Abstract
Novel engineered nanomaterials (ENMs) are being developed to enhance therapy. The physicochemical properties of ENMs can be manipulated to control/direct biodistribution and target delivery, but these alterations also have implications for toxicity. It is well known that size plays a significant role in determining ENM effects since simply nanosizing a safe bulk material can render it toxic. However, charge, shape, rigidity, and surface modifications also have a significant influence on the biodistribution and toxicity of nanoscale drug delivery systems (NDDSs). In this review, NDDSs are considered in terms of platform technologies, materials, and physical properties that impart their pharmaceutical and toxicological effects. Moving forward, the development of safe and effective nanomedicines requires standardized protocols for determining the physical characteristics of ENMs as well as assessing their potential long-term toxicity. When such protocols are established, the remarkable promise of nanomedicine to improve the diagnosis and treatment of human disease can be fulfilled.
Keywords: nanotechnology, drug delivery systems, biodistribution, elimination, toxicity
INTRODUCTION
Engineered nanomaterials (ENMs) offer numerous therapeutic advantages over traditional formulations; however, their unique properties have also led to concerns about human safety. Although the term nano originates from the Greek word ναν0σ, meaning dwarf, its scientific meaning is one-billionth (1). In 1908, Lohmann first used the term nano in the scientific literature to describe a small organism (2). The concept of nanotechnology was introduced by Richard Feynman during his talk “There’s Plenty of Room at the Bottom,” given at an American Physical Society meeting on December 29, 1959 (3). He coined the term nanotechnology and its use to refer to the construction of things from the bottom with atomic precision. According to the US National Nanotechnology Initiative, nanotechnology involves matter at dimensions roughly between 1 and 100 nanometers (nm) (4). Bawa proposed an alternative definition of nanotechnology that is not constrained by an arbitrary size limitation: “the design, characterization, production, and application of structures, devices, and systems by controlled manipulation of size and shape at the nanometer scale (atomic, molecular, and macromolecular scale) that produces structures, devices, and systems with at least one novel/superior characteristic or property” (5, p. 354). Interestingly, although thousands of patents and published papers suggest the therapeutic and pharmacological benefits of nanotechnology, there is a scarcity of reports demonstrating the clinical toxicity of ENMs. This review attempts to provide a critical summary of the platforms, materials, and physicochemical properties that comprise ENMs as well as relate these properties to their biopharmaceutical and toxicological potential.
ENGINEERED NANOMATERIAL PLATFORMS
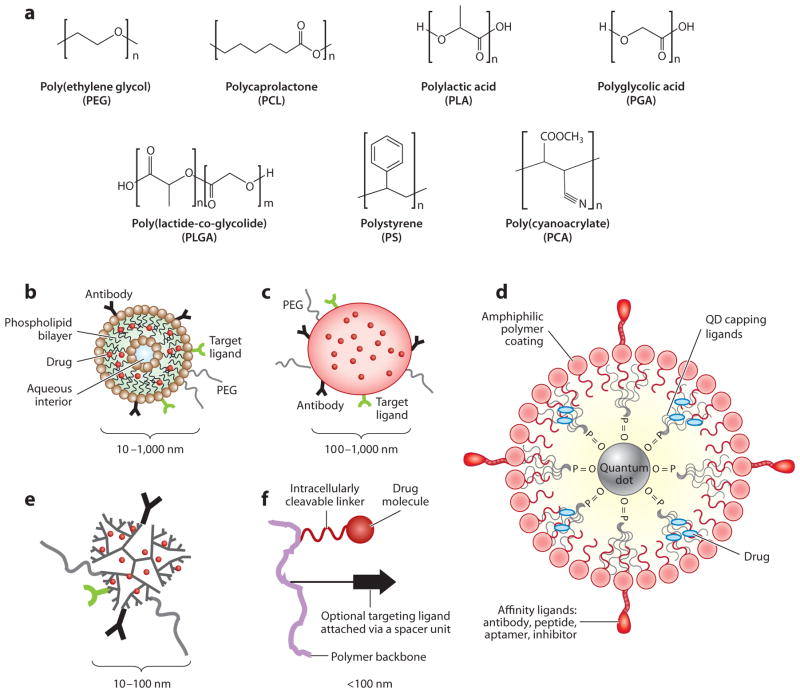
A variety of ENM platforms are constructed from a variety of materials (Figure 1) (6, 7). The most common forms include bioconjugates, nanoparticles (NPs), liposomes, dendrimers, and inorganic NPs such as quantum dots (QDs). Each platform has unique physicochemical properties and applications in nanotechnology.
Figure 1.
(a) Commonly used polymers for the assembly of a nanoscale drug delivery system (NDDS). Common NDDS platforms: (b) liposome, (c) polymeric nanoparticle, (d) quantum dot (QD), (e) dendrimer, and (f) polymeric bioconjugate nanocarrier. Adapted from Reference 115 with permission.
Bioconjugates
Bioconjugates incorporate polymeric components that act as carriers or linkers and biological components (peptides, proteins, nucleotides) that either act as ligands for targeting or elicit therapeutic effects. The covalent attachment of poly(ethylene glycol) (PEG) to drugs or therapeutic proteins is known as PEGylation. PEGylation has been extensively used to improve pharmaceutical properties such as by increasing solubility, decreasing systemic clearance, and reducing antigenicity and immunogenicity. Examples of bioconjugates include PEGylated proteins, antibody drug conjugates, and targeted nanocarriers (8–11). Because their overall structure is generally characterized as either branched or linear, bioconjugates do not possess a greater variety in shape compared with other nanoscale drug delivery system (NDDS) platforms (12). However, there are numerous examples of successfully marketed products from this platform, including Pegintron® (PEGylated interferon alfa-2b), Pegasys® (PEGylated interferon alfa-2a), and Kadcyla® (ado-trastuzumab emtansine), an antibody drug conjugate for the treatment of HER2-positive metastatic breast cancer.
Nanoparticles
NPs are stable, solid colloidal particles that are generally 1–100 nm in diameter and are fabricated using synthetic methods such as (a) polymerization, (b) emulsification and solvent evaporation, and (c) Flash NanoPrecipitation (13–15) as well as stepwise chemical synthesis. NPs can be modified to alter the release characteristics of their cargo and/or can be tailored to degrade on the basis of a biological or physicochemical stimulus. NPs are prepared from a variety of materials such as polymers, proteins, polysaccharides, and synthetic polymers. The selection of matrix materials is dependent on many factors including (a) the size of NPs required, (b) the inherent properties of the drug (e.g., aqueous solubility and stability), (c) surface characteristics such as charge, rigidity, and permeability, (d) the degree of biodegradability, biocompatibility, and toxicity, (e) the drug release profile desired, and (f) the antigenicity of the final product.
Liposomes
Liposomes are lipid bilayer composite structures composed primarily of phospholipids. They were first proposed as drug delivery vehicles by Gregoriadis (16). Laverman et al. (17), Mulder et al. (18), and Torchilin (19) have extensively studied liposomes. Several liposome-based ENMs have reached the market: Doxil® (liposomal doxorubicin), DaunoXome® (liposomal daunorubicin), and Visudyne® (liposomal verteporfin) (20). Despite some commercial success, liposomal carriers have been limited by numerous factors including (a) their relatively fast clearance, which demonstrates a pronounced dependence on size, and (b) their tendency to localize in the tissues of the mononuclear phagocyte system (MPS), particularly in the liver and spleen.
Dendrons and Dendrimers
Dendrons and dendrimers are highly branched macromolecular ENMs that can incorporate either synthetic polymeric building blocks or natural components (21). Their hierarchical factorial structure presents numerous conjugation sites for cargoes or targeting moieties. Although spherical in shape, dendrons and dendrimers possess a large cavity that can be utilized for passive entrapment and eventual release of drugs or other cargoes. The physicochemical nature of the cavity determines the entrapment efficiency and release profile of the cargo. The ability to selectively tune the cavity’s properties is considered a significant advantage of dendrons and dendrimers.
