Abstract
Maintaining crop production to feed a growing world population is a major challenge for this period of rapid global climate change. No consistent conceptual or experimental framework for crop plants integrates information at the levels of genome regulation, metabolism, physiology and response to growing environment. An important role for plasticity in plants is assisting in homeostasis in response to variable environmental conditions. Here, we outline how plant plasticity is facilitated by epigenetic processes that modulate chromatin through dynamic changes in DNA methylation, histone variants, small RNAs and transposable elements. We present examples of plant plasticity in the context of epigenetic regulation of developmental phases and transitions and map these onto the key stages of crop establishment, growth, floral initiation, pollination, seed set and maturation of harvestable product. In particular, we consider how feedback loops of environmental signals and plant nutrition affect plant ontogeny. Recent advances in understanding epigenetic processes enable us to take a fresh look at the crosstalk between regulatory systems that confer plasticity in the context of crop development. We propose that these insights into genotype × environment (G × E) interaction should underpin development of new crop management strategies, both in terms of information-led agronomy and in recognizing the role of epigenetic variation in crop breeding.
Keywords: crop plants, plasticity, epigenetics, development, climate change, adaptation
Crop plasticity, G × E and epigenetics
Maintaining crop production to feed a growing world population is a major challenge for this period of rapid global climate change. The high crop yields following the Green Revolution arose from development of new varieties, irrigation and extensive application of fertilizers and pesticides during a period of atypical climate stability. Increases in crop yields have now slowed and are likely to decline further as productive regions experience greater temperature fluctuations and increased demand for finite phosphate and water resources.
Generating climate-resilient crops will require a comprehensive understanding of the relationship between the composition of crop genomes and the underlying gene regulatory mechanisms that confer environmental plasticity and climatic adaptation. Polyploidy has underpinned the evolution of plants, providing a level of gene redundancy that has facilitated adaptation in non-domesticated plants to previous periods of climate change. Our ability to harness the plasticity of complex crop genomes demands greater insights into epigenetic processes and how these mediate signals between the environment, regulatory networks and gene function.
No consistent conceptual or experimental framework for crop plants integrates information at the levels of genome regulation, metabolism, physiology and response to growing environment. The approach outlined here is to evaluate plant plasticity in the context of epigenetic regulation of developmental phases and transitions, and map these onto the key stages of crop establishment, growth, floral initiation, pollination, seed set and maturation of harvestable product. In particular, we consider how feedback loops of environmental signals and plant nutrition affect plant ontogeny. Recent advances in understanding epigenetics enable us to take a fresh look at the crosstalk between regulatory systems that confer plasticity in the context of crop development. We propose that these insights into genotype × environment (G × E) interaction should underpin development of new crop management strategies, both in terms of information-led agronomy and in recognizing the role of epigenetic variation in crop breeding.
Biological plasticity refers to distinct or overlapping phenomena at different levels of organization, while organismal plasticity may be defined as an individual's response to environmental conditions or to biological and developmental cues. In contrast, phenotypic plasticity refers to the range of phenotypes a single genotype can express as a function of its environment, where adaptive plasticity is a form of the latter that specifically increases the global fitness of a genotype (Nicotra et al., 2010). Meanwhile, developmental plasticity is the capacity of a genotype or an individual's progeny to express phenotypic variation without DNA sequence mutations (Jablonka, 2012).
In the context of crop plants, plasticity plays a key role in adaptation and potential resilience of cultivars to specific or changing growing environments. Phenological plasticity refers to the specific ability of a plant to adjust to seasonal variations during development (Rathcke and Lacey, 1985), although during species divergence, this may not always result in selection of advantageous traits and may limit the ability of a species to invade new habitats (Levin, 2009). However, in the context of generating crops resilient to climate change, such variation may be desirable. In the case of allopolyploid crops such as canola, wheat, oat, cotton and coffee, functional plasticity is the ability of an organism to relocate functions from one tissue to another as a result of paralogous genes (Osborn et al., 2003; Zhao et al., 2011).
Plasticity in plants assists in maintaining homeostasis in response to variable environmental conditions. Of particular relevance to crops, this may occur in response to permanent (soil type, altitude, niche), regular (seasonal, day length and light quality) or intermittent (rain, ambient temperature, wind) abiotic factors. Biotic influences eliciting plastic responses include herbivory, disease and plant–plant competition (Latzel et al., 2012). Each of these factors may to some extent influence development and timing of reproductive organs and sexual reproduction. Crop cultivars are also likely to suffer the fate of plant species that lack a plastic response to environmental cues, when a change in environment may drive less plastic plant species more rapidly into decline (Böhnke and Bruelheide, 2013).
Epigenetic processes confer plasticity
Definitions of epigenetics have varied over the past 60 years, from ‘how genotypes give rise to phenotypes during development’ (Waddington and Kacser, 1957) to ‘the study of mitotically and/or meiotically heritable changes in gene function that cannot be explained by changes in DNA sequence’ (Russo et al., 1996). More recently, Bird (2007) defined epigenetics as ‘the structural adaptation of chromosomal regions so as to register, signal or perpetuate altered activity states’, which encompasses current understanding of molecular epigenetics involving components of DNA methylation, histone variants, small RNAs, transposable elements (TEs) and associated chromatin modulation. These components may be categorized as either (i) genome plasticity: structural or organizational genome changes associated with environmental signals leading to novel phenotypes (Nicotra et al., 2010) or (ii) epigenetic plasticity: the ability to modulate a genome for discrete cellular functions (Zhu and Reinberg, 2011).
Dynamic chromatin structure contributes to regulation of cell stage and identity and is influenced by environmental and endogenous factors through variation in the genomic distribution of DNA and histone epigenetic marks (Jarillo et al., 2009). These marks affect chromatin condensation and packaging, with specific molecular modifiers on histone proteins able to influence DNA transcription. Transient chromatin modifications have been suggested as the primary epigenetic system regulating epigenetic marks and machinery (Meagher, 2010).
Nucleosomes are highly conserved among eukaryotes and form the building blocks of chromatin, comprised of 147 bp DNA wound around dimers of four core histones: H2A, H2B, H3 and H4 (Sarma and Reinberg, 2005), and a further 20 bp associated with H1 linker histones forming the chromatosome (Kowalski and Palyga, 2012; Simpson, 1978). Within these five histone families, there are 13 subfamilies comprising of at least 76 variants, each of which can be chemically modified (e.g. methylation, acetylation, phosphorylation, etc.), providing an additional epigenetic code superimposed on the genome (Punta et al., 2012). Histone remodelling in situ, dimer exchange or variant replacement can confer structural and functional modifications to the chromatin resulting in altered levels of transcription (Ahmad et al., 2010; Bernstein and Hake, 2006; Kamakaka and Biggins, 2005; Sarma and Reinberg, 2005), particularly by modifying the ability of the transcription machinery to bind up- or downstream promoter regions. This rich variation underlies many aspects of orchestrated ontogeny as well as providing scope for plasticity by affecting the ability of genes to be expressed at an appropriate level both spatially, temporally (Cohen et al., 2009) and in response to environment.
Cytosine methylation (5mC), replicated by methyltransferases during mitosis, provides a stable but plastic epigenetic mark superimposed on genomic DNA (Goettel and Messing, 2013; Zhang et al., 2013b), with a key role in silencing TEs and gene expression levels (He et al., 2011; Tsuchiya and Eulgem, 2013). The methylome represents the full set of DNA methylation marks, with potential for further in vivo chemical modifications resulting in conversion to 5-hydroxymethyl, 5-formyl and 5-carboxyl cytosines, for which structural, regulatory and functional roles are currently being established (Kriukiene et al., 2012).
Transposable elements are often hypermethylated and comprise a large portion of many crop genomes, such as barley (84%) and wheat (80%), sorghum (55%) and soybean (59%; Cantu et al., 2010; Mayer et al., 2012; Paterson et al., 2011; Schmutz et al., 2010). TEs are also able to act as a source of novel genes, such as Daysleeper in Arabidopsis from the hobo/Ac/Tam superfamily of TEs (Alzohairy et al., 2013), Gary in a number of cereal genomes (Muehlbauer et al., 2006) and SchAT in sugarcane (de Jesus et al., 2012).
Silencing of TEs may involve a variety of epigenetic marks including DNA methylation, histone 3 lysine dimethylation and small interfering RNAs (Bucher et al., 2012; Sasaki et al., 2012), with perturbation of DNA methylation in TE regions able to reactivate their expression (Kantama et al., 2013). DNA methylation is generally recognized as providing a natural defence system for plants against the expansion of TEs (Kantama et al., 2013). Recent evidence in maize indicates that genes <500 bp from TEs are expressed less than genes further up- or downstream (Goettel and Messing, 2013), with repression of the gene able to be influenced by the epigenetic marks employed in silencing the TE.
In plants, cytosine methylation occurs in three sequence contexts, with decreasing abundance of CG, CHG and CHH (where H is any nucleotide except G). This contrasts with mammalian systems, where it occurs predominantly in a CG context (Jaenisch and Bird, 2003). Each methylation context in plants is thought to be regulated primarily by three different families of DNA methyltransferases MET1 for CG, CMT for CHG and DRM for CHH (Law and Jacobsen, 2010), although a more recent study has indicated the possibility of additional methyltransferases (Stroud et al., 2013). Variation in DNA methylation can have significant effects on chromatin structure, with increases in 5mC marks associated with the alignment of nucleosomes in plant genomes (Chodavarapu et al., 2010).
The distribution of epialleles superimposed on genetic maps can be revealed by methylation-sensitive AFLP and retrotransposon epimarkers. In Brassica napus biparental populations, this distribution appears nonrandom, with some clustering of parent-specific methylated alleles (Long et al., 2011). This study demonstrated a high level of stability, with 90% of mapped markers retaining their allelic pattern in contrasting environments and developmental stage, and stable transmission through multiple meioses.
Methylome maps at base pair resolution have been generated for Arabidopsis (Zhang et al., 2006), rice (Oryza; Li et al., 2012b) and tomato (Solanum lycopersicum; Zhong et al., 2013) based on bisulphite sequencing, where unmodified cytosines are converted to uracil (sequenced as T) and 5mC remains sequenced as C. Methylation states are far from static, with reproductive tissues of endosperm and embryo, from torpedo-stage seeds in particular, undergoing large-scale re-patterning in Arabidopsis (Gehring et al., 2009). Methylome mapping of tomato fruit has shown that while distinct methylation patterns are associated with specific tissues and reproductive organs, mean methylation levels remain similar (Zhong et al., 2013). However, demethylation of TE regions is observed, with otherwise variable patterns of DNA methylation throughout the developmental pathway. microRNAs (miRNAs), a class of small noncoding RNA, may also regulate initiation of fruit development in tomato, with miR393 and miR167 appearing to target a putative auxin receptor (SITIR1) and auxin response factors that consequently modulate auxin, a key regulator of fruit development (Karlova et al., 2013).
Comparison of tissue-specific methylomes can provide an indication of changing patterns of gene regulation associated with epigenetic plasticity. Actively transcribed regions of euchromatin are often associated with hypomethylated DNA, whereas condensed heterochromatin regions are frequently hypermethylated (May, 2010). The reversibility of DNA methylation states may confer plasticity to an organism by facilitating regulation of RNA transcription from genomic regions or specific genes. Hypermethylated DNA regions can lead to transcriptional silencing and result in a quiescent state, which can later be derepressed through demethylation triggered by signalling factors. For example, during rice endosperm biogenesis, DNA demethylation is crucial for correct gene transcription (Zemach et al., 2010).
Two broad classes of post-transcriptionally processed small noncoding RNAs (snRNAs), miRNA and small interfering RNA (siRNA), are associated with gene silencing as antisense compliments to untranslated genome regions, which include DNA transcription factor (TF)-binding sites (Bartel, 2004; Pattanayak et al., 2013). snRNAs are generally short lived within cells, but can play key regulatory roles in plant development and G × E interactions via pre- and post-transcriptional regulation of gene expression (Ahmad et al., 2010). Crucially, their mobility enables signal transduction beyond the immediate site of initiation via plasmodesmata or over longer distances through the phloem (Uddin and Kim, 2011). This confers considerable plasticity, particularly in relation to mediating homeostasis of mineral nutrients from root source to vegetative and reproductive sinks. Several regulatory elements are responsible for the differential expression of miRNAs among cell types (Zeng, 2006), with variations in the stepwise nature of the process. RNA-directed DNA methylation (RdDM) is an important epigenetic process that in plants involves snRNA-mediated targeting of de novo DNA methylation by DNA methyltransferase DOMAINS REARRANGED METHYLTRANSFERASE2 (DRM2; Chan et al., 2004; Henderson, 2012; Matzke et al., 2007).
A developmental framework for crop yield and quality
Prior to harvest, most crops undergo phase transitions through one or more developmental stages. These include germination, seedling establishment and root and/or tuber development, juvenility, vegetative branching, floral induction, inflorescence development, gamete production, pollination, development and maturity of fruit and seed (Figure 1). For food crops including staple cereals and oilseed grains, the latter stages of seed development and maturity represent the final stages of yield potential, whereas fruit crops depend on the timely development of carpel or associated tissues. Vegetables represent a wide range of organs that are harvested at specific stages, with some such as cauliflower or broccoli arrested at defined stages during reproductive development. For vegetatively propagated crops, the regeneration of rooting systems is a key determinant of establishment (Shepherd et al. 2013).
Figure 1.
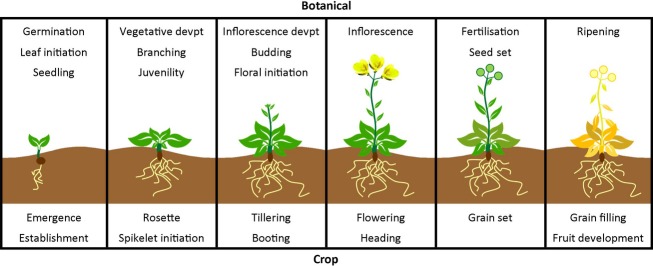
Typical stages within the crop cycle, with corresponding stages of plant development.
Crop establishment, vegetative growth and canopy architecture
The rapid and synchronized establishment of crops is dependent upon successful germination and subsequent seedling vigour that enables rapid search and acquisition of water and nutrient resources while competing with weeds. Plasticity during vegetative growth provides flexibility in decisions relating to timing and patterning of stem and root branching, meristem identity variations and individual cell responses.
It has become apparent that epigenetic mechanisms play important roles during these early growth phases. Of the more than 40 genes involved with chromatin remodelling in Arabidopsis, at least 17 are implicated in maintenance of vegetative growth (Jarillo et al., 2009). These include genes expressed in shoot apical meristems (SAMs) such as AtBRAHMA (AtBRM), BRUSHY1 (BRU1)/MGOUN3 (MGO3)/TONSOKU (TSK), FASCIATA 1/2 (FAS1/2), MULTICOPY SUPPRESSOR OF IRA 1 (MSI1) and SPLAYED (SYD; Table 1; Jarillo et al., 2009). Both vegetative and reproductive meristem identities are influenced by epigenetic regulation, such as the DNA methylation within the rice OsMADS1 promoter region, and OsLEC1 stimulation of histone H3 post-translational modifications including methylation and acetylation (Table 1; Zhang and Xue, 2013). Expression of specific histone modifiers can occur either in an organ specific manner or throughout the plant during growth phases. In tomato, 124 post-translational histone modifiers have been described that cover a broad functional range and include both novel and homologous motifs shared with Arabidopsis, maize and rice (Cigliano et al., 2013).
