70% of the mold-allergic patients showed a skin test reactivity to Alternaria [25]. A vacuolar serine protease has been identified as a major allergen in C. cladosporioides [26]. In addition, three P. cf. chrysogenum proteins have been recently identified as allergens by examining their reactivity with the IgE antibodies present in the sera of asthma patients [27]. According to the previously reported information, the identified dominant fungal species listed in Table 2 are characterized as allergenic, and exposure to their spores can provoke adverse health effects in susceptible individuals, including children with AD. Therefore, it is necessary to properly manage the filters installed inside the air cleaners in ADCPHs.
Table 2.
Identification of the dominant fungal isolates from the air cleaner HEPA filters by molecular analysis of the ITS rDNA or tef-1α gene sequences
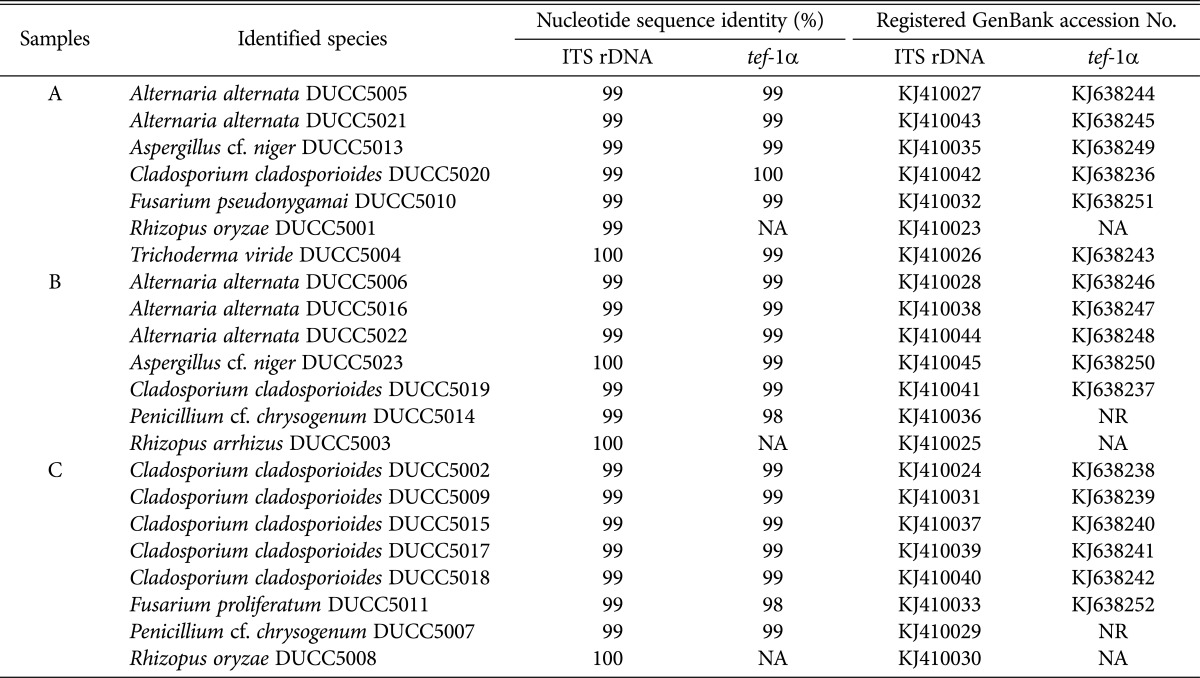
HEPA, high-efficiency particulate air; ITS, internal transcribed spacer; NA, not analyzed; NR, not registered.
Fig. 3.