Dendrons/dendrimers typically exist as single-molecular entities; i.e., one molecule could represent the entire ENM structure with no solution-phase multimolecular assembly required. Furthermore, discrete (i.e., chemically uniform) building blocks can be utilized for their synthesis, resulting in discrete single-molecule engineered nanomaterials (SMENMs). SMENMs may represent the only class of ENMs that are characterized by an absolute molecular weight and analyzed as a single molecular ion in mass spectrometry. Although chemically synthesized discrete building blocks cost significantly more than their alternative counterparts made by polymerization, the resultant SMENMs can be useful in the discovery/development stage of ENM research. The lack of polydispersity simplifies early toxicity and analytical evaluation as well as allows for control of the toxicological profile. Loading drugs and/or diagnostics into ENMs by chemical means (e.g., using covalent bonds) could result in drug-loaded ENMs that offer absolute control of stoichiometry and ultimately more uniform biological effects in vitro and in vivo.
Inorganic Nanoparticles
ENMs constructed from inorganic materials are used for both therapeutic and diagnostic purposes. Commonly used materials include QDs in addition to gold, silver, iron oxide, or mesoporous silica nanoparticles (MSNs). Each material lends unique properties based on its size, charge, and surface chemistry, as well as its core structure. These vastly different materials also display inherently different pharmacokinetic/pharmacodynamic and safety profiles. Because of these differences, each inorganic material is discussed individually below. However, the use of PEGylation is common among inorganic NPs to improve biocompatibility and increase biological half-life.
QDs are semiconductive nanocrystals composed primarily of cadmium selenide (CdSe). The structure of a QD is shown in Figure 1. QDs have great potential for in vivo imaging and diagnostic purposes, but the QD core material of Cd metal is a highly toxic heavy metal (20, 22). Cd is a bioaccumulative carcinogen that has a half-life of 15–20 years and the ability to cross the blood-brain barrier and placenta (6, 20).
Gold exists in three states: elemental gold, gold I, and gold III; elemental gold is biologically and chemically inert (20). Gold I salts are considered a biologically safe material and have been used to treat rheumatoid arthritis for more than 60 years (20). However, gold III oxidizes methionine residues in proteins, resulting in their denaturation. Gold NPs have been investigated for both therapeutic and diagnostic purposes including thermal ablation and imaging, respectively (20, 23).
MATERIALS
ENMs for drug delivery utilize an array of materials including polymers, proteins, polysaccharides, and synthetic polymers. Polymers commonly used to fabricate ENMs include PEG, polylactide (PLA), poly(lactide-co-glycolide) (PLGA), polystyrene (PS), poly(cyanoacrylate) (PCA), poly(vinylpyrrolidone), polycaprolactone (PCL), and their copolymers. These polymers are widely used owing to their biodegradable and biocompatible nature as well as their ability to entrap hydrophobic drugs (Figure 1) (24–26). To better understand the overall toxic potential of ENMs, the specific toxicities of their architectural units (i.e., building blocks, repetitive blocks/subunits, and core and shell surfaces as well as the entire structures) should be taken into account. It is anticipated that even a linear polymerization of nontoxic building blocks could form toxic or potentially unsafe products. In this review, we focus on commonly used pharmaceutical polymers such as PEG, polyamidoamine (PAMAM), PLA, and PCL, and copolymers such as PS-PEG. Biodegradable polymers such as PLA, PLGA, polyglycolic acid (PGA), and PCL have been approved by the FDA (27).
Toxicity associated with PEGylated block copolymers in ENMs is limited because NPs are usually loaded with drug, which can cause its own adverse effects (28). PEGylated PLA NPs were shown to have no toxicity in doses up to 440 mg/kg intravenously, whereas non-PEGylated PLA NPs at the same doses resulted in fatal outcomes in rats (the latter were safe at doses <75 mg/kg) (29). PLGA NPs have little effect on cell viability and generate little evidence of pathological changes or tissue damage (30, 31). However, block copolymers that are nondegradable should be given special consideration. For those materials, there is risk of accumulation in the MPS or other tissues due to lack of elimination (6, 7, 32, 33). Even for biodegradable polymers, the size of the particles, the degradability, and the site of administration can influence immunological responses (28). Microparticles injected subcutaneously can act as drug depots; however, they can also generate foreign body responses on the basis of their duration within the injection site. The inflammation generated during the foreign body response can range from acute inflammation to the development of granulation tissue depending on the degradation rate of the particles (28).
Poly(ethylene glycol)
The use of PEG as a means of imparting new or different pharmacokinetic properties to proteins was first studied at Rutgers University in Frank Davis’s laboratory in the late 1970s (34–41). PEG was conjugated to enzymatic proteins in a process commonly known as PEGylation (see above), which reduces the antigenicity and immunogenicity of injected enzymes while increasing the circulating half-life of the protein. PEGylated bovine liver catalase not only was less immunogenic in rabbits but was also resistant to degradation by the proteolytic enzymes trypsin and chymotrypsin. In addition, PEGylated bovine liver catalase retained virtually all its innate enzymatic activity despite the addition of PEG to >40% of catalase’s amino groups (41). The success of this approach represented a fundamental shift in biologic therapeutics and ultimately ENMs.
Each ethylene glycol unit of PEG has the ability to bind two to three water molecules, resulting in an apparent size of a PEG molecule that is 5–10 times greater than its actual molecular weight owing to a greater Stokes radius (8, 9, 42, 43). The PEG-bound water creates an aqueous shield that not only increases the solubility of hydrophobic compounds but also prevents their degradation in vivo (44, 45). PEG can easily be incorporated with low polydispersity into ENMs through conjugation chemistry via a variety of functional end groups for conjugation either in organic or aqueous solvents (46). In addition, PEG is available in both branched and linear architectures, with the branched versions acting as though they are larger than the linear counterparts. Our lab has utilized both linear and branched versions of PEG as scaffolds for bioconjugates utilized in a variety of applications (47–56).
PEG is generally regarded as safe with LD50 >10 g/kg, and PEGylated bioconjugates usually require toxicological evaluation only of the parent compound (57–59). PEG has long been used as an excipient in pharmaceutical formulations (including pediatric formulations), cosmetic formulations, and food formulations and is extensively used for parenteral, oral, ocular, rectal, and topical routes of administration (59). Little toxicity is associated with PEG, and exposures of 10 mg/kg for PEGs up to 10 kDa are deemed acceptable (57, 58). However, there are few long-term toxicological data on PEGs of >10 kDa that are commonly used in ENMs. In isolated incidents, potential toxicity due to PEG overexposure has been observed in kidneys, where vacuolation within the proximal tubule can occur (58, 60, 61). A similar condition was observed in PEGylated proteins, whereby protein moieties that were filtered by the glomerulus influenced the reabsorption of the PEGylated protein (62). The process leads to the accumulation of nonbiodegradable PEG within the intracellular vacuoles of the tubular epithelial cells. The hygroscopic nature of PEG then causes fluid distention of the lysosome trying to metabolize the PEGylated protein and results in intracellular sequestration of the PEG polymers. Although the condition is transient and is resolved after cessation of therapy, it could be a point to consider during chronic therapy and an area requiring more investigation owing to the ubiquitous use of PEG in ENMs.
Most of the anti-immunogenic effect of PEG is due to the decrease in opsonin adsorption to the ENM, thereby reducing phagocytosis by macrophages of the MPS. However, exposure to PEG and PEGylated ENMs has induced the expression of anti-PEG antibodies in both humans and preclinical animal models (63, 64). Anti-PEG antibodies of classes immunoglobulin M (IgM) and immunoglobulin G (IgG) have been observed with binding epitopes of four to five repeating ethoxy units (65). The IgM antibodies have low binding affinity individually, but they do form pentamers (resulting in 10 binding sites) and thus increase the affinity through the multivalent effect (20). In one specific incident, the presence of PEG IgG and IgM antibodies resulted in the increased clearance of PEG-asparaginase bioconjugates (65). The PEG-induced antibodies may be caused by the long circulating half-life of PEG-asparaginase or by the prolonged exposure to PEG as an antigen. However, a survey of healthy donors revealed that 25% of them produced anti-PEG antibodies because of the prevalent use of PEG in cosmetics, pharmaceuticals, and food products (66, 67). Ultimately, the presence of anti-PEG antibodies may require dose adjustment to overcome the accelerated clearance level (65). Site-specific PEGylation could play an important role in reducing toxicity and immunogenicity of PEG-protein conjugates (68).