Table 1.
Examples of plant genes effected by or involved with epigenetic mechanisms (Table updated from King et al., 2010)
| Species | Crop trait | Gene | Gene class | Gene function | Epigenetic mode | References |
|---|---|---|---|---|---|---|
| Arabidopsis | Vernalization | AG | MADS domain type | Reduces H3K27 trimethylation of KNU | – | Ito (2012), Lamesch et al. (2011) |
| Arabidopsis | Vernalization | AGL19 | MADS-box | H3K27 trimethylation, possibly regulated by PRC2 | – | Lamesch et al. (2011), Schonrock et al. (2006) |
| Arabidopsis | Flowering | AP1 | MADS domain protein | – | – | Lamesch et al. (2011), Saleh et al. (2008b) |
| Arabidopsis | Vegetative development and flowering | AtBRM | SWI/SNF chromatin-remodelling ATPase | Involved in chromatin remodelling | – | Jarillo and Pineiro (2011), Lamesch et al. (2011) |
| Brassica | Flowering and fertilization | BcJMJ30 | jmjC domain-containing histone demethylase | Putative histone demethylase regulating cell fate | – | Lamesch et al. (2011), Li et al. (2012c) |
| Arabidopsis | Plant architecture | BNS | Anaphase-promoting complex | Methylated | Hypermethylation induces by ddm1 dominance and abnormal floral morphology | Saze and Kakutani (2007) |
| Arabidopsis | Vegetative development | BRU1/MGO3/TSK | Tetratricopeptide repeat | Putative part of a protein complex involved in chromatin organization | – | Jarillo and Pineiro (2011), Lamesch et al. (2011) |
| Arabidopsis | – | CMT3 | Chromomethylase | DNA methylation maintenance in CHG contexts | – | Lamesch et al. (2011), Law and Jacobsen (2010) |
| Solanum lycopersicon | Fruit (pericarp) ripening | CNR | MADS TF | Hypomethylated | Hypermethylation | Manning et al. (2006) |
| Linaria vulgaris | – | CYCLOIDEA | Class II TCP transcription activator | Hypomethylated. Normal floral symmetry | Hypermethylated allele confers irregular floral symmetry | Cubas et al. (1999) |
| Oryza | Stature | D1 | – | – | Transcriptional initiation site differentially hypermethylated in metastable Epi-d1 | Miura et al. (2009b) |
| Arabidopsis | Flowering/fertilization | DDM1 | SWI2/SNF2-like chromatin-remodelling protein | DNA methylation and heterochromatin maintenance | – | Lamesch et al. (2011), Melamed-Bessudo and Levy (2012) |
| Arabidopsis | – | DME | DNA glycosylase DEMETER | Maternal-allele-specific hypomethylation at the MEDEA (MEA) gene | – | Lamesch et al. (2011) |
| Arabidopsis | – | DRM2 | Dormancy/auxin-associated family protein | DNA methylation maintenance in CHH contexts | – | Law and Jacobsen (2010) |
| Arabidopsis | – | FAS1/2 | Chromatin Assembly Factor-1 | De novo nucleosome assembly during DNA replication | Reduced heterochromatin content | Jarillo and Pineiro (2011), Lamesch et al. (2011) |
| Arabidopsis | Seed development | FIE | Polycomb protein | – | – | Ohad et al. (1996) |
| Arabidopsis | Seed development | FIS2 | TF | Methylated | Mediated by DEMETER | Luo et al. (1999) |
| Arabidopsis | Vernalization | FLC | Nuclear localized | – | Modified histone derepresses | Bastow et al. (2004), Saleh et al. (2008b) |
| Arabidopsis, Brassica | Flowering, vernalization | FT | Phosphatidylethanolamine-binding protein | Paralogues independently silenced by 5mC | – | Lamesch et al. (2011) |
| Arabidopsis thaliana | Seed development | FWA | Homeodomain-containing TF | Hypermethylated and positively regulates flowering | Hypomethylated allele confers late flowering, mediated by DEMETER | Kinoshita et al. (2004), Soppe et al. (2000) |
| Helianthus | Embryogenesis | HaL1L | CCAAT box-binding factor | Differential methylation of CG rich regions | – | Salvini et al. (2012) |
| Arabidopsis | Floral number | KNU | C2H2-type zinc finger protein | Reduction in H3K27 trimethylation resulting in its derepression | Precocious flowering | Ito and Sun (2009), Lamesch et al. (2011), Sun et al. (2009) |
| Arabidopsis | Seed development | MEA | Polycomb protein | Methylated | Mediated by DEMETER | Grossniklaus et al. (1998) |
| Zea mays | Seed development | MEG1 | Cys-rich protein | Biallelic at later stages | Maternal parent-of-origin expression during early stages of endosperm; development | Gutierrez-Marcos et al. (2004) |
| Arabidopsis | Vegetative development, seed set, ripening | MET1 | Bromo adjacent homology (BAH) domain, C-5 cytosine methyltransferase | Maternal hypomethylation patterning and transgenerational epigenetic inheritance | Perturbation of auxin gradients in embryos | Lamesch et al. (2011), Law and Jacobsen (2010), Paszkowski and Grossniklaus (2011) |
| Arabidopsis | Seed development | MPC | Poly(A)-binding protein | – | – | Tiwari et al. (2008) |
| Arabidopsis | – | MSI1 | WD-40 repeat-containing protein | Involved in de novo nucleosome assembly | – | Jarillo and Pineiro (2011), Lamesch et al. (2011) |
| Oryza | Vegetative and reproductive development | OsLEC1 | CCAAT box-binding factor | Stimulates histone post-translational modification | – | Kawahara et al. (2013), Sakai et al. (2013) |
| Oryza | Sexual reproduction | OsMADS1 | MADS-box TF | Methylated promoter region | – | Kawahara et al. (2013), Sakai et al. (2013), Zhang and Xue (2013) |
| Arabidopsis | PAI2 | Phosphoribosylanthranilate isomerase | Unmethylated. Does not fluoresce under UV light | Methylated allele leads to fluorescent shoots under UV light | Bender and Fink (1995) | |
| Arabidopsis | Seed development | PHERES1 | MADS TF | – | – | Kohler and Makarevich (2006) |
| Zea mays | Grain colour | PI | DNA binding, similar to MYB oncogenes | Unmethylated allele P-rr confers uniform pigmentation of pericarp | Methylated alleles of P-pr-1 and P-pr-2 give variegated pigmentation of pericarp | Das and Messing (1994) |
| Arabidopsis, Brassica | Nucleolar dominance | rDNA | Ribosomal RNA | – | Nucleolar dominance | Preuss and Pikaard (2007) |
| Arabidopsis | Establishment | SKP2B | F-box | Represses lateral root formation | – | Lamesch et al. (2011), Manzano et al. (2012) |
| Brassica | Self-incompatibility | SP11 | Cys-rich protein | Promoter methylated | Demethylation leads to transcription | Shiba et al. (2006) |
| Arabidopsis | Male appendages | SUPERMAN | Zinc finger protein | Unmethylated. Normal flower development | Hypermethylated allele results in excessive staminoid organs | Jacobsen and Meyerowitz (1997) |
| Arabidopsis | – | SYD | SWI2/SNF2-like protein | – | – | Jarillo and Pineiro (2011), Lamesch et al. (2011) |
| Arabidopsis | Vernalization | VRN1/VIN3 | TF | Reduces H3K9 acetylation and dimethylation of FLC | – | Bastow et al. (2004), Lamesch et al. (2011), Sung and Amasino (2004) |
TF, transcription factor.
In Arabidopsis, the overall 5mC genome content increases from cotyledon emergence through to reproductive organogenesis (Ruiz-Garcia et al., 2005). This suggests a broad range of genes are involved early in plant life cycles, while in reproductive tissues, a more specialized set of genes are required. Although increased methylation has generally been associated with a decrease in gene expression, Arabidopsis RNA expression profiles lack a corresponding decrease in overall transcription throughout vegetative growth (Richards et al., 2012).
Adverse abiotic environments can induce plant miRNA expression profiles that may be unique to a given stressor, although many miRNAs are expressed in response to more than one stressor (López et al., 2012). In sugarcane (Saccharum spp.), miRNAs are expressed additively with increasing water deficit, suggesting a progressive up-regulation of genes contributing to drought response (Ferreira et al., 2012; Gentile et al., 2013). Accumulation of miR159 triggered by abscisic acid (ABA) appears to contribute to inactivation of sugarcane axillary bud outgrowth (Ortiz-Morea et al., 2013).
Involvement of plant hormones in signalling networks can also elicit plastic responses to environmental conditions (Xu et al., 2013), with crosstalk to the epigenome system including histone modifications. For example, ABA has been shown to induce H3K4 trimethylation, which acts with H3K9 acetylation as a gene activation marker (Kim et al., 2013a). Multiple ABA receptors may affect different subsets of pathways, affecting vegetative processes such as germination, stomatal movement and osmotic regulation, depending from which tissue or subcellular compartment signals arise (Golldack et al., 2013; Nakashima and Yamaguchi-Shinozaki, 2013; Xu et al., 2013).
Shoot branching can be regulated by auxin and strigolactones (Stirnberg et al., 2010; Yaish et al., 2010), with axillary meristems undergoing an initial period of rapid cell division to form an axillary bud, often followed by temporary or permanent arrest (Leyser, 2009). In Arabidopsis, BRANCHED1 (BRC1) is a key local factor contributing to bud arrest that is up-regulated in response to shading from neighbours, which is perceived as a decrease in red : far red (R : FR) light ratio, and is negatively regulated by phytochrome B (Gonzalez-Grandio et al., 2013; Hofmann, 2013; Niwa et al., 2013). Repression of BRC1 in axillary buds allows shoot development to restart. However, simultaneous up-regulation of FLOWERING LOCUS T (FT) and repression of BRC1 can result in a transition from axillary bud to an inflorescence meristem (Hofmann, 2013; Martin-Trillo et al., 2011; Stirnberg et al., 2010). Thus, the ability of plants to exhibit plasticity in response to external stimuli may not always be a consequence of epigenetic regulation.
Nutrient acquisition and root development
Crop productivity relies on adequate and balanced supplies of nutrients to ensure timely growth and progression through developmental stages. Plants require 16 elements to complete their life cycle, and the available quantities of these elements vary widely across soils and can fluctuate with seasonal changes. Plants have thus evolved a number of mechanisms that regulate the uptake and homeostasis of these elements throughout their life cycle. Nutrient homeostasis relies on several large families of transporters to facilitate root uptake and subsequent distribution of micronutrient anions and cations (Gierth and Maser, 2007; Rausch and Bucher, 2002; White and Broadley, 2009). The regulation of these transporters typically involves a series of gene networks and signalling systems that respond to external (i.e. at the root surface) or internal stimuli. For example, root plasticity in response to variation in soil temperature (soybean) and water availability (tomato) appears to be facilitated by modulation of 5mC and histone modifications (Gonzalez et al., 2013; Stepinski, 2012). These gene networks, their molecular regulation and post-transcriptional regulation have been widely studied (see reviews by Fox and Guerinot, 1998; Glass et al., 2002; White and Broadley, 2009).
To respond to changing mineral availability and requirements during crop development, and in impoverished soils, it is important for crop cultivars to incorporate plasticity in management of source/sink relationships. It has been demonstrated in Arabidopsis that miRNAs are part of a programmed response to fluctuating nutrient availability, with several plant miRNAs found to regulate TF expression, such as miR169 targeting HAP2, and target genes involved in phosphate, sulphate and copper metabolism (Kehr, 2013; López et al., 2012; Zeng et al., 2014). For example, inorganic phosphorus deprivation leads to induced expression of miR156, miR399, miR778, miR827 and miR2111 and repression of the expression of miR169, miR395 and miR398 (Hsieh et al., 2009). To date, the role of miR399 has been elucidated (reviewed by Rouached et al., 2010), and miR395 has been shown to be induced by sulphate limitation (Kawashima et al., 2009), while miR398 is induced by copper deficiency (Beauclair et al., 2010). An overlapping web of miRNAs associated with nutrient stress response has been inferred from the response of a number of these miRNAs to nitrogen starvation in maize, rice and common bean (Fischer et al., 2013). Understanding the dynamics of epigenetic regulation affecting nutrient homeostasis will open up new approaches for crop agronomic management.
Epigenetic processes have also been strongly implicated in root development. A combination of auxin and histone modulation regulate S-PHASE KINASE-ASSOCIATED PROTEIN2B (SKP2B) expression (Table 1), a repressor of meristem cell division in Arabidopsis that can regulate root branching (Manzano et al., 2012; Saini et al., 2013). Roots tend to proliferate and physiologically enhance their nutrient uptake capacity when encountering a nutrient-rich zone (Hodge, 2004). However, the actual benefit to the plant from root plasticity, while seemingly obvious, has been challenging to quantify due to the technical difficulties in assessing traits such as quantifiable root responses to nitrogen-rich soil patches (Hodge, 2004) and their effects on root epigenetic marks and mechanisms (Manzano et al., 2012). Recent work has demonstrated that miR390 is involved in lateral root initiation and is possibly mobile within the root primordia (reviewed in Marin-Gonzalez and Suarez-Lopez, 2012). Loss of SDG2-mediated chromatin modulation resulting in reduced H3K4 methylation in root stem cells also impairs later root establishment and causes a loss of auxin gradient maximum (Yao et al., 2013).
Climate control of floral initiation
Development of resilient crop cultivars requires adaptation to increased variability in the seasonal patterns of temperature and precipitation, where significant changes in phenology have been observed over the past two decades. This leads to the prospect of optimal growing regions for particular crops migrating to different latitudes, with consequent changes in photoperiod affecting key stages of floral induction and reproductive development.
Plasticity enables over-wintering annuals and perennial crops to respond to the length and severity of winter, while retaining tightly regulation that ensures individuals are coordinated in reaching reproductive maturity. The passing of winter perceived as the vernalization signal enables promotion of the flowering programme (Ausin et al., 2005; Guzy-Wrobelska et al., 2013; Song et al., 2012; Srikanth and Schmid, 2011; Yamaguchi and Abe, 2012), with considerable variation in the cardinal temperatures (from 4 to 19 °C), reflecting regional adaptation to latitude and altitude (Wollenberg and Amasino, 2012; Wurr et al., 2004). In vernalization-sensitive species, the length of winter is perceived as an integral of daytime degrees below a threshold temperature by an additive effect of histone modifications that regulate silencing of FLOWERING LOCUS C (FLC; Table 1; Sheldon et al., 2009).
