Abstract
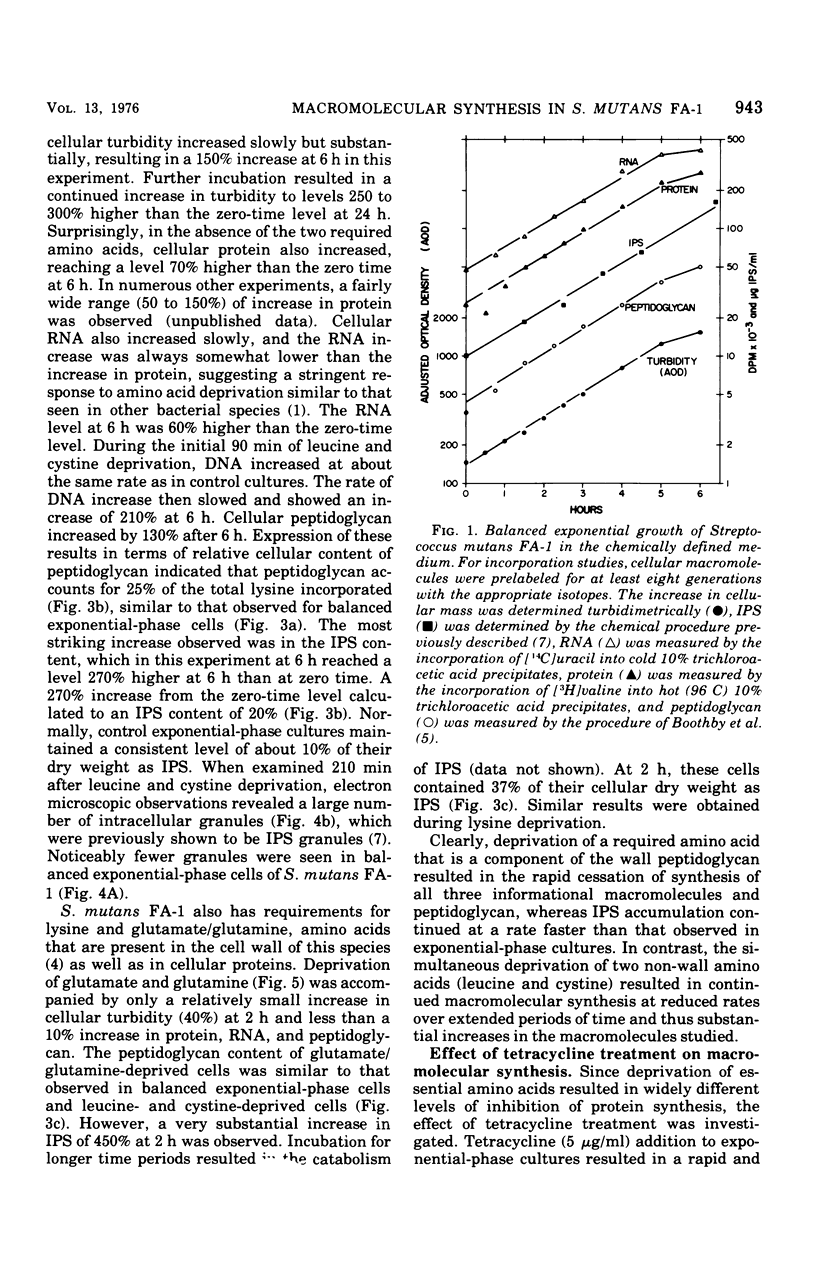
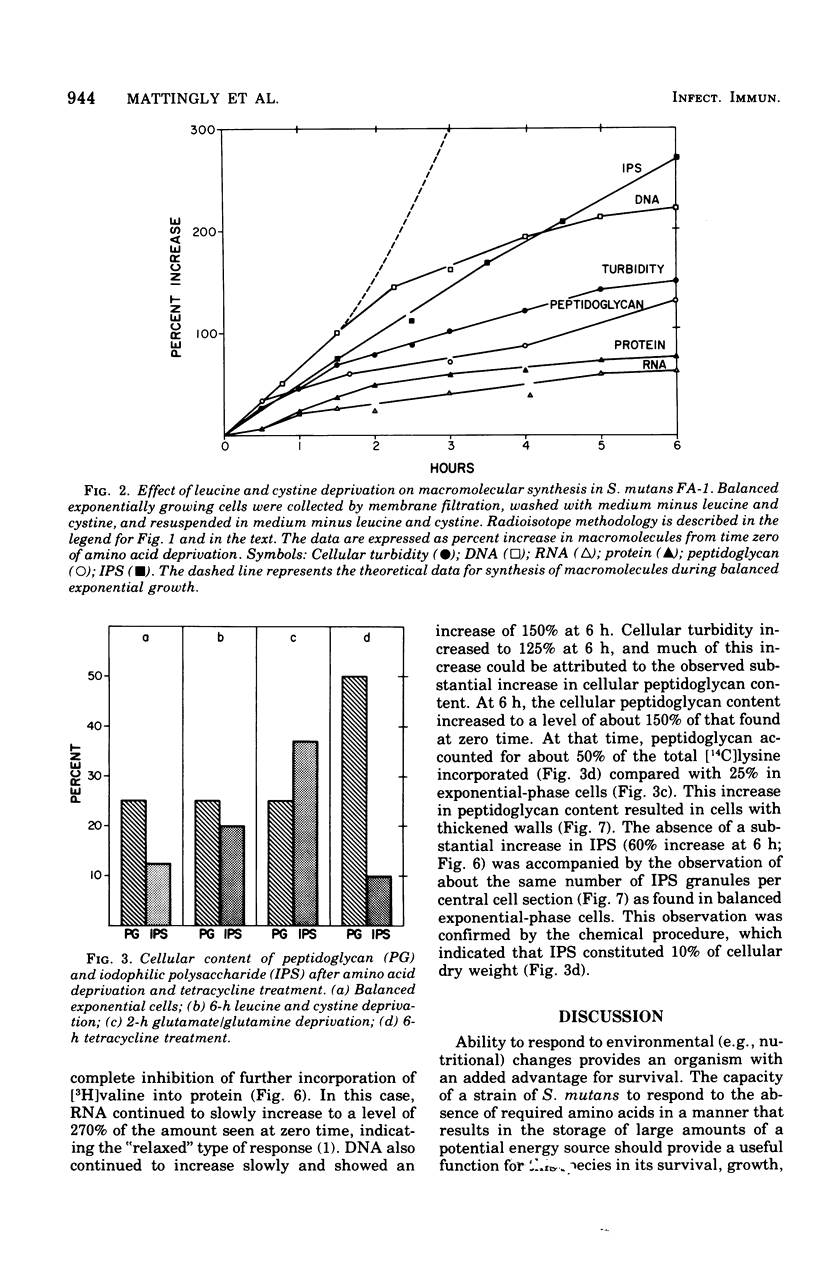
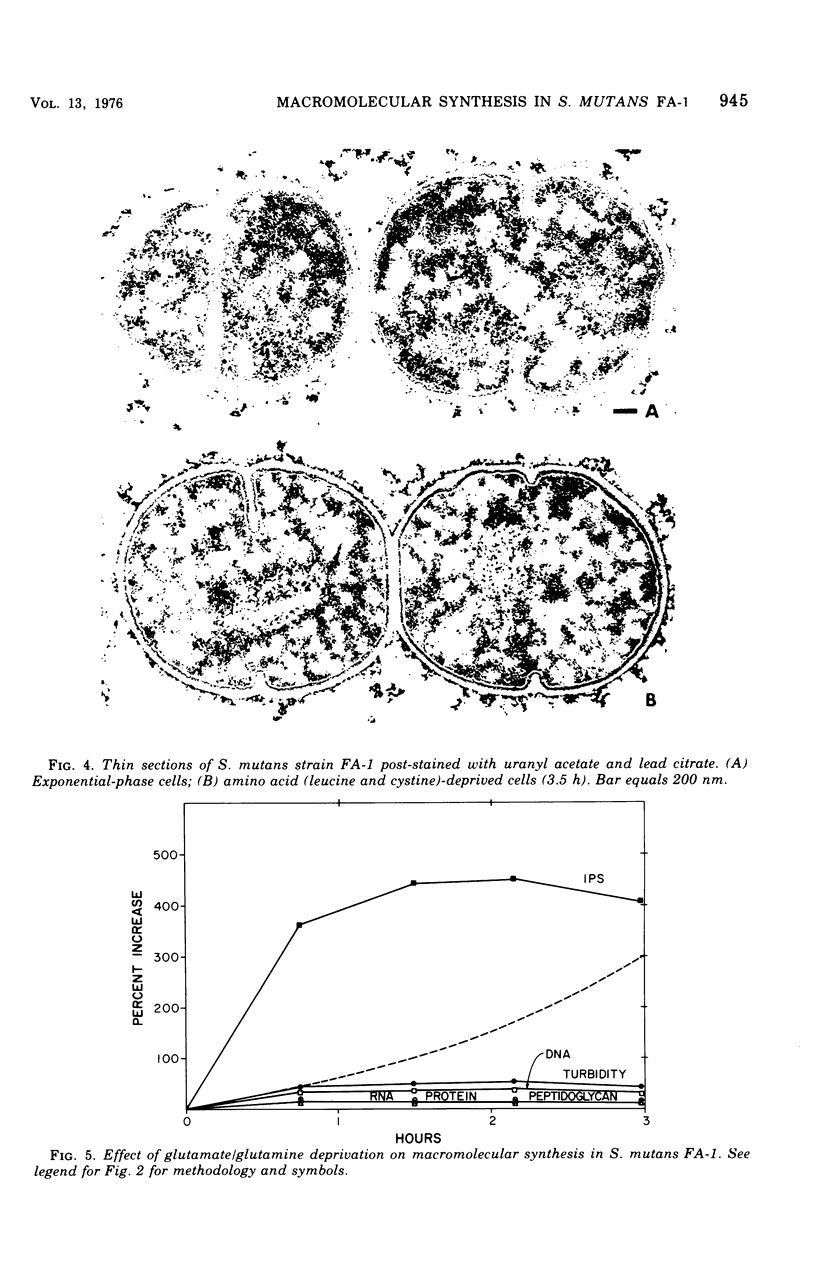
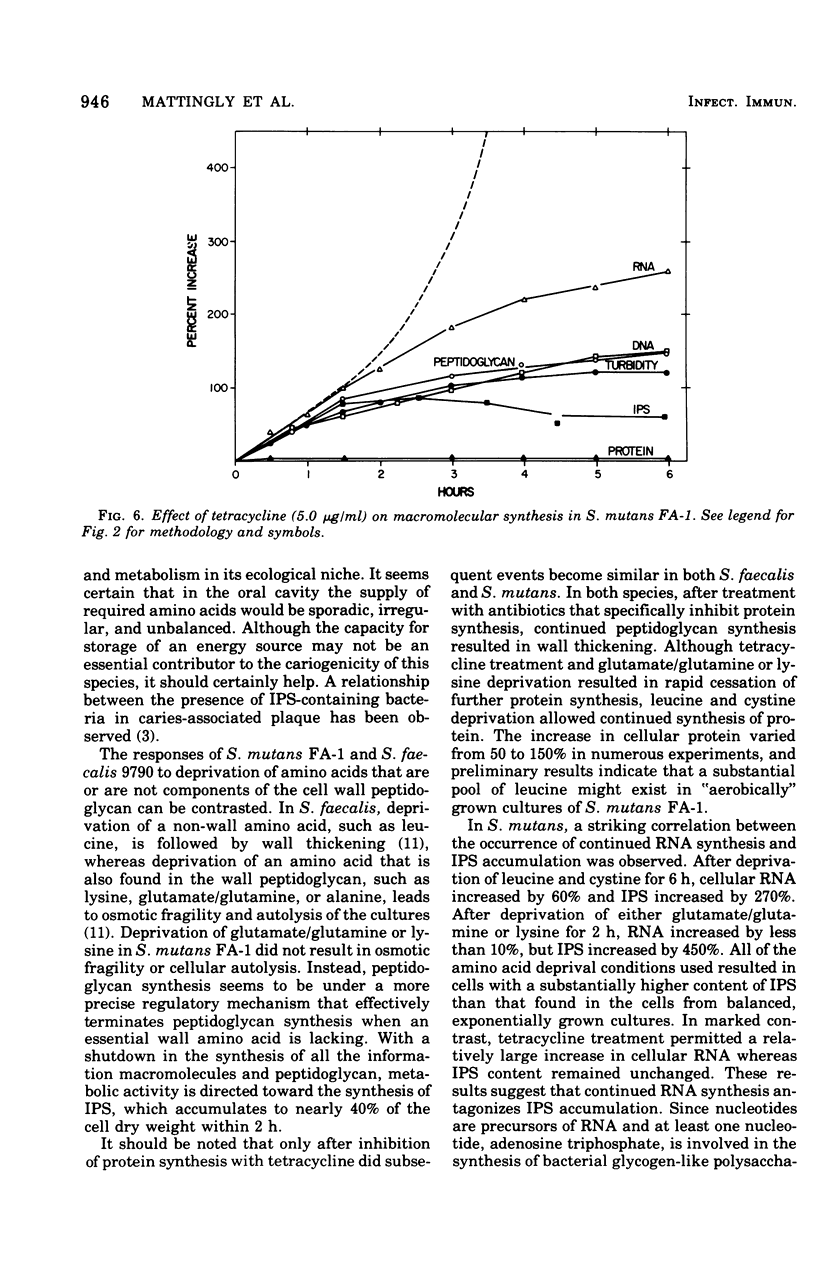
The continued synthesis of deoxyribonucleic acid, protein, cell wall peptidoglycan and intracellular iodophilic polysaccharide (IPS) by Streptococcus mutans strain FA-1 after several treatments intended to inhibit protein synthesis was studied. Exponential-phase cultures were: (i) simultaneously deprived of two required amino acids (cystine and leucine) that are not present in the cell wall peptidoglycan of this species; (ii) depreived of required amino acids (lysine or glutamate plus glutamine ) that are present in both peptidoglycan and protein; or (iii) treated with tetracycline. Each of these three types of treatment was accompanied by a different pattern of unbalanced growth. The patterns of unbalanced growth that accompanied treatments (i) or (ii) differed substantially from the patterns observed previously for Streptococcus