PEG building blocks are commercially available in three grades of molecular-weight dispersity: (a) low-polydispersity products of polymerization, (b) near-monodisperse products purified by sample displacement chromatography, and (c) discrete monodisperse products of organic synthesis. Lower polydispersity may correlate with lower toxicity, especially immunotoxicity. Recent reports emphasized the importance of dispersity when evaluating the toxicity of NPs (69). Varying grades of PEG building blocks can be used in comparative experiments to address the question of dispersity-dependent toxicity of ENMs.
Polyamidoamine
PAMAM dendrimers, especially constructs with high positive surface charges, demonstrate significant toxicities. Charge-derived toxicities can be reduced by covalent modification using a variety of methods, the most common of which is acylation. In addition, noncovalent loading of counter anions or even drug cargo such as siRNA that possesses counter ions can reduce toxicity as well (70, 71).
Recent years have seen the introduction of new chemical technologies for assembling ENMs that could increase the toxicity in numerous ways. Copper(I)-catalyzed and copper-free click chemistries are widely used not just for drug loading but also for constructing polymeric carriers (72). Residual catalysts, additives, and permanent cyclic moieties from click chemistry (1,2,3-triazole) should be strictly investigated for toxicity.
PHYSICOCHEMICAL PROPERTIES
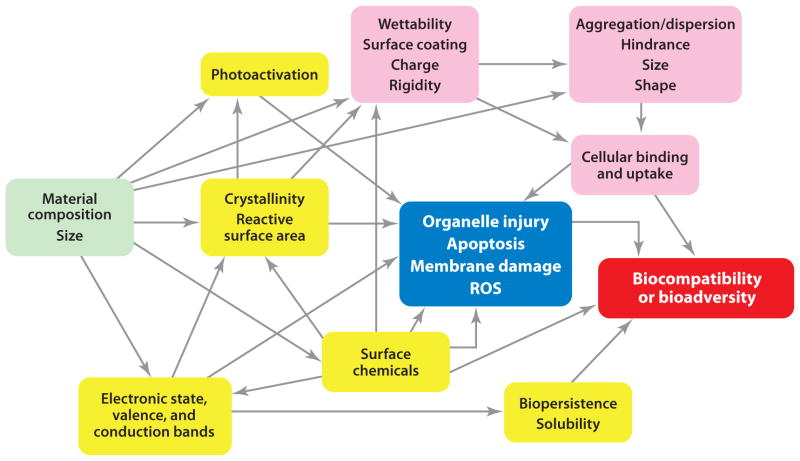
ENMs are unique owing to their surface-determined physicochemical properties and their size. The physicochemical properties of ENMs that are of the greatest significance are size, charge/hydrophobicity, shape, and rigidity. These properties are often fine-tuned, depending on the application, to achieve the appropriate drug loading, drug release, and uptake of ENMs within the body. A network of hypothetical correlations of various materials characteristics of ENMs to biological effects/outcomes such as toxicity and ultimately biocompatibility is shown in Figure 2. In this example, in addition to the material composition and size, the three major groupings of materials characteristics are surface reactivity, cellular uptake and subcellular localization, and factors that determine interactions with specific cellular compartments or processes. Furthermore, immunological parameters are important in determining the overall biocompatibility and bioadversity of ENMs. Therefore, it is important to understand the effects that these properties have on the distribution, metabolism, excretion, and toxicity of ENMs.
Figure 2.
The hypothetical correlations of various materials characteristics (MCs) of engineered nanomaterials (ENMs) to biological effects/outcomes. In addition to the material composition and size (green) that are the primary attributes determining the overall MCs of ENMs, the rest of the physicochemical characteristics of ENMs can be divided into overlapping modules as one set of MCs that determines surface reactivity (yellow), another set of MCs that determines the cellular uptake and subcellular localization (pink), and a third set of MCs that determines the interaction with specific cellular compartments or processes (blue). Biocompatibility and bioadversity (red) are two new modalities of MCs representing the concerns about using biologicals in ENMs. Abbreviation: ROS, reactive oxygen species. Modified from Reference 85 with permission.
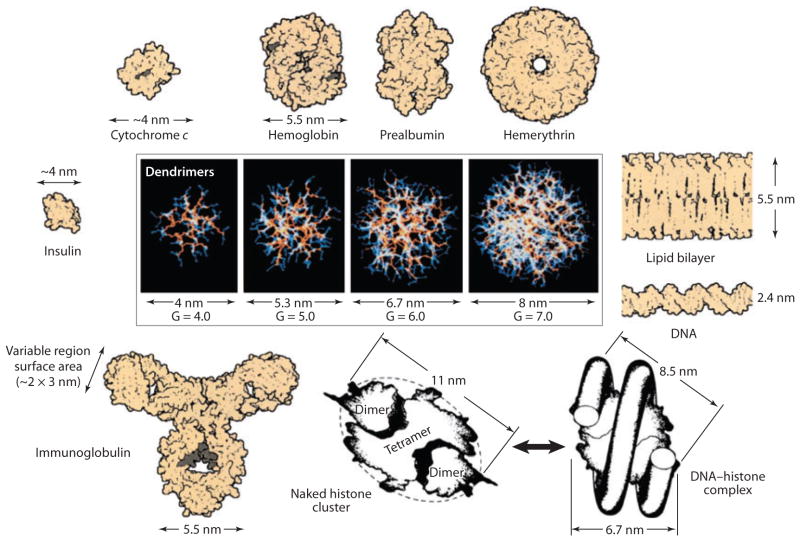
Size
NP size is a fundamental determinant of the NP’s biodistribution by tissue, but it is also influential in the rate of macrophage uptake. A size comparison between dendrimers and common biological components is shown in Figure 3. Particle sizes <100 nm have prolonged circulating half-lives and reduced opsonization compared with >100-nm particles. The adsorption of opsonins increases the recognition of foreign materials for phagocytosis (62, 73, 74). In addition, size can also affect the route and extent of uptake by target cells and phagocytes. NPs that are <200 nm are internalized via clathrin-coated pits, whereas 500-nm particles are internalized via caveolae-meditated endocytosis (75, 76). Size-dependent uptake was observed in a mouse macrophage cell line, and phagocytosis increased as the particles reached 100 nm (77). Studies involving micrometer-sized particles postulate that the size-dependent uptake is influenced by the particles’ attachment to the phagocytes, with maximal attachment occurring between 2 and 3 μm (78).
Figure 3.
It is well known that size plays a significant role in determining ENM effects since simply nanosizing a safe bulk material can render it toxic. Other factors such as charge, shape, rigidity, and surface modifications, however, also have a significant influence on NDDS biodistribution and toxicity. This figure shows a dimensionally scaled comparison of a series of PAMAM dendrimers (G = 4–7) with a variety of proteins, a typical lipid bilayer membrane, and DNA. The closely matched size and contours of important proteins and bioassemblies are indicated. Abbreviations: ENM, engineered nanomaterial; G, generation; NDDS, nanoscale drug delivery system; PAMAM, polyamidoamine. Modified from Reference 70 with permission.