The vernalization signal can occur at different developmental stages depending on species and is recorded by epigenetic switches that can be maintained through in vitro vegetative propagation, but is reset during sexual reproduction (Song et al., 2012). The mechanism typically involves active repression of FLC or FLC-like genes until a target threshold has been perceived (Sheldon et al., 2009; Xiao et al., 2013). In Arabidopsis, FLC is repressed by reducing H3 acetylation and dimethylation of H3K9 through the action of REDUCED VERNALIZATION 1/VERNALIZATION INSENSITIVE 3 (VRN1/VIN3; Table 1; Bastow et al., 2004; Sung and Amasino, 2004). After winter, derepression of FLC occurs gradually by histone remodification (Song et al., 2012). In both winter and spring rape (B. napus) 5mC is significantly decreased in response to cold treatment, with subsequent gradual re-methylation to pretreatment levels in spring rape, but only up to 70% in winter rape (Guzy-Wrobelska et al., 2013). Similar cold-induced changes in 5mC patterning has been reported in other crop species, including cotton, maize, rice and wheat, suggesting some overall mechanistic conservation of epigenetic regulation across plant taxa (Fan et al., 2013; Pan et al., 2011; Sherman and Talbert, 2002; Steward et al., 2002).
A network of TFs associated with vernalization mediates signal transduction to floral initiation. Although this control pathway is not fully understood, the AGAMOUS-like MADS-box gene AGL19 is expressed in response to vernalization (Kim et al., 2013b; Schonrock et al., 2006) and activates LEAFY (LFY) and APETALA1 (AP1), both involved in floral meristem induction and transition (Lamesch et al., 2011). Elevated H3K27 trimethylation in the AGL19 chromatin region suggests epigenetic regulation (Table 1), which may itself be regulated by POLYCOMB REPRESSIVE COMPLEX 2 (PRC2), also acting in response to vernalization (Schonrock et al., 2006). Small RNAs are involved in the transmission and regulation of flowering time signals in response to vernalization (Oh et al., 2007) and include miR399, first reported as associated with inorganic phosphorus homeostasis (Fujii et al., 2005). miR399 appears to respond to temperature and induce floral development via its target gene AT2G33770.1 (PHO2; Kim et al., 2011). This suggests that nutrient uptake may also promote flowering after a nutrient concentration switch in vegetative tissue is triggered (Matsoukas et al., 2012).
The light programme
Plants have innate pathways that lead to phenotypic variation under different conditions of day length, light quantity and quality (Song et al., 2012). The photoperiod pathway is an ancient adaptation (Valverde, 2011), with photoreceptors, including phytochromes (PHYA—PHYE) and cryptochromes (CRY1 and CRY2; Ausin et al., 2005) detecting photoperiodicity, with signalling integrated into the regulatory network of the internal circadian clock (Ausin et al., 2005; Boss et al., 2004). A variety of epigenetic mechanisms have been shown to underlie the photoperiod pathway, with modulation of DNA methylation in several plant species (Kondo et al., 2010; Thanananta et al., 2006) including B. napus (Guzy-Wrobelska et al., 2013), histone modulation in rice (Wang et al., 2013) as well as transcriptional, post-transcriptional and post-translational regulation in Arabidopsis (Ausin et al., 2005).
Circadian rhythm and ageing contribute as components of the autonomous pathway and are able to provide a more resilient route to floral initiation (Arana et al., 2011). The circadian pathway allows pre-empting of expected daily and seasonal fluctuations via multiple feedback loops and light inputs (Mouradov et al., 2002; Troein et al., 2009). Genes involved fall into two broad categories of RNA-processing proteins and chromatin modulation and maintenance (Srikanth and Schmid, 2011). Circadian rhythms are able to influence strong control of flowering time; in Arabidopsis, MADS AFFECTING FLOWERING 5 and FLC transcription is affected by the circadian clock mutation late elongated hypocotyl 1 (Fujiwara et al., 2010). For perennial crops, plant age can be a significant factor in floral initiation (Srikanth and Schmid, 2011). This can have significant economic impact in terms of the number of years taken for crop establishment prior to productive yields. In Arabidopsis and maize, miR156 and miR172 play key roles in enabling floral development in an age-dependant manner (Jarillo and Pineiro, 2011; Yamaguchi and Abe, 2012).
Managing juvenility
Many plants undergo vegetative phase change (VPC), where pronounced changes in leaf morphology, orientation, anatomy and chemistry accompany puberty (Figure 1). The duration of the juvenile growth period has considerable consequences for economic production and scheduling of many crops including sustainable forest and tree fruit production, where long periods of juvenility are often required prior to reproductive or harvestable maturity (Thomas, 2013).
Vegetative phase change is most evident in trees, where it often occurs earlier than the transition to reproductive competence and is thought to be largely regulated independent of the floral transition (Poethig, 2003), although some tree species may have the ability to initiate floral transitions on branches exhibiting juvenile foliage (Jaya et al., 2010a,b, 2011; Wiltshire et al., 1998). It has long been recognized that epigenetics plays a major role in regulation of the plasticity associated with relative stability and immunity to environmental influences, yet reversibility of VPC at gametogenesis (Greenwood and Hutchison, 1993). In Arabidopsis, VPC is regulated by a set of TFs (SQUAMOSA PROMOTER-BINDING PROTEIN-LIKE [SBP/SPL proteins]) which are repressed during the juvenile phase by miR156 and miR157. Reduction in these miRNAs leads to expression of SBP/SPL proteins (Poethig, 2003), with miR172 having an inverse expression pattern. Suppression of flowering by miR156 can be overcome, demonstrating the independence of flowering and VPC (Poethig, 2010). More recently, HYPONASTIC LEAVES1 has been shown to regulate levels of miR156-targeted SPL genes, enabling plants to maintain the juvenile phase, ensuring maximum growth and productivity (Li et al., 2012a). This miRNA-mediated regulation appears conserved across a range of annual and woody perennial plants (Wang et al., 2011), despite the diverse and taxa-specific phenotypic manifestation of VPC.
As many pathways can be influenced by epigenetic marks (Brown et al., 2012; Cazzonelli and Pogson, 2010; Chinnusamy and Zhu, 2009; McGarry and Kragler, 2013; Pauli et al., 2011; Popova et al., 2013), it may be possible to use targeted epigenetic manipulation to decouple vegetative maturation and reproductive organogenesis in crop species (McGarry and Kragler, 2013). Rapid regeneration of a mature tree in response to extensive damage (e.g. fire) can occur from primed dormant meristematic nodes and may involve phenotypic reversion to a juvenile appearance for a short period. The ability to revert to a juvenile expression programme suggests significant epigenetic similarities between adult and juvenile meristems.
Inflorescence induction
Induction of flowering represents the key transition affecting harvestable potential of many crops. The inflorescence induction pathway is highly regulated and complex (Tan and Swain, 2006) and can also exhibit extremely large plastic responses (Bernier, 1986). This has led to multiple routes to flowering, with extensive involvement of epigenetic regulation in the autonomous, vernalization, gibberellin and photoperiod induction pathways (Yaish et al., 2011). Among plant species, the location of floral induction varies. In crops such as wheat (Danilevskaya et al., 2008) or common bean (Repinski et al., 2012), the SAM develops into an inflorescence meristem (IM), resulting in terminal determinate inflorescence (Figure 1). In contrast, Brassica species develop indeterminate raceme inflorescences, while in some eucalypt trees, axillary inflorescences are induced along lateral branches (Jaya et al., 2010a).
For crops such as canola, plasticity of floral timing is apparent in the very large number of environment-specific quantitative trait loci (QTLs) identified (Shi et al., 2009). Plasticity regulated by epigenetic mechanisms is likely to have been under selection in landraces and modern cultivars adapted to different latitudes. Brassica napus FT paralogues may be independently silenced by 5mC (e.g. FT-C2) to repress expression (Table 1), with FT-A7 and FT-C6 paralogues having distinct expression profiles under vernalization-free conditions in cultivar types selected specifically for winter or spring planting (Wang et al., 2012).
The autonomous floral promoting pathway can be influenced by signals from the vernalization, photoperiod, hormonal and circadian pathways. In Arabidopsis, the autonomous pathway can itself intervene in these floral enabling pathways via FLC suppression (Adams et al., 2009). Expression of GA INSENSITIVE DWARF 1 (GID1) genes in Arabidopsis oscillates diurnally and may be a result of circadian clock activity (Arana et al., 2011). There is evidence for diurnal oscillation of gibberellic acid (GA) in both Arabidopsis (Hisamatsu et al., 2005; Zhao et al., 2007) and sorghum (Lee et al., 1998), indicating a link between autonomous and hormone pathways.
Modulation of epigenetic marks to modify and fix novel phenological profiles that accelerate onset of floral initiation and anthesis would enhance productivity in crops such as canola (Kondo et al., 2010). Conversely, bioenergy crops and potato tuber (Solanum) production would benefit from an increase in length of the juvenile growth stage. Accelerated flowering has been achieved in Solanum sp. treated with 5-Azac (Marfil et al., 2012). In rice, hypomethylation by 5-Azac and zebularine has induced stable transgenerational dwarfism (Akimoto et al., 2007; Sano et al., 1990), although the specific genomic networks involved have not been characterized.
Synchronization of flowering in crops
Precocious flowering can reduce crop yield; in Arabidopsis, this may occur where the TF WUSCHEL (WUS) is not repressed by KNUCKLES (KNU), resulting in floral stem cell over-proliferation (Ito and Sun, 2009). KNU is derepressed by reduction in the H3K27me3 chromatin mark just prior to induction of its transcription at initiation of flower development (Table 1; Ito and Sun, 2009; Sun et al., 2009). The AGAMOUS (AG) MADS domain type TF is induced by WUS in floral meristem primordia, and 2 days later, AG reaches a tipping point where its own action represses WUS (Table 1; Ikeda et al., 2009). This period of activity by AG is also responsible for the reduction in H3K27me3 in the KNU locus, although how AG itself is involved in histone demethylation is unclear (Ito and Sun, 2009). The overarching result of these interactions acts to specify reproductive organ identity, and a number of floral stem cells to ensure flowers of different individuals are all similar in size (Ito and Sun, 2009).
Phloem mobile signals such as siRNAs, miRNAs and messenger RNAs can play pivotal roles in Arabidopsis inflorescence induction (McGarry and Kragler, 2013). Of approximately 200 miRNA families in Arabidopsis, only three (miR156, miR159 and miR172) have been shown to influence flowering time control (Yamaguchi and Abe, 2012). It is possible that the negative feedback from high fruit loading influencing inflorescence induction in crop species (Samach and Smith, 2013) is regulated by phloem mobile signals such as these. This feedback system may have evolved to ensure that fitness of developing fruits is not encumbered by overcommitting to excessive fruit quantity.
The photoperiod pathway promoting flowering regulates CONSTANS (CO), which activates several gene networks. One of these gene networks involves the key TF integrators: SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1) and FLOWERING LOCUS T (FT) that together initiate IM induction (Valverde, 2011; Wigge et al., 2005). FT and FT-like genes have homologs in many angiosperms and a number of gymnosperms (Klintenas et al., 2012). However, FT/TERMINAL FLOWER1-like (FTL1 & FTL2) genes in Picea (spruces) and Pinus (pines) are thought to act conversely to FT in angiosperms as their ectopic expression in Arabidopsis represses flowering (Klintenas et al., 2012).
The FT gene encodes the transmissible ‘florigen’, a phosphatidylethanolamine-binding protein (PEBP; Klintenas et al., 2012), which is small, globular and phloem mobile. PEBP can travel from leaves to SAMs where it induces the flowering programme (McGarry and Kragler, 2013; Wigge et al., 2005). FT requires the basic-leucine zipper TF AT4G35900 (FD) for floral promotion. FD RNA is highly expressed in both leaf and floral anlagen (the cluster of meristematic tissue which develops into an organ), although after floral primordia begin to express AP1 FD transcript levels decrease (Wigge et al., 2005). The interaction between FT and FD is thought to be reciprocal. In Arabidopsis, FT integrates promotional environmental cues enabling inflorescence induction while FD expressed in SAMs provides a target for FT (Wigge et al., 2005). In Arabidopsis, the FT/FTL1 family is comprised of six genes (Kim et al., 2013c). Additional complexity is conferred in B. napus by the six primary FT paralogues, each of which may be epigenetically regulated (Wang et al., 2012), leading to scope for exquisite differences in expression profiles for cultivars adapted to different environments.
Inflorescence development
In most crops, establishment of meristem identity and reproductive organ development following floral initiation is a key determinant of harvest potential. Regulation of these phase changes by chromatin modification allows for plastic responses to the environment (Jarillo et al., 2009). Epigenetic marks influence development and relative timing of reproductive organs, with snRNA-mediated chromatin remodelling having the potential to maintain developmental patterns without positional signalling (Kidner and Martienssen, 2005).
Although the floral transition may be somewhat plastic, many of the subsequent regulatory circuits appear highly conserved within species, with around 20 chromatin-remodelling genes implicated in regulatory networks associated with floral identity and reproductive organogenesis (Jarillo et al., 2009).
Epigenetic modulation is apparent in the regulation of TFs such as AP1 and LFY, which are key to both floral meristem and floral organ identity determination during transitional development (Kidner and Martienssen, 2005; Saleh et al., 2008a). Crosstalk between hormone and epigenetic systems may also contribute to floral meristem initiation. Auxin expression can elicit floral meristem initiation, while cytokinins and other pre-existing signals from inflorescence induction may regulate positional reproductive compartment initiation in floral primordia (Chandler, 2012).
In rice, trans-acting snRNAs and their associated machinery contribute to meristem maintenance (Pautler et al., 2013), with miR166 accumulation in some rice mutants partially implicated in SAM defects (Nagasaki et al., 2007). Although LEAFY COTYLEDON 1 (LEC1) is fundamental to late embryo development in Arabidopsis, in rice, it is thought to regulate fine control of meristem identity, indicating different functions in monocotyledon and dicotyledon systems (Zhang and Xue, 2013), with OsLEC1 regulating meristem identity by hypermethylating specific regions of OsMADS1 (Zhang and Xue, 2013).
Photoperiod- and thermo-sensitive genic male sterility (PGMS/TGMS) is important for hybrid rice breeding. A novel spontaneous PGMS mutant was characterized in 1973, which is sterile under long-day conditions and has been incorporated into rice breeding germplasm (Zhang et al., 1994). A single base mutation has been identified, which may lead to loss of function of a unique snRNA that regulates key genetic networks involved in male reproductive organ development (Zhou et al., 2012).
Fertilization, orchestration of the reproductive programme and seed set
Timing of flower opening can exhibit substantial plasticity in response to external stimuli, with light and temperature able to independently regulate opening and closing of petals (van Doorn and van Meeteren, 2003). This may be important to reduce pollen loss when pollinators are not operating or to limit rain damage. The number of hours a flower remains open and receptive can vary in response to pollination (Arathi et al., 2002; Frund et al., 2011). Flower longevity can exhibit considerable plasticity, such as in Mimulus guttatus (monkey flower), where plasticity facilitates unpollinated flowers to remain open longer (Arathi et al., 2002).