faecalis ATCC 9790, a noncariogenic organism that does not contain IPS. In contrast to S. faecalis 9790, S. mutans FA-1 failed to accumulate peptidoglycan and thicken its wall when deprived of non-wall amino acids. Instead, S. mutans FA-1 continued to accumulate IPS to levels substantially higher than those found in exponential-phase cells. Again, in contrast to S. faecalis, S. mutans FA-1 failed to autolyze upon deprivation of essential precursors of wall peptidoglycan. Under conditions of lysine of glutamate/glutamine deprivation, S. mutans FA-1 continued to accumulate IPS to very high levels. Treatment with tetracycline did result in peptidoglycan accumulation and wall thickening in a manner very similar to that observed previously for inhibition of protein synthesis in S. faecalis. Realtively little IPS synthesis continued after tetracycline treatment. Accumulation of IPS appeared to occur when both ribonucleic acid and peptidoglycan synthesis were severely inhibited. The observations are discussed in terms of the survival of cariogenic organisms in the oral environment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALFOLDI L., STENT G. S., CLOWES R. C. The chromosomal site of the RNA control (RC) locus in Escherichia coli. J Mol Biol. 1962 Sep;5:348–355. doi: 10.1016/s0022-2836(62)80077-1. [DOI] [PubMed] [Google Scholar]
- BATTISTONE G. C., BURNETT G. W. The free amino acid composition of human saliva. Arch Oral Biol. 1961 Apr;3:161–170. doi: 10.1016/0003-9969(61)90133-9. [DOI] [PubMed] [Google Scholar]
- Berman K. S., Gibbons R. J. Iodophilic polysaccharide synthesis by human and rodent oral bacteria. Arch Oral Biol. 1966 May;11(5):533–542. doi: 10.1016/0003-9969(66)90160-9. [DOI] [PubMed] [Google Scholar]
- Bleiweis A. S., Craig R. A., Zinner D. D., Jablon J. M. Chemical composition of purified cell walls of cariogenic streptococci. Infect Immun. 1971 Jan;3(1):189–191. doi: 10.1128/iai.3.1.189-191.