Collectively, the lungs, liver, spleen, and kidneys act as a series of filter organs that ultimately determine the body persistence and biodistribution of ENMs. ENMs that are smaller than the size of serum albumin (~40–50 kDa or a diameter of ≲4–6 nm) are eliminated primarily through the kidneys (47, 50, 62, 79). Particles or aggregates of particles >10 μm become passively entrapped within the capillaries of the lung (47, 80–83). These particles persist within the lung until degraded to smaller components, whereas particles that are >3 μm are transiently entrapped in the lung and subsequently move to the liver (47). Particles that are in the range of 3–6 μm accumulate in the liver and spleen. From a pure filtering perspective, these organs have little effect on the biodistribution of ENMs. It is well known that bulk materials (>1 μm) that are relatively inert become toxic when their size is reduced to the nanoscale (84). This toxicity results from (a) an unusually high ratio of surface area to volume that makes the ENM surfaces highly reactive and (b) the fact that the ENMs’ subcellular, cellular, and body distribution may be enhanced owing to their ability to traverse cell barriers. The significant increase in exposed functional groups on the material may lead to increased surface reactivity, further increasing the potential for interactions with other nanoscale biological molecules such as DNA, proteins, and cell membranes (85). Oxidative stress occurs through the interaction of molecular oxygen and electron donor or acceptor groups on the ENM surface. The interaction produces either superoxide or hydrogen peroxide, and either species can oxidize other compounds through an electron transfer mechanism (30). Propagation of reactive oxygen species (ROS) is associated with nanosized materials and is, therefore, a potential cause of toxicity (86). ENMs also have a higher potential than larger-sized particles to interact with biological components such as cells and subcellular organelles owing to their much greater biodistribution. This greater biodistribution is due primarily to the size of endothelial junctions in normal blood vessels (~2–6 nm) and the lymphatic vessels (80–1,000 nm) (87). In addition, ENM-biological interactions could lead to membrane permeability changes that allow for an even wider biodistribution and greater possible toxicity of ENMs (85).
As with all other ENMs, size plays a large role in the disposition of QDs. QDs that were 2.2 nm in diameter had the ability to localize intracellularly in the nucleus, causing toxicity (88). QDs that were slightly larger (5.2 nm) accumulated within the cytosol and were generally less deleterious than the smaller QDs (88). The toxicity of QDs is generated by the production of free radicals capable of nicking DNA (89). Therefore, QDs must be coated to entrap the CdSe and prevent leaching into the biological milieu. Entrapment is often achieved using PEG-silica or ZnS surface coatings to shield the QD core in order to protect against toxicity and prevent aggregation (88). ENMs must be small enough to penetrate the desired tissues as well as to be excreted by the kidneys to avoid accumulation of toxic materials (79).
PEGylated gold NPs (13 nm in size) demonstrated long circulating half-lives, ultimately accumulating within the liver and spleen over the course of 1 week. The sequestration of gold NPs within lysosomes of Kupffer cells and spleen macrophages resulted in acute hepatic inflammation and apoptosis in mice (90). Expression of inflammatory cytokines, adhesion molecules, and chemokines in vivo 24 h postdose confirmed the acute inflammatory response to the gold NPs. Further size-based investigation of gold NPs demonstrated that 100-nm NPs quickly accumulated within the liver (within 24 h), whereas 4- and 13-nm NPs remained in the blood for ~1 week (91). All of the gold NPs, regardless of their size, were detectable in the liver, spleen, and mesenteric lymph nodes for up to 6 months postdose, indicating a lack of elimination over time. Detectable levels of gold were observed for the better part of 1 month in the bile and urine, but the concentration remained higher in the tissues of the MPS. Furthermore, the induction of Phase I metabolizing enzymes (CYP1A1 and CYP2B1) and apoptotic and inflammation genes by gold NPs indicates the production of oxidative stress within the liver (91, 92).
PEG can decrease the rate of clearance of NDDSs because of its flexibility and its ability to bind water. In aqueous solutions, PEG exists in an extended conformation and is fully hydrated; thus, it provides steric hindrance to enzymatic degradation as well as the adsorption of serum proteins to the surface of foreign bodies identifying them for phagocytosis by macrophages (opsonization) (93–95). When proteins attempt to adsorb to a PEGylated ENM, the PEG chains are compressed and thus forced into a higher-energy conformation. This higher-energy conformation creates a repulsive force that is either equal to or greater than the magnitude of the attractive force of adsorption, thereby protecting the ENM from phagocytosis during circulation and reducing metabolic elimination (43). ENM shielding by PEG increases its residence time, leading to pharmacological benefits.
PEG itself is nonbiodegradable, and the majority of it is renally excreted at sizes below the glomerular filtration cutoff (<30 kDa for PEG) or excreted in the feces after biliary excretion (PEG >50 kDa) (59, 96). Although PEG is largely unchanged within the body, there is some evidence that it can be metabolized by Phase I enzymes such as alcohol dehydrogenase and cytochrome P450s to smaller oligomers of PEG, carbon dioxide, and traces of oxalic acid (57). Manipulating the size of the PEG chains coupled to the bioconjugates can be utilized to reduce the renal filtration and increase the circulating half-life (8, 34, 41, 43, 96).
Shape
Particle shape is another physical characteristic that can determine the biological fate of an ENM in the body. In particular, phagocytosis of foreign materials such as ENMs can be influenced by their aspect ratios. Particles of ellipsoid shape are more readily engulfed by macrophages than are spherical particles (97). However, NPs with dramatically high aspect ratios (tubular in shape versus spherical) resist uptake by macrophages because of their high curvature angles (98, 99). Short-rod (aspect ratio = 1.5) MSNs are easily trapped in the liver, whereas long-rod (aspect ratio = 5) MSNs distribute in the spleen (100). Thus, particles with smaller aspect ratios exhibit more rapid clearance. The difference in clearance between Pegintron and Pegasys (see above) also demonstrates the effect of ENM shape. Pegintron, composed of a linear 12-kDa PEG, is primarily eliminated by the kidneys, whereas Pegasys, which contains a branched 40-kDa PEG, is metabolized by nonspecific proteases in the liver. The larger, branched structure of Pegasys allows for a greater biological half-life because metabolism is necessary for its clearance (101). In addition to the overall shape of the NP, the smoothness/roughness of the particle’s surface also affects the opsonization of the particle and its subsequent uptake by the MPS (62). Particle shape also affects potential toxicities. For example, alteration of a relatively inert material, such as TiO2, into a fiber structure that has a length of >15 μm results in a toxic particle that provokes an inflammatory response in alveolar macrophages (102). Materials altered into shapes that are difficult to process by phagocytic cells can result in toxicity by lysosomal disruption (102).
Charge and Hydrophobicity
Surface properties of ENMs, such as the charge (zeta potential) and hydrophobicity, which is closely related to wettability, directly affect ENM interactions with biological surfaces, cell membranes, and proteins. Biodistribution, cell uptake, and the extent of protein adsorption on ENMs are all related to their surface chemistry. Charged NPs (positive or negative) undergo greater opsonization than do neutral NPs and show greater accumulation in the MPS (103). In mice, undesirable liver uptake has been observed for micellar NPs with highly positive or highly negative surfaces, whereas liver uptake was low for slightly negatively charged NPs. These NPs showed a greater ability to accumulate in ovarian tumors (104). Owing to the negative charge of cell surfaces, positively charged ENMs exhibit greater cell uptake, leading to high rates of nonspecific internalization and a shorter half-life in the circulation. Particles with positive charges are more likely to accumulate within macrophages (105, 106). Conversely, negatively charged or neutral materials experience less nonspecific uptake owing to steric or electrostatic repulsion (74). This repulsion, in the case of negatively charged ENMs, creates a barrier to cytotoxicity. The presence of a strong electrostatic barrier created by charge repulsion may even trump other factors affecting toxicity, such as size or shape (107).
Rigidity
The flexibility or rigidity of ENMs can also influence their biodistribution (47, 87, 108). Altering the intra- and intermolecular architecture of an ENM can control rigidity. For example, the flexibility of PEGylated materials can be affected by the level of hydration, which is modulated by the amount of PEG and/or the length of PEG chains on the surface. The level of rigidity of an ENM can also influence the ability of the material to deform to conformations that allow it to pass through physiologic pores (87). However, highly rigid architectures can compromise the biocompatibility of a material. Thin (~50 nm in diameter) multiwalled carbon nanotubes with a highly crystalline structure pierce cell membranes and induce cytotoxicity in mesothelial cells, whereas those that are thick (~150 nm in diameter) or tangled (~2–20 nm in diameter) exhibit reduced toxicity (109).