Epigenetic marks accumulated in the SAM during growth are to a large extent removed, reset or reprogrammed during meiosis (Paszkowski and Grossniklaus, 2011). In Arabidopsis, MET1 expression is down-regulated during gametogenesis in pollen sperm and egg cells, resulting in hypomethylation compared with the parental genomes (Table 1; Saze et al., 2003; Schmidt et al., 2013). However, not all 5mC marks are removed during reproduction as a number of important genomic regions can remain imprinted (Borges and Martienssen, 2013; Calarco et al., 2012). Although epigenetic regulation is critical in this stage of reproductive development, little plasticity has been directly observed.
Maintaining pollen viability until fertilization is a complex process. In maize pollen, over 20 000 genes appear to be expressed in the short period prior to fertilization (Walbot and Evans, 2003). DECREASED DNA METHYLATION 1 (DDM1) appears to be part of the pollen maintenance system as it is expressed in the sperm cell nuclei but not the vegetative nuclei (Law and Jacobsen, 2010). In Arabidopsis sperm cells, DNA methylation levels at most TE loci are hypermethylated, with those repressed solely by DNA methylation becoming expressed and mobile (Wollmann and Berger, 2012). This suggests that a more permanent repression of TEs requires additional epigenetic controls.
Diploid Brassica crops are able to limit self-fertilization via the sporophytic self-incompatibility system, with the SP11 locus in anther tapetal tissue regulated by de novo 5mC (Table 1; Henderson and Jacobsen, 2007). Here, plasticity provides variation in strength of incompatibility dominance under different conditions (Shiba et al., 2003).
Pollen recognition on the stigma involves protein–protein interactions followed by pollen hydration, recognition, pollination, germination and penetration into the style (Edlund et al., 2004). Self-pollination in self-incompatible species inhibits pollen germination. The CENTRAL CELL GUIDANCE (CCG) domain is associated with long-distance guidance of germinated pollen tubes (Chen et al., 2007). Within the central cell, specific reproductive genes are derepressed (i.e. hypomethylated) by suppression of MET1 and activation of DME (DEMETER; Wollmann and Berger, 2012), although chromatin modulation may precede genome-wide DNA hypomethylation (Gutierrez-Marcos and Dickinson, 2012).
DEMETER regulates at least five parent-of-origin genes, and another 40 candidates remain to be experimentally verified (Law and Jacobsen, 2010). The central cell is a participant in double fertilization and initiates the development of endosperm tissue after fertilization, both distinguishing features of angiosperms (Wollmann and Berger, 2012). In the Arabidopsis central cell prior to fertilization, expression of DME results in hypomethylation of the endosperm genome (Lamesch et al., 2011), although to what extent is still unclear (Wollmann and Berger, 2012; Zemach et al., 2010). Moreover, whole-genome demethylation by DME appears primarily to affect CG sites (Wollmann and Berger, 2012). Although DME is rapidly down-regulated after fertilization, it is present during endosperm development, perhaps imprinting at the MEDEA (MEA) locus (Garnier et al., 2008). MEA in Arabidopsis is thought to encode a TF that represses fruit development in the absence of fertilization (Lamesch et al., 2011).
In plants, 5mC in CHG contexts can be maintained by CHROMOMETHYLASE3 (CMT3) and CHH by DRM2 (Table 1; Law and Jacobsen, 2010). Both CMT3 and DRM2 are expressed in the embryo and seed, but only DRM2 is expressed in the pollen sperm cell (Lamesch et al., 2011). The Arabidopsis INCREASE IN BONSAI METHYLATION 1 (IBM1) has been associated with 5mC in both CHG and CHH contexts (Miura et al., 2009a).
Yields and seed viability of grain crops are dependent on the outcome of a battle between maternal and paternal genomes over expression of respective alleles, which is mediated by differential maintenance of epigenetic marks. Maternal DME activity can mediate the activation of maternal specific alleles, while paternal alleles are silenced by MET1 and may be regulated by RdDM via FLOWERING WAGENINGEN (FWA) and FERTILIZATION INDEPENDENT SEED 2 (FIS2) or histone modulation by MEA on H3Lys27 (Table 1; Kohler and Makarevich, 2006; Vu et al., 2013). The challenge for plant sexual reproduction is to reprogramme DNA methylation in each set of chromosomes while ensuring maintenance of TE silencing (Wollmann and Berger, 2012), with the latter in rice achieved by mechanisms such as demethylation of H3K4 by JUMONJI703 (JMJ703; Cui et al., 2013).
Gamete fusion and seed development
Both histone (H3K27me3) and DNA methylation regulate repression of TEs and genes in Arabidopsis endosperm (Schmidt et al., 2013), with significant variation in methylation patterning between embryo and endosperm tissues (Gehring et al., 2009) including overall hypomethylation in both tissues, although some CHH hypermethylation occurs, which may result from RdDM (Law and Jacobsen, 2010). In rice, endosperm hypomethylation occurs in all sequence contexts, although CHG and CHH contexts are hypomethylated similarly across the genome in comparison with targeted CG methylation (Zemach et al., 2010). Arabidopsis embryogenesis requires activity of both MET1 and CMT3 for seed viability (Xiao et al., 2006). This leads to preferential maternal hypomethylation in the endosperm while paternal methylated alleles are maintained. However, the function of the remaining methylated genes is largely unknown (Zhang et al., 2013a).
At the earliest stages of embryo development, epigenetic regulation can play a crucial role in directing the partitioning of tissues into distinct anlagen cell lineages and driving inheritance of each transcriptional programme through mitosis (Bantignies and Cavalli, 2006). MET1 is not only expressed following gametogenesis in the egg cell, but may also be expressed in the developing embryo, endosperm and seed coat (Schmidt et al., 2013). In Brassica rapa, BcJMJ30 (Table 1) encodes the jmjC domain-containing histone demethylase, which is associated with pollen development and fertilization (Li et al., 2012c), while the Helianthus LEAFY COTYLEDON1-LIKE (HaL1L) is involved in early stages of zygotic and somatic embryogenesis, with multiplexed transcriptional regulation by DNA methylation, TFs, auxin and ABA (Table 1; Salvini et al., 2012). The MATERNALLY EXPRESSED GENE 1 (MEG1) in maize is expressed only in the basal nutrient transfer region of the endosperm (Table 1; Gutierrez-Marcos et al., 2004), with genomic imprinting of MEG1 assisting nutrient transfer from endosperm to the developing embryo (Costa et al., 2012).
Genomic imprinting
Transgenerational inheritance of epigenetic marks involved in genomic imprinting can result in a greater than expected expression of alleles from either maternal or paternal origin (Mosher and Melnyk, 2010). Imprinting ensures TEs remain epigenetically silenced throughout plant gametogenic reprogramming (Wollmann and Berger, 2012) and can also facilitate seed germination. Pathogen exposure can initiate differential 5mC patterning, with transgenerational priming of a genome appearing to elicit pre-emptive pathogen-associated molecular patterns, such as the activation of the defence regulatory gene NON EXPRESSOR OF PR GENES (NPR1; Dowen et al., 2012; Luna and Ton, 2012). It has been suggested that post-translational histone modifications and expression of RNA Polymerase V act alongside siRNA to recruit methylation machinery, leading to transgenerational genomic imprinting of NPR1 (Luna and Ton, 2012; You et al., 2013). This defence system is similar to priming the human immune system by immunization, with a significant difference being its heritability.
Maternally produced RNAs in plant reproductive tissue can be mobile and may target specific loci in developing embryos for genomic imprinting (Gutierrez-Marcos et al., 2012; Mosher et al., 2009). Environmentally induced RNA in maternal somatic cells may also contribute to embryo genomic imprinting. In fact, a range of miRNAs, including at least four involved in nutrient homeostasis (miR169, miR395, miR398 and miR399), are mobile, present in the phloem and graft-transmissible (Marin-Gonzalez and Suarez-Lopez, 2012), adding to growing evidence that maternally produced RNAs may be present in the next generation. RNA present at fertilization can persist during seed maturation (Mosher et al., 2009) and possibly during seed dormancy. Maternal RNA may therefore influence gene regulation during germination to assist seedling establishment (Figure 1). This is an aspect of maternally influenced genomic imprinting, which may have evolved to impart a fitness advantage to offspring that germinate within the maternal niche (Gorecki et al., 2012).
METHYLTRANSFERASE 1 has been suggested as a key integrator maintaining transgenerational inheritance of epigenetic marks throughout vegetative growth (Paszkowski and Grossniklaus, 2011). Hypomethylation is less noticeable during plant embryogenesis compared with mammalian systems, with a higher proportion of parental DNA methylation states transmitted to the next generation (Reinders et al., 2009). In Arabidopsis, the methylation state of the PHE1 (PHERES1) paternal allele is methylated in contrast to the hypomethylated maternal allele (Table 1; Kohler and Makarevich, 2006; Makarevich et al., 2008). Where salt tolerance in rice has been induced and transgenerationally inherited in selfed progenies, methylation profiles suggested that salt-tolerant genotypes were hypermethylated while salt-sensitive genotypes were hypomethylated (Feng et al., 2012).
Imprinting in plants was thought only to occur in the triploid endosperm and therefore expected that gymnosperms would lack imprinting mechanisms (Garnier et al., 2008). However, more recently, it has been shown that genomic imprinting can occur in angiosperm embryos (Scholten, 2010). The gymnosperm Norway spruce also has capacity to store environmental conditions in an epigenetic memory during embryogenesis, with exposure to different temperatures during embryo development fixing epigenetic marks prior to seed maturation, leading to modified germination time and seedling development (Yakovlev et al., 2010). The epigenetic memory in long-lived plant species may confer adaptive plasticity to environmental drift in a single generation, with important consequences for perennial and clonally propagated crops.
Fruit development and ripening
Fruit development from the ovule normally commences following fertilization of the egg cell (Figure 1), with suppression of endosperm growth following fertilization signalling the start of fruit development. MEA and MET1 are thought to be involved in the repression of both endosperm development and central cell proliferation (Schmidt et al., 2013). Parthenocarpic fruit also demonstrates the independence of fruit ripening from signalling arising from oocyte fertilization (Mesejo et al., 2013; Wang et al., 2009).
The development of the complex structures of fruit is regulated by a network of TFs. In the dry dehiscent fruit of Brassicaceae, a network of genes, TFs and hormones are involved in regulating the development of the carpel tissue compartments into valve, lignified layer, separation layer and replum (Alonso-Cantabrana et al., 2007; Girin et al., 2010; Lewis et al., 2006; Muhlhausen et al., 2013; Ripoll et al., 2011; Romera-Branchat et al., 2013; Seymour et al., 2013). In crops with indehiscent fruit, such as canola and soybean, development is general tightly regulated and programmed, with the primary plastic trait being the decision to abort individual embryos or even the entire silique. The decision to abort embryos in the Brassicaceae can come from at least two abiotic stimuli, water and nutrient availability (Guo et al., 2013).
In fleshy fruits such as tomato and peach, development and ripening of carpel tissues is regulated by a subset of conserved TF networks (Chapman et al., 2012; Fujisawa et al., 2013; Karlova et al., 2013; Luo et al., 2013; Manning et al., 2006; Seymour et al., 2013a; Zhong et al., 2013), with additional taxon-specific regulators. In tomato, the MADS-box gene RIPENING INHIBITOR (RIN) induces the expression of COLOURLESS NONRIPENING (CNR), FRUITFULL homolog TDR4/FUL1, Solyc07g052960 and a network of at least another 22 genes with TF activity thought to influence aroma and flavour, pathogen defence, stress response, fruit softening and pigmentation during ripening (Fujisawa et al., 2012). The SBP-box (SQUAMOSA promoter-binding protein-like) gene CNR (Manning et al., 2006; Seymour et al., 2013) was first identified in a commercial crop and characterized as a dominant mutant allele (Cnr) that had undergone spontaneous hypermethylation in the promoter region. Comparative deep methylome sequencing at four stages of tomato fruit development has identified several candidate genes underlying phenotypic traits important in breeding programme. Notably, this epiallelic variation is within genes having little available genetic variation and so are potential targets for crop improvement via epiallelic screening (Zhong et al., 2013).
COLOURLESS NONRIPENING targets AP2, a major regulator of tomato fruit ripening (Karlova et al., 2011), and cultivar-specific variation in the pattern of 5mC in the CNR promoter region affects the rate of ripening (Table 1). CNR is also targeted by miR156/157 (Moxon et al., 2008; Zuo et al., 2011), although there is only partial overlap of expression between miR156 and CNR, with the consequence of suppressing CNR in specific cell types (Zuo et al., 2011). A wider range of miRNAs target key TFs and hormonal regulators of tomato fruit development (Karlova et al., 2013).
Concluding remarks
In this review, we have explored the conventional understanding of crop plant G × E interactions and plasticity and outlined the extent to which epigenetic mechanisms contribute to adaptive plasticity. We have mapped key stages of plant development onto the corresponding crop phases and described many transitions and responses modulated by epigenetic marks and processes. We note that while the model plant Arabidopsis has provided startling insights into vegetative and reproductive development, it often is unable to provide a viable model for studying many aspects of crop responses to field growing environments (Seymour et al., 2013b).
The domestication of crop plants has involved selection of a wide range of phenotypes adapted to human requirements (Ross-Ibarra et al., 2007; Vaughan et al., 2007). This selection is characterized by strong pressure for stability of key ‘domestication traits’ associated with the crop products such as food and fibre. In many cases, the value and popularity of a crop in one location has stimulated radiation to other eco-agronomic regions, with a requirement for a wider range of adaptations to local growing environments. While the genetic canalization associated with crop domestication is well characterized, the extent to which breeding germplasm has inadvertently been selected for epiallelic variation has yet to be established (Amoah et al., 2012; King et al., 2010).
We have shown that a significant proportion of phenotypic variation and plasticity observed in crop plants appears to be influenced by modulation of epigenetic marks. Although in many cases the architecture of regulatory networks is sufficiently complex to provide developmental plasticity, the additional complexity and behaviour of epigenetic processes allows a more dynamic response to environmental signals. It has recently been suggested that phenotypes influenced by epigenotypes are primarily facilitated by gene expression (Springer, 2013). However, our broader examination of epigenetic processes suggests a complex web of negative and positive feedback, which can act independently of specific gene expression. Epigenetic regulation is now understood to be a key factor in homeostasis of developmental and response networks in plants, with considerable crosstalk affecting hormonal, TF and other genomic regulatory networks. This inherent complexity provides plants with additional plasticity and enabled evolution of diverse vegetative and reproductive structures that contribute to crop productivity.
The extent to which epialleles may affect trans-regulation of gene expression remains poorly characterized, particularly in complex crop genomes. The few studies to date that have taken a genetical genomics approach indicate that a large proportion of expression QTL (eQTL) have trans-regulatory effects (Cubillos et al., 2012; Hammond et al., 2011). The complexity of these pleiotropic effects is likely to be confounded by the tissue- or environment-specific epiallelic status of trans-regulated loci. Simultaneous characterization of eQTL and methylome mapping in crop plant systems will provide greater insights into the molecular basis of G × E interactions.