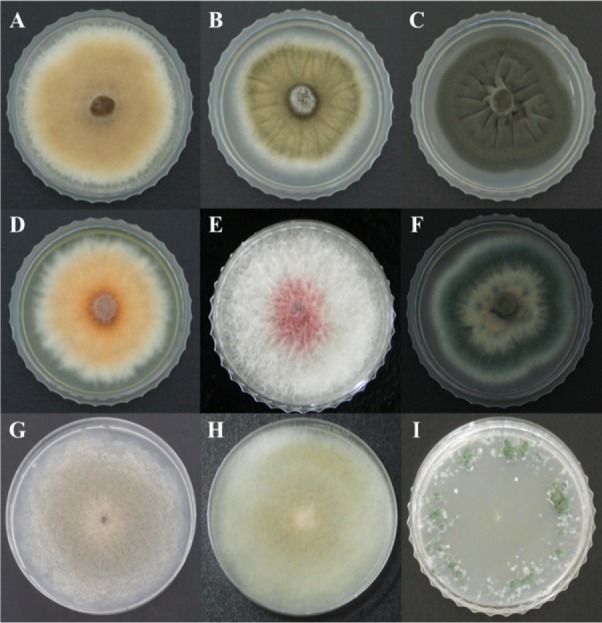
Colony morphology of the nine fungal species detected in the air cleaner high-efficiency particulate air filters obtained from the three atopic dermatitis-inflicted child patient's houses. A, Alternaria alternata DUCC5005; B, Aspergillus cf. niger DUCC5013; C, Cladosporium cladosporioides DUCC5020; D, Fusarium proliferatum DUCC5011; E, Fusarium pseudonygamai DUCC5010; F, Penicillium cf. chrysogenum DUCC5014; G, Rhizopus arrhizus DUCC5003; H, Rhizopus oryzae DUCC5001; and I, Trichoderma viride DUCC5004.
Overall, our work on air cleaner HEPA filters allowed us to identify the allergenic fungal species present in the indoor environment of ADCPHs. This is presumably the first report identifying the molds present on air cleaner HEPA filters in Korea. The fungi filtered on the HEPA filters are expected to be in a stressful condition, considering the fact that these filters are always subject to receiving air wind from the air cleaners. Thus, it could be possible that air wind stress has an effect on the survival of filtered fungi. Consequently, it is expected that a larger number of fungal species and/or spores would be present in the indoor environment than on the air cleaner filters. To better identify the fungal contaminants present in the indoor air of ADCPHs, further studies on the diversity of these airborne fungi need to be conducted.
ACKNOWLEDGEMENTS
This study was supported by the project on survey and excavation of Korean indigenous species of National Institute of Biological Resources under the Ministry of Environment, and by the Converging Research Center Program through the Ministry of Science, ICT, and Future Planning (2013K000393), Korea.
References
- 1.Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112(6 Suppl):S118–S127. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 2.Jones AP. Indoor air quality and health. Atmos Environ. 1999;33:4535–4564. [Google Scholar]
- 3.Miller JD. Fungi as contaminants in indoor air. Atmos Environ A Gen Top. 1992;26:2163–2172. [Google Scholar]
- 4.Rao CY, Burge HA, Chang JC. Review of quantitative standards and guidelines for fungi in indoor air. J Air Waste Manag Assoc. 1996;46:899–908. doi: 10.1080/10473289.1996.10467526. [DOI] [PubMed] [Google Scholar]
- 5.Mitakakis TZ, Barnes C, Tovey ER. Spore germination increases allergen release from Alternaria. J Allergy Clin Immunol. 2001;107:388–390. doi: 10.1067/mai.2001.112602. [DOI] [PubMed] [Google Scholar]
- 6.Green BJ, Mitakakis TZ, Tovey ER. Allergen detection from 11 fungal species before and after germination. J Allergy Clin Immunol. 2003;111:285–289. doi: 10.1067/mai.2003.57. [DOI] [PubMed] [Google Scholar]
- 7.Green BJ, Sercombe JK, Tovey ER. Fungal fragments and undocumented conidia function as new aeroallergen sources. J Allergy Clin Immunol. 2005;115:1043–1048. doi: 10.1016/j.jaci.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. 2004;113:227–234. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Bush RK, Portnoy JM. The role and abatement of fungal allergens in allergic diseases. J Allergy Clin Immunol. 2001;107(3 Suppl):S430–S440. doi: 10.1067/mai.2001.113669. [DOI] [PubMed] [Google Scholar]