CONCLUSIONS AND PERSPECTIVES
Nanomedicine is a growing field of research that offers remarkable prospects for the improvement of the diagnosis and treatment of human disease. NDDSs are able to overcome the poor solubility of hydrophobic drugs while achieving cell- or tissue-specific targeted delivery. This allows for reductions in overall dosage, and because only affected tissues are treated, the risk of harmful interactions with healthy tissues is minimized. Owing to the flexibility in ENM design (e.g., size, shape, surface modifications), it is possible to achieve transcytosis across epithelial barriers and deliver both small-molecule and macromolecular drugs to specific sites of action within cells. In addition, the ability to deliver combinations of drugs and imaging modalities offers precise visualization of delivery targets. Depending on the application, some ENM platforms may be more suitable than others. For example, liposomes are able to encapsulate both hydrophobic and hydrophilic compounds owing to their lipid bilayer structure. The lipid head groups can be easily modified with PEG or targeting ligands to improve circulation and achieve site-specific delivery. When targeting harsh body environments (e.g., the gut), however, more stable nanocarriers (such as polymeric NPs) may be necessary. The use of bioconjugates can enhance drug delivery, but they suffer from low payload compared with other nanocarriers such as NPs. Advantages of NDDSs are comprehensively discussed in other reviews (110, 111).
Unfortunately, the development of ENMs remains controversial. Britain’s Royal Society and Royal Academy of Engineering have taken the stance that NPs should be deemed harmful until proven safe (112). Alternatively, a draft guidance document from the FDA states that nanomaterials will be regarded as neither safe nor harmful and that review of applications will be considered on a case-by-case basis and safety determined on the available science (113). In addition, the proper safety of the scale-up and manufacturing of ENMs is a concern in terms of environmental and occupational safety. However, the benefits and potential of ENMs, especially NDDSs, are undeniable. Advantages such as reduced dosing, improved therapeutic index, enhanced solubility, and targeted delivery to disease cells/tissues are worthy reasons to continue pursuing the development of ENMs. Emerging nanotechnologies have raised the potential for the development of materials with unexpected and unpredictable toxicities. From a toxicological standpoint, nanosizing/nanoformulating nontoxic materials should be considered similar to synthesizing new chemical entities (NCEs) owing to the changes in their materials characteristics upon manufacturing. The toxicological characterization of ENMs could be even more challenging because their physicochemical properties will likely undergo more significant changes than will those of NCEs. Varying independent physicochemical characteristics, i.e., size and charge, could synergistically enhance or decrease overall toxicities. Therefore, toxicological screening should be implemented from the early stages of ENM development, just as early toxicological evaluation is implemented in NCE development. Owing to the unique and complex properties of ENMs compared with chemicals or therapeutic agents, novel approaches and methods will need to be developed in order to perform safety assessments and manage risk. Although current testing for ENMs aims to establish a link between in vitro and in vivo toxicological parameters, the methods to predict and ultimately determine toxicity, fate, and transport of ENMs have yet to be elaborated and standardized (114).
Novel ENMs are being designed and evaluated for the optimization of drug delivery and diagnostic platforms, and researchers are manipulating their physicochemical properties to control/direct biodistribution and elimination while maximizing therapeutic potential and minimizing toxicity. The ultimate goal is to produce targeted and possibly even personalized therapeutic regimens to treat disease. However, as we begin to understand more about the properties of ENMs, new challenges and tensions are surfacing in an already complicated field. For example, Bonner et al. observed that “[s]ize alone is a major determinant because many bulk materials that are relatively inert become toxic when produced at the nanoscale” (84, p. 676). On one hand, micrometer-sized DDSs are typically trapped in blood or lymphatic vessels and are unable to leave the circulation, whereas NDDSs can penetrate cells and, if small enough, can leave the cytosol and enter the nucleus where they can exert significant toxic effects. On the other hand, nuclear delivery of anti-HIV drugs is a goal of drug delivery scientists. The tension between maximizing therapeutic benefit and minimizing toxic potential will continue to be a significant challenge in the development of new targeted nanomedicines for the foreseeable future. Although the fact that size is a major determinant of the functional properties of ENMs has been known for some time, how ENM size is characterized and reported is being reexamined. The effect of polydispersity is increasingly influencing our understanding of therapeutic benefit and toxicity, along with the interpretation of the variability observed in both (69). What is clear today is that standardized protocols for determining the physical characteristics of ENMs as well as assessing their toxicity need to be established in the near future (69, 84).
SUMMARY POINTS.
Although engineered nanomaterials (ENMs) provide therapeutic advantages over conventional formulations and drug delivery systems, they also possess unique and complex physicochemical properties that can lead to potential toxicities.
ENMs can be classified into several different platforms that have different physicochemical properties and applications including bioconjugates, nanoparticles, liposomes, dendrimers, and inorganic materials. Each platform provides distinct advantages for delivering small- or large-molecule therapeutic or diagnostic agents.
The physicochemical properties of ENMs, such as size, shape, charge, and rigidity, are critical in determining their overall biodistribution, body persistence/clearance, and potential for toxicity.
Relatively inert bulk materials become toxic when formulated at the nanoscale.
The specific toxicities of common polymeric entities used in the formulation of ENMs are also important in determining their overall toxic potential.
FUTURE ISSUES.
Because ENMs are far more complex than chemicals and therapeutic agents, novel approaches and methods will need to be developed in order to perform thorough safety assessments and manage risk.
Implementation of toxicological screening at the early stages of ENM development would be beneficial toward understanding unknown or unusual toxicities.
Standardized protocols for determining the physical characteristics and toxicities of ENMs must be established if the potential of nanomedicine is to be fully realized.
Acknowledgments
This research is supported by the National Institutes of Health CounterACT Program through the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Award U54AR055073), the National Cancer Institute (Award CA155061), and the National Institute of Allergy and Infectious Diseases (Award AI051214 and Award AI084137).