As a more detailed understanding of the specific molecular mechanisms underlying crop plasticity emerges, increasingly more sophisticated strategies are likely to be developed to intervene in the epigenetic status of cultivars and contribute to developing climate-resilient crops. These approaches may involve epiallelic selection including retrospective characterization of DNA methylation (Hauben et al., 2009), chemical hypomethylation and selection (Amoah et al., 2012), tissue-culture-induced epiallelic variation (Rhee et al., 2010), targeted modulation of epigenetic status or miRNA engineering (Zhou and Luo, 2013).
Recognition of the potential impact and value of epigenetic selection is increasing, with new strategies for managing epialleles being developed alongside conventional and transgenic programmes. However, any approach to crop improvement based on modification of epiallelic status ideally requires knowledge of the specific molecular changes and potential epistatic consequences on gene regulation. Although we have referred to a number of studies that have shown genome-wide hypo- or hypermethylation in crop plants, to date, many of these failed to distinguish between direct modulation of 5mC affecting gene transcription and trans-effects resulting from activation of TEs. The availability of new sequencing technologies that are able to rapidly generate comprehensive methylome profiles associated with different tissues and cultivation environments will contribute to the platforms required for predictive crop epi-breeding.
Acknowledgments
The authors were supported by Southern Cross Plant Science and Division of Research at Southern Cross University. GK is also supported by a Chutian scholarship from Hubei Province, China.
References
- Adams S, Allen T, Whitelam GC. Interaction between the light quality and flowering time pathways in Arabidopsis. Plant J. 2009;60:257–267. doi: 10.1111/j.1365-313X.2009.03962.x. [DOI] [PubMed] [Google Scholar]
- Ahmad A, Zhang Y, Cao X-F. Decoding the epigenetic language of plant development. Mol. Plant. 2010;3:719–728. doi: 10.1093/mp/ssq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimoto K, Katakami H, Kim HJ, Ogawa E, Sano CM, Wada Y, Sano H. Epigenetic inheritance in rice plants. Ann. Bot. 2007;100:205–217. doi: 10.1093/aob/mcm110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Cantabrana H, Ripoll JJ, Ochando I, Vera A, Ferrandiz C, Martinez-Laborda A. Common regulatory networks in leaf and fruit patterning revealed by mutations in the Arabidopsis ASYMMETRIC LEAVES1 gene. Development. 2007;134:2663–2671. doi: 10.1242/dev.02864. [DOI] [PubMed] [Google Scholar]
- Alzohairy AM, Gyulai G, Jansen RK, Bahieldin A. Transposable elements domesticated and neofunctionalized by eukaryotic genomes. Plasmid. 2013;69:1–15. doi: 10.1016/j.plasmid.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Amoah S, Kurup S, Lopez CMR, Welham SJ, Powers SJ, Hopkins CJ, Wilkinson MJ, King GJ. A hypomethylated population of Brassica rapa for forward and reverse epi-genetics. BMC Plant Biol. 2012;12:193. doi: 10.1186/1471-2229-12-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arana MV, Marin-de la Rosa N, Maloof JN, Blazquez MA, Alabadi D. Circadian oscillation of gibberellin signaling in Arabidopsis. Proc. Natl Acad. Sci. USA. 2011;108:9292–9297. doi: 10.1073/pnas.1101050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arathi HS, Rasch A, Cox C, Kelly JK. Autogamy and floral longevity in Mimulus guttatus. Int. J. Plant Sci. 2002;163:567–573. [Google Scholar]
- Ausin I, Alonso-Blanco C, Martinez-Zapater JM. Environmental regulation of flowering. Int. J. Dev. Biol. 2005;49:689–705. doi: 10.1387/ijdb.052022ia. [DOI] [PubMed] [Google Scholar]
- Bantignies F, Cavalli G. Cellular memory and dynamic regulation of polycomb group proteins. Curr. Opin. Cell Biol. 2006;18:275–283. doi: 10.1016/j.ceb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bastow R, Mylne JS, Lister C, Lippman Z, Martienssen RA, Dean C. Vernalization requires epigenetic silencing of FLC by histone methylation. Nature. 2004;427:164–167. doi: 10.1038/nature02269. [DOI] [PubMed] [Google Scholar]
- Beauclair L, Yu A, Bouche N. microRNA-directed cleavage and translational repression of the copper chaperone for superoxide dismutase mRNA in Arabidopsis. Plant J. 2010;62:454–462. doi: 10.1111/j.1365-313X.2010.04162.x. [DOI] [PubMed] [Google Scholar]
- Bender J, Fink GR. Epigenetic control of an endogenous gene family is revealed by a novel blue fluorescent mutant of Arabidopsis. Cell. 1995;83:725–734. doi: 10.1016/0092-8674(95)90185-x. [DOI] [PubMed] [Google Scholar]
- Bernier G. The flowering process as an example of plastic development. Symp. Soc. Exp. Biol. 1986;40:257–286. [PubMed] [Google Scholar]
- Bernstein E, Hake SB. The nucleosome: a little variation goes a long way. Biochem. Cell Biol. 2006;84:505–507. doi: 10.1139/o06-085. [DOI] [PubMed] [Google Scholar]
- Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- Böhnke M, Bruelheide H. How do evergreen and deciduous species respond to shade?—Tolerance and plasticity of subtropical tree and shrub species of South-East China. Environ. Exp. Bot. 2013;87:179–190. [Google Scholar]
- Borges F, Martienssen RA. Establishing epigenetic variation during genome reprogramming. RNA Biol. 2013;10:490–494. doi: 10.4161/rna.24085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss PK, Bastow RM, Mylne JS, Dean C. Multiple pathways in the decision to flower: enabling, promoting, and resetting. Plant Cell. 2004;16:S18–S31. doi: 10.1105/tpc.015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SJ, Stoilov P, Xing Y. Chromatin and epigenetic regulation of pre-mRNA processing. Hum. Mol. Genet. 2012;21:R90–R96. doi: 10.1093/hmg/dds353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher E, Reinders J, Mirouze M. Epigenetic control of transposon transcription and mobility in Arabidopsis. Curr. Opin. Plant Biol. 2012;15:503–510. doi: 10.1016/j.pbi.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Calarco JP, Borges F, Donoghue MTA, Van Ex F, Jullien PE, Lopes T, Gardner R, Berger F, Feijo JA, Becker JD, Martienssen RA. Reprogramming of DNA methylation in pollen guides epigenetic inheritance via small RNA. Cell. 2012;151:194–205. doi: 10.1016/j.cell.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantu D, Vanzetti LS, Sumner A, Dubcovsky M, Matvienko M, Distelfeld A, Michelmore RW, Dubcovsky J. Small RNAs, DNA methylation and transposable elements in wheat. BMC Genomics. 2010;11:408. doi: 10.1186/1471-2164-11-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzonelli CI, Pogson BJ. Source to sink: regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010;15:266–274. doi: 10.1016/j.tplants.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Chan SWL, Zilberman D, Xie Z, Johansen LK, Carrington JC, Jacobsen SE. RNA silencing genes control de novo DNA methylation. Science. 2004;303:1336. doi: 10.1126/science.1095989. [DOI] [PubMed] [Google Scholar]
- Chandler JW. Floral meristem initiation and emergence in plants. Cell. Mol. Life Sci. 2012;69:3807–3818. doi: 10.1007/s00018-012-0999-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman NH, Bonnet J, Grivet L, Lynn J, Graham N, Smith R, Sun GP, Walley PG, Poole M, Causse M, King GJ, Baxter C, Seymour GB. High-resolution mapping of a fruit firmness-related quantitative trait locus in tomato reveals epistatic interactions associated with a complex combinatorial locus. Plant Physiol. 2012;159:1644–1657. doi: 10.1104/pp.112.200634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-H, Li H-J, Shi D-Q, Yuan L, Liu J, Sreenivasan R, Baskar R, Grossniklaus U, Yang W-C. The central cell plays a critical role in pollen tube guidance in Arabidopsis. Plant Cell. 2007;19:3563–3577. doi: 10.1105/tpc.107.053967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Zhu J-K. Epigenetic regulation of stress responses in plants. Curr. Opin. Plant Biol. 2009;12:133–139. doi: 10.1016/j.pbi.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodavarapu RK, Feng SH, Bernatavichute YV, Chen PY, Stroud H, Yu YC, Hetzel JA, Kuo F, Kim J, Cokus SJ, Casero D, Bernal M, Huijser P, Clark AT, Kramer U, Merchant SS, Zhang XY, Jacobsen SE, Pellegrini M. Relationship between nucleosome positioning and DNA methylation. Nature. 2010;466:388–392. doi: 10.1038/nature09147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigliano RA, Sanseverino W, Cremona G, Ercolano MR, Conicella C, Consiglio FM. Genome-wide analysis of histone modifiers in tomato: gaining an insight into their developmental roles. BMC Genomics. 2013;14:562. doi: 10.1186/1471-2164-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R, Schocken J, Kaldis A, Vlachonasios KE, Hark AT, McCain ER. The histone acetyltransferase GCN5 affects the inflorescence meristem and stamen development in Arabidopsis. Planta. 2009;230:1207–1221. doi: 10.1007/s00425-009-1012-5. [DOI] [PubMed] [Google Scholar]
- Costa LM, Yuan J, Rouster J, Paul W, Dickinson H, Gutierrez-Marcos JF. Maternal control of nutrient allocation in plant seeds by genomic imprinting. Curr. Biol. 2012;22:160–165. doi: 10.1016/j.cub.2011.11.059. [DOI] [PubMed] [Google Scholar]
- Cubas P, Vincent C, Coen E. An epigenetic mutation responsible for natural variation in floral symmetry. Nature. 1999;401:157–161. doi: 10.1038/43657. [DOI] [PubMed] [Google Scholar]
- Cubillos FA, Coustham V, Loudet O. Lessons from eQTL mapping studies: non-coding regions and their role behind natural phenotypic variation in plants. Curr. Opin. Plant Biol. 2012;15:192–198. doi: 10.1016/j.pbi.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Cui XK, Jin P, Cui X, Gu LF, Lu ZK, Xue YM, Wei LY, Qi JF, Song XW, Luo M, An G, Cao XF. Control of transposon activity by a histone H3K4 demethylase in rice. Proc. Natl Acad. Sci. USA. 2013;110:1953–1958. doi: 10.1073/pnas.1217020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilevskaya ON, Meng X, Selinger DA, Deschamps S, Hermon P, Vansant G, Gupta R, Ananiev EV, Muszynski MG. Involvement of the MADS-Box gene ZMM4 in floral induction and inflorescence development in maize. Plant Physiol. 2008;147:2054–2069. doi: 10.1104/pp.107.115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das OP, Messing J. Variegated phenotype and developmental methylation changes of a maize allele originating from epimutation. Genetics. 1994;136:1121–1141. doi: 10.1093/genetics/136.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn WG, van Meeteren U. Flower opening and closure: a review. J. Exp. Bot. 2003;54:1801–1812. doi: 10.1093/jxb/erg213. [DOI] [PubMed] [Google Scholar]
- Dowen RH, Pelizzola M, Schmitz RJ, Lister R, Dowen JM, Nery JR, Dixon JE, Ecker JR. Widespread dynamic DNA methylation in response to biotic stress. Proc. Natl Acad. Sci. USA. 2012;109:E2183–E2191. doi: 10.1073/pnas.1209329109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund AF, Swanson R, Preuss D. Pollen and stigma structure and function: the role of diversity in pollination. Plant Cell. 2004;16:S84–S97. doi: 10.1105/tpc.015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HH, Wei J, Li TC, Li ZP, Guo N, Cai YP, Lin Y. DNA methylation alterations of upland cotton (Gossypium hirsutum) in response to cold stress. Acta Physiol. Plant. 2013;35:2445–2453. [Google Scholar]
- Feng Q, Yang C, Lin X, Wang J, Ou X, Zhang C, Chen Y, Liu B. Salt and alkaline stress induced transgenerational alteration in DNA methylation of rice (Oryza sativa. Aust J. Crop Sci. 2012;6:877–883. [Google Scholar]
- Ferreira TH, Gentile A, Vilela RD, Costa GGL, Dias LI, Endres L, Menossi M. microRNAs associated with drought response in the bioenergy crop sugarcane (Saccharum spp.) PLoS ONE. 2012;7:1–14. doi: 10.1371/journal.pone.0046703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer JJ, Beatty PH, Good AG, Muench DG. Manipulation of microRNA expression to improve nitrogen use efficiency. Plant Sci. 2013;210:70–81. doi: 10.1016/j.plantsci.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Fox TC, Guerinot ML. Molecular biology of cation transport in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998;49:669–696. doi: 10.1146/annurev.arplant.49.1.669. [DOI] [PubMed] [Google Scholar]
- Frund J, Dormann CF, Tscharntke T. Linne's floral clock is slow without pollinators—flower closure and plant-pollinator interaction webs. Ecol. Lett. 2011;14:896–904. doi: 10.1111/j.1461-0248.2011.01654.x. [DOI] [PubMed] [Google Scholar]
- Fujii H, Chiou TJ, Lin SI, Aung K, Zhu JK. A miRNA involved in phosphate-starvation response in Arabidopsis. Curr. Biol. 2005;15:2038–2043. doi: 10.1016/j.cub.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Fujisawa M, Shima Y, Higuchi N, Nakano T, Koyama Y, Kasumi T, Ito Y. Direct targets of the tomato-ripening regulator RIN identified by transcriptome and chromatin immunoprecipitation analyses. Planta. 2012;235:1107–1122. doi: 10.1007/s00425-011-1561-2. [DOI] [PubMed] [Google Scholar]
- Fujisawa M, Nakano T, Shima Y, Ito Y. A large-scale identification of direct targets of the tomato MADS box transcription factor RIPENING INHIBITOR reveals the regulation of fruit ripening. Plant Cell. 2013;25:371–386. doi: 10.1105/tpc.112.108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S, Nakagawa M, Oda A, Kato K, Mizoguchi T. Photoperiod pathway regulates expression of MAF5 and FLC that encode MADS-box transcription factors of the FLC family in Arabidopsis. Plant Biotechnol. 2010;27:447–454. [Google Scholar]
- Garnier O, Laouielle-Duprat S, Spillane C. Genomic imprinting in plants. Genomic Imprinting. 2008;626:89–100. doi: 10.1007/978-0-387-77576-0_7. [DOI] [PubMed] [Google Scholar]