- 10.Bobbitt RC, Jr, Crandall MS, Venkataraman A, Bernstein JA. Characterization of a population presenting with suspected mold-related health effects. Ann Allergy Asthma Immunol. 2005;94:39–44. doi: 10.1016/S1081-1206(10)61283-5. [DOI] [PubMed] [Google Scholar]
- 11.Hamada N, Fujita T. Effect of air-conditioner on fungal contamination. Atmos Environ. 2002;36:5443–5448. [Google Scholar]
- 12.Woudenberg JH, Groenewald JZ, Binder M, Crous PW. Alternaria redefined. Stud Mycol. 2013;75:171–212. doi: 10.3114/sim0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varga J, Frisvad JC, Kocsubé S, Brankovics B, Tóth B, Szigeti G, Samson RA. New and revisited species in Aspergillus section Nigri. Stud Mycol. 2011;69:1–17. doi: 10.3114/sim.2011.69.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bensch K, Braun U, Groenewald JZ, Crous PW. The genus Cladosporium. Stud Mycol. 2012;72:1–401. doi: 10.3114/sim0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samson RA, Frisvad JC. Penicillium subgenus Penicillium: new taxonomic schemes, mycotoxins and other extrolites. Stud Mycol. 2004;49:1–260. [Google Scholar]
- 16.Bissett J. A revision of the genus Trichoderma I. Section Longibrachiatum sect. nov. Can J Bot. 1984;62:924–931. [Google Scholar]
- 17.Bissett J. A revision of the genus Trichoderma. II. Infrageneric classification. Can J Bot. 1991;69:2357–2372. [Google Scholar]
- 18.Bissett J. A revision of the genus Trichoderma. III. Section Pachybasium. Can J Bot. 1991;69:2373–2417. [Google Scholar]
- 19.Tang L, Hyun MW, Yun YH, Sun DY, Kim SH, Sung GH. New record of Mariannaea elegans var. elegans in Korea. Mycobiology. 2012;40:14–19. doi: 10.5941/MYCO.2012.40.1.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego: Academic Press; 1990. pp. 315–322. [Google Scholar]
- 21.Carbone I, Kohn LM. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. [Google Scholar]
- 22.Samuels GJ, Dodd SL, Gams W, Castlebury LA, Petrini O. Trichoderma species associated with the green mold epidemic of commercially grown Agaricus bisporus. Mycologia. 2002;94:146–170. [PubMed] [Google Scholar]
- 23.Sander I, Raulf-Heimsoth M, Siethoff C, Lohaus C, Meyer HE, Baur X. Allergy to Aspergillus-derived enzymes in the baking industry: identification of β-xylosidase from Aspergillus niger as a new allergen (Asp n 14) J Allergy Clin Immunol. 1998;102:256–264. doi: 10.1016/s0091-6749(98)70109-5. [DOI] [PubMed] [Google Scholar]
- 24.Halonen M, Stern DA, Wright AL, Taussig LM, Martinez FD. Alternaria as a major allergen for asthma in children raised in a desert environment. Am J Respir Crit Care Med. 1997;155:1356–1361. doi: 10.1164/ajrccm.155.4.9105079. [DOI] [PubMed] [Google Scholar]
- 25.Schonwald P. Allergenic molds in the Pacific Northwest. J Allergy. 1938;9:175–179. [Google Scholar]
- 26.Chou H, Tam MF, Lee LH, Chiang CH, Tai HY, Panzani RC, Shen HD. Vacuolar serine protease is a major allergen of Cladosporium cladosporioides. Int Arch Allergy Immunol. 2008;146:277–286. doi: 10.1159/000121462. [DOI] [PubMed] [Google Scholar]
- 27.Shen HD, Chou H, Tam MF, Chang CY, Lai HY, Wang SR. Molecular and immunological characterization of Pen ch 18, the vacuolar serine protease major allergen of Penicillium chrysogenum. Allergy. 2003;58:993–1002. doi: 10.1034/j.1398-9995.2003.00107.x. [DOI] [PubMed] [Google Scholar]