Glossary
- ENM
engineered nanomaterial
- NP
nanoparticle
- PEG
poly(ethylene glycol)
- NDDS
nanoscale drug delivery system
- MPS
mononuclear phagocyte system
- SMENM
single-molecule engineered nanomaterial
- PLA
polylactide or polylactic acid
- PLGA
poly(lactide-co-glycolide)
- PS
polystyrene
- PCA
poly(cyanoacrylate)
- PCL
polycaprolactone
- PAMAM
polyamidoamine
- IgM
immunoglobulin M
- IgG
immunoglobulin G
- ROS
reactive oxygen species
- NCE
new chemical entity
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Joachim C. To be nano or not to be nano? Nat Mater. 2005;4:107–9. doi: 10.1038/nmat1319. [DOI] [PubMed] [Google Scholar]
- 2.Lohmann H. Untersuchungen zur Feststellund des vollstandigen Gehaltes des Meeres an Plankton. Wiss Meeresunters Kiel, NF. 1908;10:129–370. [Google Scholar]
- 3.Feynman RP. There’s plenty of room at the bottom. Eng Sci. 1960;23:22–36. [Google Scholar]
- 4.Natl. Nanotechnol. Initiat. Nanotechnol. Vol. 101. US Natl. Nanotechnol. Initiat; 2000. What is nanotechnology? http://www.nano.gov/nanotech-101/what/definition. [Google Scholar]
- 5.Bawa R. Patents and nanomedicine. Nanomedicine. 2007;2:351–74. doi: 10.2217/17435889.2.3.351. [DOI] [PubMed] [Google Scholar]
- 6.Aillon KL, Xie Y, El-Gendy N, Berkland CJ, Forrest ML. Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv Drug Deliv Rev. 2009;61:457–66. doi: 10.1016/j.addr.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buzea C, Pacheco, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2:MR17–71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 8.Jevsevar S, Kunstelj M, Porekar VG. PEGylation of therapeutic proteins. Biotechnol J. 2010;5:113–28. doi: 10.1002/biot.200900218. [DOI] [PubMed] [Google Scholar]
- 9.Veronese FM, Pasut G. PEGylation, successful approach to drug delivery. Drug Discov Today. 2005;10:1451–58. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- 10.Larson N, Ghandehari H. Polymeric conjugates for drug delivery. Chem Mater. 2012;24:840–53. doi: 10.1021/cm2031569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palombo MS, Singh Y, Sinko PJ. Prodrug and conjugate drug delivery strategies for improving HIV/AIDS therapy. J Drug Deliv Sci Technol. 2009;19:3–14. doi: 10.1016/s1773-2247(09)50001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh Y, Palombo M, Sinko PJ. Recent trends in targeted anticancer prodrug and conjugate design. Curr Med Chem. 2008;15:1802–26. doi: 10.2174/092986708785132997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bazile D, Prud’homme C, Bassoullet M-T, Marlard M, Spenlehauer G, Veillard M. Stealth Me. PEG-PLA nanoparticles avoid uptake by the mononuclear phagocytes system. J Pharm Sci. 1995;84:493–98. doi: 10.1002/jps.2600840420. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, Pansare VJ, Prud’homme RK, Priestley RD. Flash nanoprecipitation of polystyrene nanoparticles. Soft Matter. 2012;8:86–93. [Google Scholar]
- 15.Johnson BK, Prud’homme RK. Flash nanoprecipitation of organic actives and block copolymers using a confined impinging jets mixer. Aust J Chem. 2003;56:1021–24. [Google Scholar]
- 16.Gregoriadis G. Overview of liposomes. J Antimicrob Chemother. 1991;28:39–48. doi: 10.1093/jac/28.suppl_b.39. [DOI] [PubMed] [Google Scholar]
- 17.Laverman P, Carstens MG, Boerman OC, Dams ETM, Oyen WJ, et al. Factors affecting the accelerated blood clearance of polyethylene glycol-liposomes upon repeated injection. J Pharmacol Exp Ther. 2001;298:607–12. [PubMed] [Google Scholar]
- 18.Mulder WJ, Strijkers GJ, Griffioen AW, van Bloois L, Molema G, et al. A liposomal system for contrast-enhanced magnetic resonance imaging of molecular targets. Bioconjug Chem. 2004;15:799–806. doi: 10.1021/bc049949r. [DOI] [PubMed] [Google Scholar]
- 19.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–60. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 20.Webster TJ, editor. Safety of Nanoparticles: From Manufacturing to Medical Applications. New York: Springer; 2009. [Google Scholar]
- 21.Baker JR., Jr Dendrimer-based nanoparticles for cancer therapy. Hematology. 2009;2009:708–19. doi: 10.1182/asheducation-2009.1.708. [DOI] [PubMed] [Google Scholar]
- 22.Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004;4:11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerrero S, Araya E, Fiedler JL, Arias JI, Adura C, et al. Improving the brain delivery of gold nanoparticles by conjugation with an amphipathic peptide. Nanomedicine. 2010;5:897–913. doi: 10.2217/nnm.10.74. Describes effective surface modifications of NPs for improved brain delivery. [DOI] [PubMed] [Google Scholar]
- 24.Duan Y, Sun X, Gong T, Wang Q, Zhang Z. Preparation of DHAQ-loaded mPEG-PLGA-mPEG nanoparticles and evaluation of drug release behaviors in vitro/in vivo. J Mater Sci Mater Med. 2006;17:509–16. doi: 10.1007/s10856-006-8933-3. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Feng SS. Nanoparticles of poly(lactide)/vitamin E TPGS copolymer for cancer chemotherapy: synthesis, formulation, characterization and in vitro drug release. Biomaterials. 2006;27:262–70. doi: 10.1016/j.biomaterials.2005.05.104. [DOI] [PubMed] [Google Scholar]
- 26.Brannon-Peppas L. Recent advances on the use of biodegradable microparticles and nanoparticles in controlled drug delivery. Int J Pharm. 1995;116:1–9. [Google Scholar]
- 27.Thomas V, Dean DR, Vohra YK. Nanostructured biomaterials for regenerative medicine. Curr Nanosci. 2006;2:155–77. [Google Scholar]
- 28.Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 29.Plard JP, Bazile D. Comparison of the safety profiles of PLA50 and Me. PEG-PLA50 nanoparticles after single dose intravenous administration to rat. Colloids Surf B. 1999;16:173–83. [Google Scholar]
- 30.Semete B, Booysen L, Lemmer Y, Kalombo L, Katata L, et al. In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomedicine. 2010;6:662–71. doi: 10.1016/j.nano.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Mura S, Hillaireau H, Nicolas J, Le Droumaguet B, Gueutin C, et al. Influence of surface charge on the potential toxicity of PLGA nanoparticles towards Calu-3 cells. Int J Nanomed. 2011;6:2591–605. doi: 10.2147/IJN.S24552. Demonstrates limited cytotoxicity of NPs of various charge states. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garnett MC, Kallinteri P. Nanomedicines and nanotoxicology: some physiological principles. Occup Med. 2006;56:307–11. doi: 10.1093/occmed/kql052. [DOI] [PubMed] [Google Scholar]
- 33.Kunzmann A, Andersson B, Thurnherr T, Krug H, Scheynius A, Fadeel B. Toxicology of engineered nanomaterials: focus on biocompatibility, biodistribution and biodegradation. Biochim Biophys Acta. 2011;1810:361–73. doi: 10.1016/j.bbagen.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Pyatak PS, Abuchowski A, Davis FF. Preparation of a polyethylene glycol: superoxide dismutase adduct, and an examination of its blood circulation life and anti-inflammatory activity. Res Commun Chem Pathol Pharmacol. 1980;29:113–27. [PubMed] [Google Scholar]
- 35.Wieder KJ, Palczuk NC, van Es T, Davis FF. Some properties of polyethylene glycol:phenylalanine ammonialyase adducts. J Biol Chem. 1979;254:12579–87. [PubMed] [Google Scholar]
- 36.Savoca KV, Abuchowski A, van Es T, Davis FF, Palczuk NC. Preparation of a non-immunogenic arginase by the covalent attachment of polyethylene glycol. Biochim Biophys Acta. 1979;578:47–53. doi: 10.1016/0005-2795(79)90111-9. [DOI] [PubMed] [Google Scholar]
- 37.Abuchowski A, Davis FF. Preparation and properties of polyethylene glycol-trypsin adducts. Biochim Biophys Acta. 1979;578:41–46. doi: 10.1016/0005-2795(79)90110-7. [DOI] [PubMed] [Google Scholar]
- 38.Abuchowski A, van Es T, Palczuk N, McCoy J, Davis F. Treatment of L5178Y tumor-bearing BDF1 mice with a nonimmunogenic L-glutaminase-L-asparaginase. Cancer Treat Rep. 1979;63:1127–32. [PubMed] [Google Scholar]
- 39.Davis FF, Abuchowski A, van Es T, Palczuk NC, Chen R, et al. Enzyme-polyethylene glycol adducts: modified enzymes with unique properties. In: Broun G, Manecke G, Wingard L Jr, editors. Enzyme Engineering. New York: Springer; 1978. pp. 169–73. [Google Scholar]
- 40.Abuchowski A, van Es T, Palczuk NC, Davis FF. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J Biol Chem. 1977;252:3578–81. [PubMed] [Google Scholar]