- Gehring M, Bubb KL, Henikoff S. Extensive demethylation of repetitive elements during seed development underlies gene imprinting. Science. 2009;324:1447–1451. doi: 10.1126/science.1171609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile A, Ferreira TH, Mattos RS, Dias LI, Hoshino AA, Carneiro MS, Souza GM, Calsa T, Nogueira RM, Endres L, Menossi M. Effects of drought on the microtranscriptome of field-grown sugarcane plants. Planta. 2013;237:783–798. doi: 10.1007/s00425-012-1795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierth M, Maser P. Potassium transporters in plants—involvement in K+ acquisition, redistribution and homeostasis. FEBS Lett. 2007;581:2348–2356. doi: 10.1016/j.febslet.2007.03.035. [DOI] [PubMed] [Google Scholar]
- Girin T, Stephenson P, Goldsack CMP, Kempin SA, Perez A, Pires N, Sparrow PA, Wood TA, Yanofsky MF, Ostergaard L. Brassicaceae INDEHISCENT genes specify valve margin cell fate and repress replum formation. Plant J. 2010;63:329–338. doi: 10.1111/j.1365-313X.2010.04244.x. [DOI] [PubMed] [Google Scholar]
- Glass ADM, Britto DT, Kaiser BN, Kinghorn JR, Kronzucker HJ, Kumar A, Okamoto M, Rawat S, Siddiqi MY, Unkles SE, Vidmar JJ. The regulation of nitrate and ammonium transport systems in plants. J. Exp. Bot. 2002;53:855–864. doi: 10.1093/jexbot/53.370.855. [DOI] [PubMed] [Google Scholar]
- Goettel W, Messing J. Epiallele biogenesis in maize. Gene. 2013;516:8–23. doi: 10.1016/j.gene.2012.12.034. [DOI] [PubMed] [Google Scholar]
- Golldack D, Li C, Mohan H, Probst N. Gibberellins and abscisic acid signal crosstalk: living and developing under unfavorable conditions. Plant Cell Rep. 2013;32:1007–1016. doi: 10.1007/s00299-013-1409-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez RM, Ricardi MM, Iusem ND. Epigenetic marks in an adaptive water stress-responsive gene in tomato roots under normal and drought conditions. Epigenetics. 2013;8:864–872. doi: 10.4161/epi.25524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Grandio E, Poza-Carrion C, Sorzano COS, Cubas P. BRANCHED1 promotes axillary bud dormancy in response to shade in Arabidopsis. Plant Cell. 2013;25:834–850. doi: 10.1105/tpc.112.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorecki MJ, Long RL, Flematti GR, Stevens JC. Parental environment changes the dormancy state and karrikinolide response of Brassica tournefortii seeds. Ann. Bot. 2012;109:1369–1378. doi: 10.1093/aob/mcs067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood MS, Hutchison KW. Maturation as a developmental process. In: Ahuja MR, Libby WJ, editors. Clonal Forestry I—Genetics and Biotechnology. Berlin: Springer-Verlag; 1993. pp. 14–33. [Google Scholar]
- Grossniklaus U, Vielle-Calzada JP, Hoeppner MA, Gagliano WB. Maternal control of embryogenesis by medea, a Polycomb group gene in Arabidopsis. Science. 1998;280:446–450. doi: 10.1126/science.280.5362.446. [DOI] [PubMed] [Google Scholar]
- Guo YM, Chen S, Nelson MN, Cowling W, Turner NC. Delayed water loss and temperature rise in floral buds compared with leaves of Brassica rapa subjected to a transient water stress during reproductive development. Funct. Plant Biol. 2013;40:690–699. doi: 10.1071/FP12335. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Marcos JF, Dickinson HG. Epigenetic reprogramming in plant reproductive lineages. Plant Cell Physiol. 2012;53:817–823. doi: 10.1093/pcp/pcs052. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Marcos JF, Costa LM, Biderre-Petit C, Khbaya B, O'Sullivan DM, Wormald M, Perez P, Dickinson HG. Maternally expressed gene 1 is a novel maize endosperm transfer cell-specific gene with a maternal parent-of-origin pattern of expression. Plant Cell. 2004;16:1288–1301. doi: 10.1105/tpc.019778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Marcos JF, Constância M, Burton GJ. Maternal to offspring resource allocation in plants and mammals. Placenta. 2012;33(Suppl. 2):e3–e10. doi: 10.1016/j.placenta.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Guzy-Wrobelska J, Filek M, Kaliciak A, Szarejko I, Machackova I, Krekule J, Barciszewska M. Vernalization and photoperiod-related changes in the DNA methylation state in winter and spring rapeseed. Acta Physiol. Plant. 2013;35:817–827. [Google Scholar]
- Hammond JP, Mayes S, Bowen HC, Graham NS, Hayden RM, Love CG, Spracklen WP, Wang J, Welham SJ, White PJ, King GJ, Broadley MR. Regulatory hotspots are associated with plant gene expression under varying soil phosphorus supply in Brassica rapa. Plant Physiol. 2011;156:1230–1241. doi: 10.1104/pp.111.175612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauben M, Haesendonckx B, Standaert E, Van Der Kelen K, Azmi A, Akpo H, Van Breusegem F, Guisez Y, Bots M, Lambert B, Laga B, De Block M. Energy use efficiency is characterized by an epigenetic component that can be directed through artificial selection to increase yield. Proc. Natl Acad. Sci. USA. 2009;106:20109–20114. doi: 10.1073/pnas.0908755106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He GM, Elling AA, Deng XW. The epigenome and plant development. In: Merchant SS, Briggs WR, Ort D, editors. Annual Review of Plant Biology. Vol. 62. Palo Alto, CA: Annual Reviews; 2011. pp. 411–435. [DOI] [PubMed] [Google Scholar]
- Henderson IR. Control of meiotic recombination frequency in plant genomes. Curr. Opin. Plant Biol. 2012;15:556–561. doi: 10.1016/j.pbi.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Henderson IR, Jacobsen SE. Epigenetic inheritance in plants. Nature. 2007;447:418–424. doi: 10.1038/nature05917. [DOI] [PubMed] [Google Scholar]
- Hisamatsu T, King RW, Helliwell CA, Koshioka M. The involvement of gibberellin 20-oxidase genes in phytochrome-regulated petiole elongation of Arabidopsis. Plant Physiol. 2005;138:1106–1116. doi: 10.1104/pp.104.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge A. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol. 2004;162:9–24. [Google Scholar]
- Hofmann NR. A new function for BRC1 in axillary buds: suppression of floral identity. Plant Cell. 2013;25:1191. [Google Scholar]
- Hsieh LC, Lin SI, Shih ACC, Chen JW, Lin WY, Tseng CY, Li WH, Chiou TJ. Uncovering small RNA-mediated responses to phosphate deficiency in Arabidopsis by deep sequencing. Plant Physiol. 2009;151:2120–2132. doi: 10.1104/pp.109.147280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Mitsuda N, Ohme-Takagi M. Arabidopsis WUSCHEL is a bifunctional transcription factor that acts as a repressor in stem cell regulation and as an activator in floral patterning. Plant Cell. 2009;21:3493–3505. doi: 10.1105/tpc.109.069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H. Small RNAs and transposon silencing in plants. Dev. Growth Differ. 2012;54:100–107. doi: 10.1111/j.1440-169X.2011.01309.x. [DOI] [PubMed] [Google Scholar]
- Ito T, Sun B. Epigenetic regulation of developmental timing in floral stem cells. Epigenetics. 2009;4:564–567. doi: 10.4161/epi.4.8.10351. [DOI] [PubMed] [Google Scholar]
- Jablonka E. Epigenetic inheritance and plasticity: the responsive germline. Prog. Biophys. Mol. Biol. 2012;111:99–107. doi: 10.1016/j.pbiomolbio.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Jacobsen SE, Meyerowitz EM. Hypermethylated SUPERMAN epigenetic alleles in Arabidopsis. Science. 1997;277:1100–1103. doi: 10.1126/science.277.5329.1100. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 2003;33:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jarillo JA, Pineiro M. Timing is everything in plant development. The central role of floral repressors. Plant Sci. 2011;181:364–378. doi: 10.1016/j.plantsci.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Jarillo JA, Pineiro M, Cubas P, Martinez-Zapater JM. Chromatin remodeling in plant development. Int. J. Dev. Biol. 2009;53:1581–1596. doi: 10.1387/ijdb.072460jj. [DOI] [PubMed] [Google Scholar]
- Jaya E, Clemens J, Song JC, Zhang HB, Jameson PE. Quantitative expression analysis of meristem identity genes in Eucalyptus occidentalis: AP1 is an expression marker for flowering. Tree Physiol. 2010a;30:304–312. doi: 10.1093/treephys/tpp117. [DOI] [PubMed] [Google Scholar]
- Jaya E, Kubien DS, Jameson PE, Clemens J. Vegetative phase change and photosynthesis in Eucalyptus occidentalis: architectural simplification prolongs juvenile traits. Tree Physiol. 2010b;30:393–403. doi: 10.1093/treephys/tpp128. [DOI] [PubMed] [Google Scholar]
- Jaya E, Song J, Clemens J, Jameson PE. Effect of environment and shoot architecture on floral transition and gene expression in Eucalyptus occidentalis and Metrosideros excelsa. Plant Growth Regul. 2011;64:53–61. [Google Scholar]
- de Jesus EM, Cruz EAO, Cruz GMQ, Van Sluys MA. Diversification of hAT transposase paralogues in the sugarcane genome. Mol. Genet. Genomics. 2012;287:205–219. doi: 10.1007/s00438-011-0670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamakaka RT, Biggins S. Histone variants: deviants? Genes Dev. 2005;19:295–316. doi: 10.1101/gad.1272805. [DOI] [PubMed] [Google Scholar]
- Kantama L, Junbuathong S, Sakulkoo J, de Jong H, Apisitwanich S. Epigenetic changes and transposon reactivation in Thai rice hybrids. Mol. Breed. 2013;31:815–827. [Google Scholar]
- Karlova R, Rosin FM, Busscher-Lange J, Parapunova V, Do PT, Fernie AR, Fraser PD, Baxter C, Angenent GC, de Maagd RA. Transcriptome and metabolite profiling show that APETALA2a is a major regulator of tomato fruit ripening. Plant Cell. 2011;23:923–941. doi: 10.1105/tpc.110.081273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlova R, van Haarst JC, Maliepaard C, van de Geest H, Bovy AG, Lammers M, Angenent GC, de Maagd RA. Identification of microRNA targets in tomato fruit development using high-throughput sequencing and degradome analysis. J. Exp. Bot. 2013;64:1863–1878. doi: 10.1093/jxb/ert049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, de la Bastide M, Hamilton JP, Kanamori H, McCombie WR, Ouyang S, Schwartz DC, Tanaka T, Wu JZ, Zhou SG, Childs KL, Davidson RM, Lin HN, Quesada-Ocampo L, Vaillancourt B, Sakai H, Lee SS, Kim J, Numa H, Itoh T, Buell CR, Matsumoto T. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice. 2013;6:4. doi: 10.1186/1939-8433-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima CG, Yoshimoto N, Maruyama-Nakashita A, Tsuchiya YN, Saito K, Takahashi H, Dalmay T. Sulphur starvation induces the expression of microRNA-395 and one of its target genes but in different cell types. Plant J. 2009;57:313–321. doi: 10.1111/j.1365-313X.2008.03690.x. [DOI] [PubMed] [Google Scholar]
- Kehr J. Systemic regulation of mineral homeostasis by micro RNAs. Front. Plant Sci. 2013;4:145. doi: 10.3389/fpls.2013.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidner CA, Martienssen RA. The role of ARGONAUTE1 (AGO1) in meristem formation and identity. Dev. Biol. 2005;280:504–517. doi: 10.1016/j.ydbio.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Kim W, Ahn HJ, Chiou TJ, Ahn JH. The role of the miR399-PHO2 module in the regulation of flowering time in response to different ambient temperatures in Arabidopsis thaliana. Mol. Cells. 2011;32:83–88. doi: 10.1007/s10059-011-1043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Lee J, Yang JY, Jung C, Chua NH. Arabidopsis histone methyltransferase SET DOMAIN GROUP2 is required for regulation of various hormone responsive genes. J. Plant Biol. 2013a;56:39–48. [Google Scholar]
- Kim W, Latrasse D, Servet C, Zhou D-X. Arabidopsis histone deacetylase HDA9 regulates flowering time through repression of AGL19. Biochem. Biophys. Res. Commun. 2013b;432:394–398. doi: 10.1016/j.bbrc.2012.11.102. [DOI] [PubMed] [Google Scholar]
- Kim W, Park TI, Yoo SJ, Jun AR, Ahn JH. Generation and analysis of a complete mutant set for the Arabidopsis FT/TFL1 family shows specific effects on thermo-sensitive flowering regulation. J. Exp. Bot. 2013c;64:1715–1729. doi: 10.1093/jxb/ert036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GJ, Amoah S, Kurup S. Exploring and exploiting epigenetic variation in crops. Genome. 2010;53:856–868. doi: 10.1139/G10-059. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Miura A, Choi YH, Kinoshita Y, Cao XF, Jacobsen SE, Fischer RL, Kakutani T. One-way control of FWA imprinting in Arabidopsis endosperm by DNA methylation. Science. 2004;303:521–523. doi: 10.1126/science.1089835. [DOI] [PubMed] [Google Scholar]
- Klintenas M, Pin PA, Benlloch R, Ingvarsson PK, Nilsson O. Analysis of conifer FLOWERING LOCUS T/TERMINAL FLOWER1-like genes provides evidence for dramatic biochemical evolution in the angiosperm FT lineage. New Phytol. 2012;196:1260–1273. doi: 10.1111/j.1469-8137.2012.04332.x. [DOI] [PubMed] [Google Scholar]
- Kohler C, Makarevich G. Epigenetic mechanisms governing seed development in plants. EMBO Rep. 2006;7:1223–1227. doi: 10.1038/sj.embor.7400854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, Shiraya T, Wada KC, Takeno K. Induction of flowering by DNA demethylation in Perilla frutescens and Silene armeria: heritability of 5-azacytidine-induced effects and alteration of the DNA methylation state by photoperiodic conditions. Plant Sci. 2010;178:321–326. [Google Scholar]
- Kowalski A, Palyga J. Linker histone subtypes and their allelic variants. Cell Biol. Int. 2012;36:981–996. doi: 10.1042/CBI20120133. [DOI] [PubMed] [Google Scholar]