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothby D., Daneo-Moore L., Shockman G. D. A rapid, guantitative, and selective estimation of radioactively labeled peptidoglycan in gram-positive bacteria. Anal Biochem. 1971 Dec;44(2):645–653. doi: 10.1016/0003-2697(71)90255-7. [DOI] [PubMed] [Google Scholar]
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- DiPersio J. R., Mattingly S. J., Higgins M. L., Shockman G. D. Measurement of intracellular iodophilic polysaccharide in two cariogenic strains of Streptococcus mutans by cytochemical and chemical methods. Infect Immun. 1974 Sep;10(3):597–604. doi: 10.1128/iai.10.3.597-604.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryefus P. M., Levy H. L., Efron M. L. Concerning amino acids in human saliva. Experientia. 1968 May 15;24(5):447–448. doi: 10.1007/BF02144382. [DOI] [PubMed] [Google Scholar]
- Roth G. S., Shockman G. D., Daneo-Moore L. Balanced macromolecular biosynthesis in "protoplasts" of Streptococcus faecalis. J Bacteriol. 1971 Mar;105(3):710–717. doi: 10.1128/jb.105.3.710-717.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayare M., Daneo-Moore L., Shockman G. D. Influence of macromolecular biosynthesis on cellular autolysis in Streptococcus faecalis. J Bacteriol. 1972 Oct;112(1):337–344. doi: 10.1128/jb.112.1.337-344.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D. Symposium on the fine structure and replication of bacteria and their parts. IV. Unbalanced cell-wall synthesis: autolysis and cell-wall thickening. Bacteriol Rev. 1965 Sep;29(3):345–358. doi: 10.1128/br.29.3.345-358.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Shockman G. D. Amino acid requirements of Streptococcus mutans and other oral streptococci. Infect Immun. 1975 Apr;11(4):656–664. doi: 10.1128/iai.11.4.656-664.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
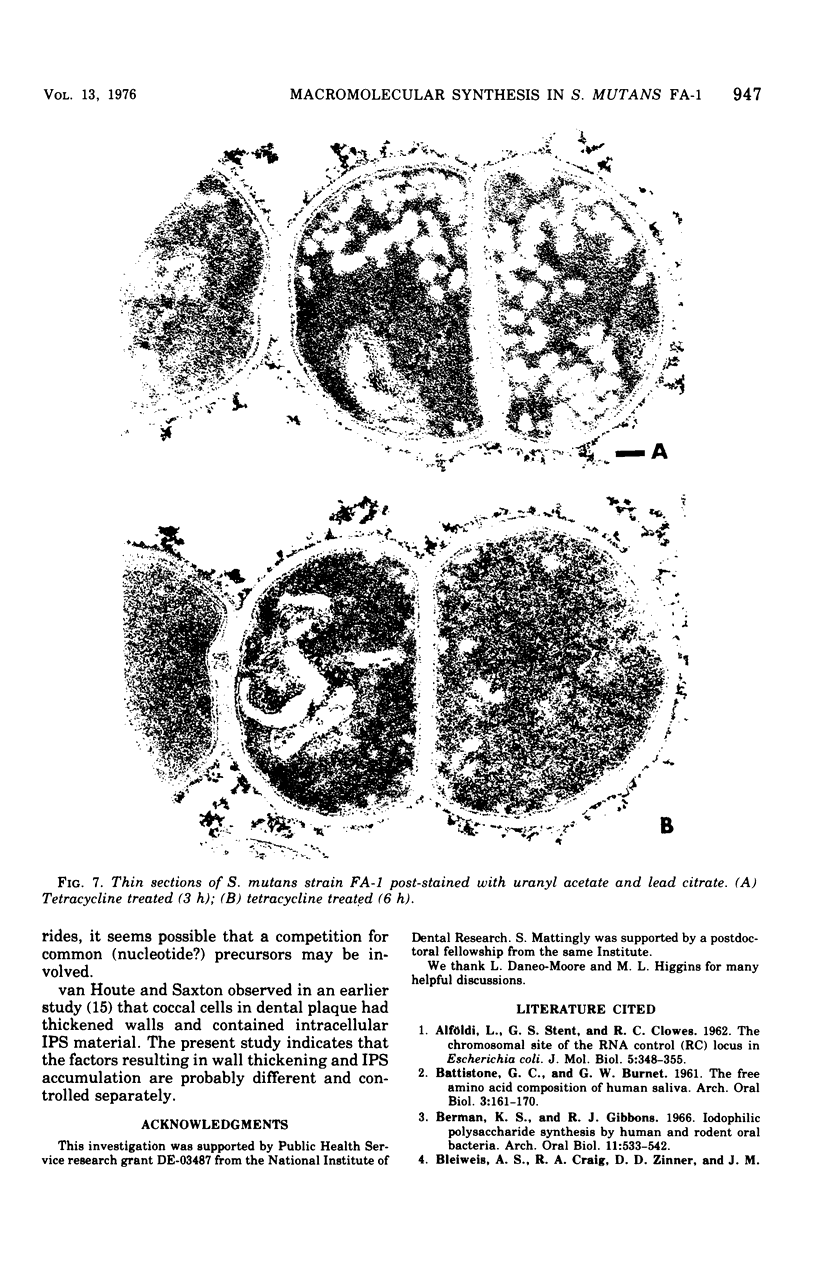
- van Houte J., Saxton C. A. Cell wall thickening and intracellular polysaccharide in microorganisms of the dental plaque. Caries Res. 1971;5(1):30–43. doi: 10.1159/000259730. [DOI] [PubMed] [Google Scholar]