- 41.Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977;252:3582–86. [PubMed] [Google Scholar]
- 42.Harris JM, Martin NE, Modi M. Pegylation: a novel process for modifying pharmacokinetics. Clin Pharmacokinet. 2001;40:539–51. doi: 10.2165/00003088-200140070-00005. [DOI] [PubMed] [Google Scholar]
- 43.Chen C, Constantinou A, Deonarain M. Modulating antibody pharmacokinetics using hydrophilic polymers. Expert Opin Drug Deliv. 2011;8:1221–36. doi: 10.1517/17425247.2011.602399. [DOI] [PubMed] [Google Scholar]
- 44.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214–21. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 45.Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed Engl. 2010;49:6288–308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 46.Veronese FM, Sacca B, Polverino de Laureto P, Sergi M, Caliceti P, et al. New PEGs for peptide and protein modification, suitable for identification of the PEGylation site. Bioconjug Chem. 2001;12:62–70. doi: 10.1021/bc000061m. [DOI] [PubMed] [Google Scholar]
- 47.Deshmukh M, Kutscher HL, Gao D, Sunil VR, Malaviya R, et al. Biodistribution and renal clearance of biocompatible lung targeted poly(ethylene glycol) (PEG) nanogel aggregates. J Control Release. 2012;164:65–73. doi: 10.1016/j.jconrel.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh Y, Gao D, Gu Z, Li S, Rivera KA, et al. Influence of molecular size on the retention of polymeric nanocarrier diagnostic agents in breast ducts. Pharm Res. 2012;29:2377–88. doi: 10.1007/s11095-012-0763-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh Y, Gao D, Gu Z, Li S, Stein S, Sinko PJ. Noninvasive detection of passively targeted poly(ethylene glycol) nanocarriers in tumors. Mol Pharm. 2012;9:144–55. doi: 10.1021/mp2003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deshmukh M, Singh Y, Gunaseelan S, Gao D, Stein S, Sinko PJ. Biodegradable poly(ethylene glycol) hydrogels based on a self-elimination degradation mechanism. Biomaterials. 2010;31:6675–84. doi: 10.1016/j.biomaterials.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gunaseelan S, Pooyan S, Chen P, Samizadeh M, Palombo MS, et al. Multimeric peptide-based PEG nanocarriers with programmable elimination properties. Biomaterials. 2009;30:5649–59. doi: 10.1016/j.biomaterials.2009.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wan L, Zhang X, Pooyan S, Palombo MS, Leibowitz MJ, et al. Optimizing size and copy number for PEG-fMLF (N-formyl-methionyl-leucyl-phenylalanine) nanocarrier uptake by macrophages. Bioconjug Chem. 2008;19:28–38. doi: 10.1021/bc070066k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wan L, Zhang X, Gunaseelan S, Pooyan S, Debrah O, et al. Novel multi-component nanopharmaceuticals derived from poly(ethylene) glycol, retro-inverso-Tat nonapeptide and saquinavir demonstrate combined anti-HIV effects. AIDS Res Ther. 2006;3:12. doi: 10.1186/1742-6405-3-12. Exhibits an early example of a targeted, multicomponent NDDS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gunaseelan S, Debrah O, Wan L, Leibowitz MJ, Rabson AB, et al. Synthesis of poly(ethylene glycol)-based saquinavir prodrug conjugates and assessment of release and anti-HIV-1 bioactivity using a novel protease inhibition assay. Bioconjug Chem. 2004;15:1322–33. doi: 10.1021/bc0498875. [DOI] [PubMed] [Google Scholar]
- 55.Pooyan S, Qiu B, Chan MM, Fong D, Sinko PJ, et al. Conjugates bearing multiple formyl-methionyl peptides display enhanced binding to but not activation of phagocytic cells. Bioconjug Chem. 2002;13:216–23. doi: 10.1021/bc0100657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramanathan S, Qiu B, Pooyan S, Zhang G, Stein S, et al. Targeted PEG-based bioconjugates enhance the cellular uptake and transport of a HIV-1 TAT nonapeptide. J Control Release. 2001;77:199–212. doi: 10.1016/s0168-3659(01)00474-6. [DOI] [PubMed] [Google Scholar]
- 57.Fruijtier-Polloth C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology. 2005;214:1–38. doi: 10.1016/j.tox.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 58.Webster R, Elliott V, Park BK, Walker D, Hankin M, Taupin P. PEG and PEG conjugates toxicity: towards an understanding of the toxicity of PEG and its relevance to PEGylated biologicals. In: Veronese FM, editor. PEGylated Protein Drugs: Basic Science and Clinical Applications. Basel, Switz: Birkhäuser; 2009. pp. 127–46. [Google Scholar]
- 59.Webster R, Didier E, Harris P, Siegel N, Stadler J, et al. PEGylated proteins: evaluation of their safety in the absence of definitive metabolism studies. Drug Metab Dispos. 2007;35:9–16. doi: 10.1124/dmd.106.012419. [DOI] [PubMed] [Google Scholar]
- 60.Herold DA, Rodeheaver GT, Bellamy WT, Fitton LA, Bruns DE, Edlich RF. Toxicity of topical polyethylene glycol. Toxicol Appl Pharmacol. 1982;65:329–35. doi: 10.1016/0041-008x(82)90016-3. [DOI] [PubMed] [Google Scholar]
- 61.Bruns DE, Herold DA, Rodeheaver GT, Edlich RF. Polyethylene glycol intoxication in burn patients. Burns Incl Therm Inj. 1982;9:49–52. doi: 10.1016/0305-4179(82)90136-x. [DOI] [PubMed] [Google Scholar]
- 62.Bertrand N, Leroux JC. The journey of a drug-carrier in the body: an anatomo-physiological perspective. J Control Release. 2012;161:152–63. doi: 10.1016/j.jconrel.2011.09.098. [DOI] [PubMed] [Google Scholar]
- 63.Richter AW, Akerblom E. Polyethylene glycol reactive antibodies in man: titer distribution in allergic patients treated with monomethoxy polyethylene glycol modified allergens or placebo, and in healthy blood donors. Int Arch Allergy Appl Immunol. 1984;74:36–39. doi: 10.1159/000233512. [DOI] [PubMed] [Google Scholar]
- 64.Richter AW, Akerblom E. Antibodies against polyethylene glycol produced in animals by immunization with monomethoxy polyethylene glycol modified proteins. Int Arch Allergy Appl Immunol. 1983;70:124–31. doi: 10.1159/000233309. [DOI] [PubMed] [Google Scholar]
- 65.Armstrong JK, Hempel G, Koling S, Chan LS, Fisher T, et al. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer. 2007;110:103–11. doi: 10.1002/cncr.22739. [DOI] [PubMed] [Google Scholar]
- 66.Armstrong JK, Leger R, Wenby RB, Meiselman HJ, Garratty G, Fisher TC. Occurrence of an antibody to poly(ethylene glycol) in normal donors. Presented at Am. Soc. Hematol. Annu. Meet., 45th; San Diego. 2003. p. Abstr. 556A. [Google Scholar]
- 67.Fisher TC, Armstrong JK, Wenby RB, Meiselman HJ, Leger R, Garratty G. Isolation and identification of a human antibody to poly(ethylene glycol). Presented at Am. Soc. Hematol. Annu. Meet., 45th; San Diego. 2003. p. Abstr. 559A. [Google Scholar]
- 68.Tsutsumi Y, Onda M, Nagata S, Lee B, Kreitman RJ, Pastan I. Site-specific chemical modification with polyethylene glycol of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) improves antitumor activity and reduces animal toxicity and immunogenicity. Proc Natl Acad Sci USA. 2000;97:8548–53. doi: 10.1073/pnas.140210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baalousha M, Lead JR. Nanoparticle dispersity in toxicology. Nat Nanotechnol. 2013;8:308–9. doi: 10.1038/nnano.2013.78. Emphasizes the importance of dispersity in toxicological profiling of ENMs. [DOI] [PubMed] [Google Scholar]
- 70.Esfand R, Tomalia DA. Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov Today. 2001;6:427–36. doi: 10.1016/s1359-6446(01)01757-3. [DOI] [PubMed] [Google Scholar]
- 71.Sadekar S, Ghandehari H. Transepithelial transport and toxicity of PAMAM dendrimers: implications for oral drug delivery. Adv Drug Deliv Rev. 2012;64:571–88. doi: 10.1016/j.addr.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Golas PL, Matyjaszewski K. Marrying click chemistry with polymerization: expanding the scope of polymeric materials. Chem Soc Rev. 2010;39:1338–54. doi: 10.1039/b901978m. [DOI] [PubMed] [Google Scholar]