- Kriukiene E, Liutkeviciute Z, Klimasauskas S. 5-Hydroxymethylcytosine—the elusive epigenetic mark in mammalian DNA. Chem. Soc. Rev. 2012;41:6916–6930. doi: 10.1039/c2cs35104h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamesch P, Berardini TZ, Li D, Swarbreck D, Wilks C, Sasidharan R, Muller R, Dreher K, Alexander DL, Garcia-Hernandez M, Karthikeyan AS, Lee CH, Nelson WD, Ploetz L, Singh S, Wensel A, Huala E. The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Res. 2011;40:D1202–D1210. doi: 10.1093/nar/gkr1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzel V, Zhang Y, Moritz KK, Fischer M, Bossdorf O. Epigenetic variation in plant responses to defence hormones. Ann. Bot. 2012;110:1423–1428. doi: 10.1093/aob/mcs088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010;11:204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IJ, Foster KR, Morgan PW. Photoperiod control of gibberellin levels and flowering in sorghum. Plant Physiol. 1998;116:1003–1011. doi: 10.1104/pp.116.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DA. Flowering-time plasticity facilitates niche shifts in adjacent populations. New Phytol. 2009;183:661–666. doi: 10.1111/j.1469-8137.2009.02889.x. [DOI] [PubMed] [Google Scholar]
- Lewis MW, Leslie ME, Liljegren SJ. Plant separation: 50 ways to leave your mother. Curr. Opin. Plant Biol. 2006;9:59–65. doi: 10.1016/j.pbi.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Leyser O. The control of shoot branching: an example of plant information processing. Plant Cell Environ. 2009;32:694–703. doi: 10.1111/j.1365-3040.2009.01930.x. [DOI] [PubMed] [Google Scholar]
- Li S, Yang X, Wu F, He Y. HYL1 controls the miR156-mediated juvenile phase of vegetative growth. J. Exp. Bot. 2012a;63:2787–2798. doi: 10.1093/jxb/err465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhu JD, Hu FY, Ge S, Ye MZ, Xiang H, Zhang GJ, Zheng XM, Zhang HY, Zhang SL, Li Q, Luo RB, Yu C, Yu J, Sun JF, Zou XY, Cao XF, Xie XF, Wang J, Wang W. Single-base resolution maps of cultivated and wild rice methylomes and regulatory roles of DNA methylation in plant gene expression. BMC Genomics. 2012b;13:300–314. doi: 10.1186/1471-2164-13-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YF, Qiu L, Huang L, Cao JS. BcJMJ30, the gene encoding jmjC domain-containing histone demethylase is associated with pollen development and fertilization in Brassica campestris ssp chinensis. Plant Mol. Biol. Rep. 2012c;30:529–538. [Google Scholar]
- López C, Pérez-Quintero A, lvaro L. The micromics revolution: microRNA-mediated approaches to develop stress-resistant crops. In: Tuteja N, Gill SS, Tiburcio AF, Tuteja R, editors. Improving Crop Resistance to Abiotic Stress. 1 & 2. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2012. pp. 559–590. [Google Scholar]
- Long Y, Xia W, Li R, Wang J, Shao M, Feng J, King GJ, Meng J. Epigenetic QTL Mapping in Brassica napus. Genetics. 2011;189:1093–1102. doi: 10.1534/genetics.111.131615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna E, Ton J. The epigenetic machinery controlling transgenerational systemic acquired resistance. Plant Signal. Behav. 2012;7:615–618. doi: 10.4161/psb.20155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Bilodeau P, Koltunow A, Dennis ES, Peacock WJ, Chaudhury AM. Genes controlling fertilization-independent seed development in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA. 1999;96:296–301. doi: 10.1073/pnas.96.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZD, Zhang JH, Li JH, Yang CX, Wang TT, Ouyang B, Li HX, Giovannoni J, Ye ZB. A STAY-GREEN protein SlSGR1 regulates lycopene and beta-carotene accumulation by interacting directly with SlPSY1 during ripening processes in tomato. New Phytol. 2013;198:442–452. doi: 10.1111/nph.12175. [DOI] [PubMed] [Google Scholar]
- Makarevich G, Villar CBR, Erilova A, Kohler C. Mechanism of PHERES1 imprinting in Arabidopsis. J. Cell Sci. 2008;121:906–912. doi: 10.1242/jcs.023077. [DOI] [PubMed] [Google Scholar]
- Manning K, Tör M, Poole M, Hong Y, Thompson A, King G, Giovannoni J, Seymour G. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat. Genet. 2006;38:948–952. doi: 10.1038/ng1841. [DOI] [PubMed] [Google Scholar]
- Manzano C, Ramirez-Parra E, Casimiro I, Otero S, Desvoyes B, De Rybel B, Beeckman T, Casero P, Gutierrez C, del Pozo JC. Auxin and epigenetic regulation of SKP2B, an F-Box that represses lateral root formation. Plant Physiol. 2012;160:749–762. doi: 10.1104/pp.112.198341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfil CF, Asurmendi S, Masuelli RW. Changes in micro RNA expression in a wild tuber-bearing Solanum species induced by 5-Azacytidine treatment. Plant Cell Rep. 2012;31:1449–1461. doi: 10.1007/s00299-012-1260-x. [DOI] [PubMed] [Google Scholar]
- Marin-Gonzalez E, Suarez-Lopez P. “And yet it moves”: cell-to-cell and long-distance signaling by plant microRNAs. Plant Sci. 2012;196:18–30. doi: 10.1016/j.plantsci.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Martin-Trillo M, Grandio EG, Serra F, Marcel F, Rodriguez-Buey ML, Schmitz G, Theres K, Bendahmane A, Dopazo H, Cubas P. Role of tomato BRANCHED1-like genes in the control of shoot branching. Plant J. 2011;67:701–714. doi: 10.1111/j.1365-313X.2011.04629.x. [DOI] [PubMed] [Google Scholar]
- Matsoukas IG, Massiah AJ, Thomas B. Florigenic and antiflorigenic signaling in plants. Plant Cell Physiol. 2012;53:1827–1842. doi: 10.1093/pcp/pcs130. [DOI] [PubMed] [Google Scholar]
- Matzke M, Kanno T, Huettel B, Daxinger L, Matzke AJM. Targets of RNA-directed DNA methylation. Curr. Opin. Plant Biol. 2007;10:512–519. doi: 10.1016/j.pbi.2007.06.007. [DOI] [PubMed] [Google Scholar]
- May O. Epigenetics & Gene Regulation. Vol. 8. Ann Arbor, MI, USA: Cayman Chemical; 2010. DNA methylation: fingerprints of the (epi)genome; pp. 22–23. [Google Scholar]
- Mayer KFX, Waugh R, Langridge P, Close TJ, Wise RP, Graner A, Matsumoto T, Sato K, Schulman A, Muehlbauer GJ, Stein N, Ariyadasa R, Schulte D, Poursarebani N, Zhou RN, Steuernagel B, Mascher M, Scholz U, Shi BJ, Langridge P, Madishetty K, Svensson JT, Bhat P, Moscou M, Resnik J, Close TJ, Muehlbauer GJ, Hedley P, Liu H, Morris J, Waugh R, Frenkel Z, Korol A, Berges H, Graner A, Stein N, Steuernagel B, Taudien S, Groth M, Felder M, Platzer M, Brown JWS, Schulman A, Platzer M, Fincher GB, Muehlbauer GJ, Sato K, Taudien S, Sampath D, Swarbreck D, Scalabrin S, Zuccolo A, Vendramin V, Morgante M, Mayer KFX, Schulman A International Barley Genome Sequencing Consortium. A physical, genetic and functional sequence assembly of the barley genome. Nature. 2012;491:711–716. doi: 10.1038/nature11543. [DOI] [PubMed] [Google Scholar]
- McGarry RC, Kragler F. Phloem-mobile signals affecting flowers: applications for crop breeding. Trends Plant Sci. 2013;18:198–206. doi: 10.1016/j.tplants.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Meagher RB. The evolution of epitype. Plant Cell. 2010;22:1658–1666. doi: 10.1105/tpc.110.075481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed-Bessudo C, Levy AA. Deficiency in DNA methylation increases meiotic crossover rates in euchromatic but not in heterochromatic regions in Arabidopsis. Proc. Natl Acad. Sci. USA. 2012;109:E981–E988. doi: 10.1073/pnas.1120742109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesejo C, Yuste R, Martínez-Fuentes A, Reig C, Iglesias DJ, Primo-Millo E, Agustí M. Self-pollination and parthenocarpic ability in developing ovaries of self-incompatible Clementine mandarins (Citrus clementina) Physiol. Plant. 2013;148:87–96. doi: 10.1111/j.1399-3054.2012.01697.x. [DOI] [PubMed] [Google Scholar]
- Miura A, Nakamura M, Inagaki S, Kobayashi A, Saze H, Kakutani T. An Arabidopsis jmjC domain protein protects transcribed genes from DNA methylation at CHG sites. EMBO J. 2009a;28:1078–1086. doi: 10.1038/emboj.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Agetsuma M, Kitano H, Yoshimura A, Matsuoka M, Jacobsen SE, Ashikari M. A metastable DWARF1 epigenetic mutant affecting plant stature in rice. Proc. Natl Acad. Sci. USA. 2009b;106:11218–11223. doi: 10.1073/pnas.0901942106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher RA, Melnyk CW. siRNAs and DNA methylation: seedy epigenetics. Trends Plant Sci. 2010;15:204–210. doi: 10.1016/j.tplants.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Mosher RA, Melnyk CW, Kelly KA, Dunn RM, Studholme DJ, Baulcombe DC. Uniparental expression of PolIV-dependent siRNAs in developing endosperm of Arabidopsis. Nature. 2009;460:283–286. doi: 10.1038/nature08084. [DOI] [PubMed] [Google Scholar]
- Mouradov A, Cremer F, Coupland G. Control of flowering time: interacting pathways as a basis for diversity. Plant Cell. 2002;14:S111–S130. doi: 10.1105/tpc.001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon S, Jing RC, Szittya G, Schwach F, Pilcher RLR, Moulton V, Dalmay T. Deep sequencing of tomato short RNAs identifies microRNAs targeting genes involved in fruit ripening. Genome Res. 2008;18:1602–1609. doi: 10.1101/gr.080127.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlbauer GJ, Bhau BS, Syed NH, Heinen S, Cho S, Marshall D, Pateyron S, Buisine N, Chalhoub B, Flavell AJ. A hAT superfamily transposase recruited by the cereal grass genome. Mol. Genet. Genomics. 2006;275:553–563. doi: 10.1007/s00438-006-0098-8. [DOI] [PubMed] [Google Scholar]
- Muhlhausen A, Lenser T, Mummenhoff K, Theissen G. Evidence that an evolutionary transition from dehiscent to indehiscent fruits in Lepidium (Brassicaceae) was caused by a change in the control of valve margin identity genes. Plant J. 2013;73:824–835. doi: 10.1111/tpj.12079. [DOI] [PubMed] [Google Scholar]
- Nagasaki H, Itoh JI, Hayashi K, Hibara KI, Satoh-Nagasawa N, Nosaka M, Mukouhata M, Ashikari M, Kitano H, Matsuoka M, Nagato Y, Sato Y. The small interfering RNA production pathway is required for shoot meristem initiation in rice. Proc. Natl Acad. Sci. USA. 2007;104:14867–14871. doi: 10.1073/pnas.0704339104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Yamaguchi-Shinozaki K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013;32:959–970. doi: 10.1007/s00299-013-1418-1. [DOI] [PubMed] [Google Scholar]
- Nicotra AB, Atkin OK, Bonser SP, Davidson AM, Finnegan EJ, Mathesius U, Poot P, Purugganan MD, Richards CL, Valladares F, van Kleunen M. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010;15:684–692. doi: 10.1016/j.tplants.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Niwa M, Daimon Y, Kurotani K-I, Higo A, Pruneda-Paz JL, Breton G, Mitsuda N, Kay SA, Ohme-Takagi M, Endo M, Araki T. BRANCHED1 interacts with FLOWERING LOCUS T to repress the floral transition of the axillary meristems in Arabidopsis. Plant Cell. 2013;25:1228–1242. doi: 10.1105/tpc.112.109090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M, Lee H, Kim YK, Nam JW, Rhee JK, Zhang BT, Kim VN, Lee I. Identification and characterization of small RNAs from vernalized Arabidopsis thaliana. J. Plant Biol. 2007;50:562–572. [Google Scholar]
- Ohad N, Margossian L, Hsu YC, Williams C, Repetti P, Fischer RL. A mutation that allows endosperm development without fertilization. Proc. Natl Acad. Sci. USA. 1996;93:5319–5324. doi: 10.1073/pnas.93.11.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Morea FA, Vicentini R, Silva GFF, Silva EM, Carrer H, Rodrigues AP, Nogueira FTS. Global analysis of the sugarcane microtranscriptome reveals a unique composition of small RNAs associated with axillary bud outgrowth. J. Exp. Bot. 2013;64:2307–2320. doi: 10.1093/jxb/ert089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn TC, Chris Pires J, Birchler JA, Auger DL, Jeffery Chen Z, Lee H-S, Comai L, Madlung A, Doerge RW, Colot V, Martienssen RA. Understanding mechanisms of novel gene expression in polyploids. Trends Genet. 2003;19:141–147. doi: 10.1016/s0168-9525(03)00015-5. [DOI] [PubMed] [Google Scholar]
- Pan YJ, Wang WS, Zhao XQ, Zhu LH, Fu BY, Li ZK. DNA methylation alterations of rice in response to cold stress. Plant OMICS. 2011;4:364–369. [Google Scholar]
- Paszkowski J, Grossniklaus U. Selected aspects of transgenerational epigenetic inheritance and resetting in plants. Curr. Opin. Plant Biol. 2011;14:195–203. doi: 10.1016/j.pbi.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H, Haberer G, Hellsten U, Mitros T, Poliakov A, Schmutz J, Spannagl M, Tang HB, Wang XY, Wicker T, Bharti AK, Chapman J, Feltus FA, Gowik U, Grigoriev IV, Lyons E, Maher CA, Martis M, Narechania A, Otillar RP, Penning BW, Salamov AA, Wang Y, Zhang LF, Carpita NC, Freeling M, Gingle AR, Hash CT, Keller B, Klein P, Kresovich S, McCann MC, Ming R, Peterson DG, Mehboob ur R, Ware D, Westhoff P, Mayer KFX, Messing J, Rokhsar DS. The Sorghum bicolor genome and the diversification of grasses. Nature. 2009;457:551–556. doi: 10.1038/nature07723. [DOI] [PubMed] [Google Scholar]
- Pattanayak D, Solanke AU, Kumar PA. Plant RNA interference pathways: diversity in function, similarity in action. Plant Mol. Biol. Rep. 2013;31:493–506. [Google Scholar]
- Pauli A, Rinn JL, Schier AF. Non-coding RNAs as regulators of embryogenesis. Nat. Rev. Genet. 2011;12:136–149. doi: 10.1038/nrg2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautler M, Tanaka W, Hirano H-Y, Jackson D. Grass meristems I: shoot apical meristem maintenance, axillary meristem determinacy and the floral transition. Plant Cell Physiol. 2013;54:302–312. doi: 10.1093/pcp/pct025. [DOI] [PubMed] [Google Scholar]
- Poethig RS. Phase change and the regulation of developmental timing in plants. Science. 2003;301:334–336. doi: 10.1126/science.1085328. [DOI] [PubMed] [Google Scholar]