- 73.Fang C, Shi B, Pei YY, Hong MH, Wu J, Chen HZ. In vivo tumor targeting of tumor necrosis factor-α-loaded stealth nanoparticles: effect of MePEG molecular weight and particle size. Eur J Pharm Sci. 2006;27:27–36. doi: 10.1016/j.ejps.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 74.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5:505–15. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377:159–69. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 77.Walkey CD, Olsen JB, Guo H, Emili A, Chan WCW. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J Am Chem Soc. 2011;134:2139–47. doi: 10.1021/ja2084338. Depicts the complex relationships of NP size and surface chemistry in cellular uptake. [DOI] [PubMed] [Google Scholar]
- 78.Champion J, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res. 2008;25:1815–21. doi: 10.1007/s11095-008-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith AM, Gao X, Nie S. Quantum dot nanocrystals for in vivo molecular and cellular imaging. Photochem Photobiol. 2004;80:377–85. doi: 10.1562/0031-8655(2004)080<0377:QDNFIV>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 80.Kutscher HL, Gao D, Li S, Massa CB, Cervelli J, et al. Toxicodynamics of rigid polystyrene microparticles on pulmonary gas exchange in mice: implications for microemboli-based drug delivery systems. Toxicol Appl Pharmacol. 2013;266:214–23. doi: 10.1016/j.taap.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kutscher HL, Chao P, Deshmukh M, Sundara Rajan S, Singh Y, et al. Enhanced passive pulmonary targeting and retention of PEGylated rigid microparticles in rats. Int J Pharm. 2010;402:64–71. doi: 10.1016/j.ijpharm.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kutscher HL, Chao P, Deshmukh M, Singh Y, Hu P, et al. Threshold size for optimal passive pulmonary targeting and retention of rigid microparticles in rats. J Control Release. 2010;143:31–37. doi: 10.1016/j.jconrel.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chao P, Deshmukh M, Kutscher HL, Gao D, Sundara Rajan S, et al. Pulmonary targeting microparticulate camptothecin delivery system: anticancer evaluation in a rat orthotopic lung cancer model. Anti-Cancer Drugs. 2010;21:65–76. doi: 10.1097/CAD.0b013e328332a322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bonner JC, Silva RM, Taylor AJ, Brown JM, Hilderbrand SC, et al. Interlaboratory evaluation of rodent pulmonary responses to engineered nanomaterials: the NIEHS Nano GO Consortium. Environ Health Perspect. 2013;121:676–82. doi: 10.1289/ehp.1205693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xia T, Li N, Nel AE. Potential health impact of nanoparticles. Annu Rev Public Health. 2009;30:137–50. doi: 10.1146/annurev.publhealth.031308.100155. [DOI] [PubMed] [Google Scholar]
- 86.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–27. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 87.Fox ME, Szoka FC, Frechet JM. Soluble polymer carriers for the treatment of cancer: the importance of molecular architecture. Acc Chem Res. 2009;42:1141–51. doi: 10.1021/ar900035f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lovric J, Bazzi H, Cuie Y, Fortin GA, Winnik F, Maysinger D. Differences in subcellular distribution and toxicity of green and red emitting CdTe quantum dots. J Mol Med. 2005;83:377–85. doi: 10.1007/s00109-004-0629-x. [DOI] [PubMed] [Google Scholar]
- 89.Green M, Howman E. Semiconductor quantum dots and free radical induced DNA nicking. Chem Commun. 2005;2005:121–23. doi: 10.1039/b413175d. [DOI] [PubMed] [Google Scholar]
- 90.Cho WS, Cho M, Jeong J, Choi M, Cho HY, et al. Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicol Appl Pharmacol. 2009;236:16–24. doi: 10.1016/j.taap.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 91.Cho WS, Cho M, Jeong J, Choi M, Han BS, et al. Size-dependent tissue kinetics of PEG-coated gold nanoparticles. Toxicol Appl Pharmacol. 2010;245:116–23. doi: 10.1016/j.taap.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 92.Cho WS, Kim S, Han BS, Son WC, Jeong J. Comparison of gene expression profiles in mice liver following intravenous injection of 4 and 100 nm-sized PEG-coated gold nanoparticles. Toxicol Lett. 2009;191:96–102. doi: 10.1016/j.toxlet.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 93.Jeon SI, Andrade JD. Protein-surface interactions in the presence of polyethylene oxide: II. Effect of protein size. J Colloid Interface Sci. 1991;142:159–66. [Google Scholar]
- 94.Jeon SI, Lee JH, Andrade JD, De Gennes PG. Protein-surface interactions in the presence of polyethylene oxide: I. Simplified theory. J Colloid Interface Sci. 1991;142:149–58. [Google Scholar]
- 95.Owens DE, III, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 96.Yamaoka T, Tabata Y, Ikada Y. Distribution and tissue uptake of poly(ethylene glycol) with different molecular weights after intravenous administration to mice. J Pharm Sci. 1994;83:601–6. doi: 10.1002/jps.2600830432. [DOI] [PubMed] [Google Scholar]
- 97.Sharma G, Valenta DT, Altman Y, Harvey S, Xie H, et al. Polymer particle shape independently influences binding and internalization by macrophages. J Control Release. 2010;147:408–12. doi: 10.1016/j.jconrel.2010.07.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res. 2009;26:244–49. doi: 10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006;103:4930–34. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang X, Li L, Liu T, Hao N, Liu H, et al. The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano. 2011;5:5390–99. doi: 10.1021/nn200365a. [DOI] [PubMed] [Google Scholar]
- 101.Pedder SCJ. Pegylation of interferon alfa: structural and pharmacokinetic properties. Semin Liver Dis. 2003;23(Suppl 1):19–22. doi: 10.1055/s-2003-41635. [DOI] [PubMed] [Google Scholar]
- 102.Hamilton RF, Wu N, Porter D, Buford M, Wolfarth M, Holian A. Particle length-dependent titanium dioxide nanomaterials toxicity and bioactivity. Part Fibre Toxicol. 2009;6:35. doi: 10.1186/1743-8977-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chonn A, Cullis PR, Devine DV. The role of surface charge in the activation of the classical and alternative pathways of complement by liposomes. J Immunol. 1991;146:4234–41. [PubMed] [Google Scholar]
- 104.Xiao K, Li Y, Luo J, Lee JS, Xiao W, et al. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials. 2011;32:3435–46. doi: 10.1016/j.biomaterials.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamaoka T, Kuroda M, Tabata Y, Ikada Y. Body distribution of dextran derivatives with electric charges after intravenous administration. Int J Pharm. 1995;113:149–57. [Google Scholar]
- 106.He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31:3657–66. doi: 10.1016/j.biomaterials.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 107.El Badawy AM, Silva RG, Morris B, Scheckel KG, Suidan MT, Tolaymat TM. Surface charge-dependent toxicity of silver nanoparticles. Environ Sci Technol. 2011;45:283–87. doi: 10.1021/es1034188. [DOI] [PubMed] [Google Scholar]
- 108.Davis M. Particulate radiopharmaceuticals for pulmonary studies. In: Subramanian G, editor. Radiopharmaceuticals. New York: Soc. Nucl. Med; 1975. pp. 267–81. [Google Scholar]
- 109.Nagai H, Okazaki Y, Chew SH, Misawa N, Yamashita Y, et al. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc Natl Acad Sci USA. 2011;108:E1330–38. doi: 10.1073/pnas.1110013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 111.Koo OM, Rubinstein I, Onyuksel H. Role of nanotechnology in targeted drug delivery and imaging: a concise review. Nanomedicine. 2005;1:193–212. doi: 10.1016/j.nano.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 112.Giles J. Size matters when it comes to safety, report warns. Nature. 2004;430:599. doi: 10.1038/430599b. [DOI] [PubMed] [Google Scholar]
- 113.US Food Drug Admin. Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology. Silver Spring, MD: US Food Drug Admin; 2011. http://www.fda.gov/regulatoryinformation/guidances/ucm257698.htm. [Google Scholar]
- 114.Nel A, Xia T, Meng H, Wang X, Lin S, et al. Nanomaterial toxicity testing in the 21st century: use of a predictive toxicological approach and high-throughput screening. Acc Chem Res. 2013;46:607–21. doi: 10.1021/ar300022h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gunaseelan S, Gunaseelan K, Deshmukh M, Zhang X, Sinko PJ. Surface modifications of nanocarriers for effective intracellular delivery of anti-HIV drugs. Adv Drug Deliv Rev. 2010;62:518–31. doi: 10.1016/j.addr.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]