- Poethig RS. The past, present, and future of vegetative phase change. Plant Physiol. 2010;154:541–544. doi: 10.1104/pp.110.161620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova OV, Dinh HQ, Aufsatz W, Jonak C. The RdDM pathway is required for basal heat tolerance in Arabidopsis. Mol. Plant. 2013;6:396–410. doi: 10.1093/mp/sst023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss S, Pikaard CS. RRNA gene silencing and nucleolar dominance: insights into a chromosome-scale epigenetic on/off switch. Biochim. Biophys. Acta. 2007;1769:383–392. doi: 10.1016/j.bbaexp.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, Boursnell C, Pang N, Forslund K, Ceric G, Clements J, Heger A, Holm L, Sonnhammer ELL, Eddy SR, Bateman A, Finn RD. The Pfam protein families database. Nucleic Acids Res. 2012;40:D290–D301. doi: 10.1093/nar/gkr1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathcke B, Lacey E. Phenological patterns of terrestrial plants. Annu. Rev. Ecol. Syst. 1985;16:179–214. [Google Scholar]
- Rausch C, Bucher M. Molecular mechanisms of phosphate transport in plants. Planta. 2002;216:23–37. doi: 10.1007/s00425-002-0921-3. [DOI] [PubMed] [Google Scholar]
- Reinders J, Wulff BB, Mirouze M, Marí-Ordóñez A, Dapp M, Rozhon W, Bucher E, Theiler G, Paszkowski J. Compromised stability of DNA methylation and transposon immobilization in mosaic Arabidopsis epigenomes. Genes Dev. 2009;23:939–950. doi: 10.1101/gad.524609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repinski SL, Kwak M, Gepts P. The common bean growth habit gene PvTFL1y is a functional homolog of Arabidopsis TFL1. Theor. Appl. Genet. 2012;124:1539–1547. doi: 10.1007/s00122-012-1808-8. [DOI] [PubMed] [Google Scholar]
- Rhee Y, Sekhon RS, Chopra S, Kaeppler S. Tissue culture-induced novel epialleles of a Myb transcription factor encoded by pericarp color1 in Maize. Genetics. 2010;186:843–855. doi: 10.1534/genetics.110.117929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards CL, Rosas U, Banta J, Bhambhra N, Purugganan MD. Genome-wide patterns of Arabidopsis gene expression in nature. PLoS Genet. 2012;8:e1002662. doi: 10.1371/journal.pgen.1002662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoll JJ, Roeder AHK, Ditta GS, Yanofsky MF. A novel role for the floral homeotic gene APETALA2 during Arabidopsis fruit development. Development. 2011;138:5167–5176. doi: 10.1242/dev.073031. [DOI] [PubMed] [Google Scholar]
- Romera-Branchat M, Ripoll JJ, Yanofsky MF, Pelaz S. The WOX13 homeobox gene promotes replum formation in the Arabidopsis thaliana fruit. Plant J. 2013;73:37–49. doi: 10.1111/tpj.12010. [DOI] [PubMed] [Google Scholar]
- Ross-Ibarra J, Morrell PL, Gaut BS. Plant domestication, a unique opportunity to identify the genetic basis of adaptation. Proc. Natl Acad. Sci. USA. 2007;104:8641–8648. doi: 10.1073/pnas.0700643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouached H, Arpat AB, Poirier Y. Regulation of phosphate starvation responses in plants: signaling players and cross-talks. Mol. Plant. 2010;3:288–299. doi: 10.1093/mp/ssp120. [DOI] [PubMed] [Google Scholar]
- Ruiz-Garcia L, Cervera MT, Martinez-Zapater JM. DNA methylation increases throughout Arabidopsis development. Planta. 2005;222:301–306. doi: 10.1007/s00425-005-1524-6. [DOI] [PubMed] [Google Scholar]
- Russo VEA, Martienssen RA, Riggs AD. Epigenetic Mechanisms of Gene Regulation. New York, NY, USA: Cold Spring Harbor Laboratory Press; 1996. [Google Scholar]
- Saini S, Sharma I, Kaur N, Pati PK. Auxin: a master regulator in plant root development. Plant Cell Rep. 2013;32:741–757. doi: 10.1007/s00299-013-1430-5. [DOI] [PubMed] [Google Scholar]
- Sakai H, Lee SS, Tanaka T, Numa H, Kim J, Kawahara Y, Wakimoto H, Yang C-C, Iwamoto M, Abe T, Yamada Y, Muto A, Inokuchi H, Ikemura T, Matsumoto T, Sasaki T, Itoh T. Rice Annotation Project Database (RAP-DB): an integrative and interactive database for rice genomics. Plant Cell Physiol. 2013;54:e6. doi: 10.1093/pcp/pcs183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A, Alvarez-Venegas R, Avramova Z. Dynamic and stable histone H3 methylation patterns at the Arabidopsis FLC and AP1 loci. Gene. 2008a;423:43–47. doi: 10.1016/j.gene.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Saleh A, Alvarez-venegas R, Avramova Z. An efficient chromatin immunoprecipitation (ChIP) protocol for studying histone modifications in Arabidopsis plants. Nat. Protoc. 2008b;3:1018–1025. doi: 10.1038/nprot.2008.66. [DOI] [PubMed] [Google Scholar]
- Salvini M, Sani E, Fambrini M, Pistelli L, Pucciariello C, Pugliesi C. Molecular analysis of a sunflower gene encoding an homologous of the B subunit of a CAAT binding factor. Mol. Biol. Rep. 2012;39:6449–6465. doi: 10.1007/s11033-012-1463-9. [DOI] [PubMed] [Google Scholar]
- Samach A, Smith HM. Constraints to obtaining consistent annual yields in perennials II: environment and fruit load affect induction of flowering. Plant Sci. 2013;207:168–176. doi: 10.1016/j.plantsci.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Sano H, Kamada I, Youssefian S, Katsumi M, Wabiko H. A single treatment of rice seedlings with 5-Azacytidine induces heritable dwarfism and undermethylation of genomic DNA. Mol. Gen. Genet. 1990;220:441–447. [Google Scholar]
- Sarma K, Reinberg D. Histone variants meet their match. Nat. Rev. Mol. Cell Biol. 2005;6:139–149. doi: 10.1038/nrm1567. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kobayashi A, Saze H, Kakutani T. RNAi-independent de novo DNA methylation revealed in Arabidopsis mutants of chromatin remodeling gene DDM1. Plant J. 2012;70:750–758. doi: 10.1111/j.1365-313X.2012.04911.x. [DOI] [PubMed] [Google Scholar]
- Saze H, Kakutani T. Heritable epigenetic mutation of a transposon-flanked Arabidopsis gene due to lack of the chromatin-remodeling factor DDM1. EMBO J. 2007;26:3641–3652. doi: 10.1038/sj.emboj.7601788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saze H, Scheid OM, Paszkowski J. Maintenance of CpG methylation is essential for epigenetic inheritance during plant gametogenesis. Nat. Genet. 2003;34:65–69. doi: 10.1038/ng1138. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Wohrmann HJP, Raissig MT, Arand J, Gheyselinck J, Gagliardini V, Heichinger C, Walter J, Grossniklaus U. The Polycomb group protein MEDEA and the DNA methyltransferase MET1 interact to repress autonomous endosperm development in Arabidopsis. Plant J. 2013;73:776–787. doi: 10.1111/tpj.12070. [DOI] [PubMed] [Google Scholar]
- Schmutz J, Cannon SB, Schlueter J, Ma JX, Mitros T, Nelson W, Hyten DL, Song QJ, Thelen JJ, Cheng JL, Xu D, Hellsten U, May GD, Yu Y, Sakurai T, Umezawa T, Bhattacharyya MK, Sandhu D, Valliyodan B, Lindquist E, Peto M, Grant D, Shu SQ, Goodstein D, Barry K, Futrell-Griggs M, Abernathy B, Du JC, Tian ZX, Zhu LC, Gill N, Joshi T, Libault M, Sethuraman A, Zhang XC, Shinozaki K, Nguyen HT, Wing RA, Cregan P, Specht J, Grimwood J, Rokhsar D, Stacey G, Shoemaker RC, Jackson SA. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463:178–183. doi: 10.1038/nature08670. [DOI] [PubMed] [Google Scholar]
- Scholten S. Genomic imprinting in plant embryos. Epigenetics. 2010;5:455–459. doi: 10.4161/epi.5.6.12313. [DOI] [PubMed] [Google Scholar]
- Schonrock N, Bouveret R, Leroy O, Borghi L, Kohler C, Gruissem W, Hennig L. Polycomb-group proteins repress the floral activator AGL19 in the FLC-independent vernalization pathway. Genes Dev. 2006;20:1667–1678. doi: 10.1101/gad.377206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour G, Poole M, Manning K, King GJ. Genetics and epigenetics of fruit development and ripening. Curr. Opin. Plant Biol. 2008;11:58–63. doi: 10.1016/j.pbi.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Seymour GB, Chapman NH, Chew BL, Rose JKC. Regulation of ripening and opportunities for control in tomato and other fruits. Plant Biotechnol. J. 2013a;11:269–278. doi: 10.1111/j.1467-7652.2012.00738.x. [DOI] [PubMed] [Google Scholar]
- Seymour GB, Ostergaard L, Chapman NH, Knapp S, Martin C. Fruit development and ripening. In: Merchant SS, editor. Annual Review of Plant Biology. Vol. 64. Palo Alto, CA: Annual Reviews; 2013b. pp. 219–241. [DOI] [PubMed] [Google Scholar]
- Sheldon CC, Finnegan EJ, Peacock WJ, Dennis ES. Mechanisms of gene repression by vernalization in Arabidopsis. Plant J. 2009;59:488–498. doi: 10.1111/j.1365-313X.2009.03883.x. [DOI] [PubMed] [Google Scholar]
- Shepherd M, Rose T, Raymond C. Rejuvenation of mature native Tea Tree (Melaleuca alternifolia (Maiden & Betche) Cheel) for vegetative propagation. Propagation of Ornamental Plants. 2013;13:103–111. [Google Scholar]
- Sherman JD, Talbert LE. Vernalization-induced changes of the DNA methylation pattern in winter wheat. Genome. 2002;45:253–260. doi: 10.1139/g01-147. [DOI] [PubMed] [Google Scholar]
- Shi JQ, Li RY, Qiu D, Jiang CC, Long Y, Morgan C, Bancroft I, Zhao JY, Meng JL. Unraveling the complex trait of crop yield with quantitative trait loci mapping in Brassica napus. Genetics. 2009;182:851–861. doi: 10.1534/genetics.109.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba H, Kenmochi M, Sugihara M, Iwano M, Kawasaki S, Suzuki G, Watanabe M, Isogai A, Takayama S. Genomic organization of the S-locus region of Brassica. Biosci. Biotechnol. Biochem. 2003;67:622–626. doi: 10.1271/bbb.67.622. [DOI] [PubMed] [Google Scholar]
- Shiba H, Kakizaki T, Iwano M, Tarutani Y, Watanabe M, Isogai A, Takayama S. Dominance relationships between self-incompatibility alleles controlled by DNA methylation. Nat. Genet. 2006;38:297–299. doi: 10.1038/ng1734. [DOI] [PubMed] [Google Scholar]
- Simpson RT. Structure of chomatosome, a chromatin particle containing 160-base pairs of DNA and all histones. Biochemistry. 1978;17:5524–5531. doi: 10.1021/bi00618a030. [DOI] [PubMed] [Google Scholar]
- Song J, Angel A, Howard M, Dean C. Vernalization—a cold-induced epigenetic switch. J. Cell Sci. 2012;125:3723–3731. doi: 10.1242/jcs.084764. [DOI] [PubMed] [Google Scholar]
- Soppe WJJ, Jacobsen SE, Alonso-Blanco C, Jackson JP, Kakutani T, Koornneef M, Peeters AJM. The late flowering phenotype of fwa mutants is caused by gain-of-function epigenetic alleles of a homeodomain gene. Mol. Cell. 2000;6:791–802. doi: 10.1016/s1097-2765(05)00090-0. [DOI] [PubMed] [Google Scholar]
- Springer NM. Epigenetics and crop improvement. Trends Genet. 2013;29:241–247. doi: 10.1016/j.tig.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Srikanth A, Schmid M. Regulation of flowering time: all roads lead to Rome. Cell. Mol. Life Sci. 2011;68:2013–2037. doi: 10.1007/s00018-011-0673-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepinski D. Levels of DNA methylation and histone methylation and acetylation change in root tip cells of soybean seedlings grown at different temperatures. Plant Physiol. Biochem. 2012;61:9–17. doi: 10.1016/j.plaphy.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Steward N, Ito M, Yamaguchi Y, Koizumi N, Sano H. Periodic DNA methylation in maize nucleosomes and demethylation by environmental stress. J. Biol. Chem. 2002;277:37741–37746. doi: 10.1074/jbc.M204050200. [DOI] [PubMed] [Google Scholar]
- Stirnberg P, Ward S, Leyser O. Auxin and strigolactones in shoot branching: intimately connected? Biochem. Soc. Trans. 2010;38:717–722. doi: 10.1042/BST0380717. [DOI] [PubMed] [Google Scholar]
- Stroud H, Greenberg MVC, Feng SH, Bernatavichute YV, Jacobsen SE. Comprehensive analysis of silencing mutants reveals complex regulation of the Arabidopsis methylome. Cell. 2013;152:352–364. doi: 10.1016/j.cell.2012.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Xu YF, Ng KH, Ito T. A timing mechanism for stem cell maintenance and differentiation in the Arabidopsis floral meristem. Genes Dev. 2009;23:1791–1804. doi: 10.1101/gad.1800409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung SB, Amasino RM. Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature. 2004;427:159–164. doi: 10.1038/nature02195. [DOI] [PubMed] [Google Scholar]
- Tan FC, Swain SM. Genetics of flower initiation and development in annual and perennial plants. Physiol. Plant. 2006;128:8–17. [Google Scholar]
- Thanananta T, Pongtongkam P, Thongpan A, Kaveeta L, Peyachoknagul S. Effect of short day photoperiod on DNA methylation and expression of a gene in rice KDML105. Afr. J. Biotechnol. 2006;5:1375–1382. [Google Scholar]
- Thomas H. Senescence, ageing and death of the whole plant. New Phytol. 2013;197:696–711. doi: 10.1111/nph.12047. [DOI] [PubMed] [Google Scholar]
- Tiwari S, Schulz R, Ikeda Y, Dytham L, Bravo J, Mathers L, Spielman M, Guzman P, Oakey RJ, Kinoshita T, Scott RJ. MATERNALLY EXPRESSED PAB C-TERMINAL, a novel imprinted gene in Arabidopsis, encodes the conserved C-terminal domain of polyadenylate binding proteins. Plant Cell. 2008;20:2387–2398. doi: 10.1105/tpc.108.061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troein C, Locke JCW, Turner MS, Millar AJ. Weather and seasons together demand complex biological clocks. Curr. Biol. 2009;19:1961–1964. doi: 10.1016/j.cub.2009.09.024. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T, Eulgem T. Mutations in EDM2 selectively affect silencing states of transposons and induce plant developmental plasticity. Sci. Rep. 2013;3:1701. doi: 10.1038/srep01701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin MN, Kim JY. Non-cell-autonomous RNA silencing spread in plants. Bot. Stud. 2011;52:129–136. [Google Scholar]
- Valverde F. CONSTANS and the evolutionary origin of photoperiodic timing of flowering. J. Exp. Bot. 2011;62:2453–2463. doi: 10.1093/jxb/erq449. [DOI] [PubMed] [Google Scholar]
- Vaughan DA, Balazs E, Heslop-Harrison JS. From crop domestication to super-domestication. Ann. Bot. 2007;100:893–901. doi: 10.1093/aob/mcm224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TM, Nakamura M, Calarco JP, Susaki D, Lim PQ, Kinoshita T, Higashiyama T, Martienssen RA, Berger F. RNA-directed DNA methylation regulates parental genomic imprinting at several loci in Arabidopsis. Development. 2013;140:2953–2960. doi: 10.1242/dev.092981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington C, Kacser H. The Strategy of Genes. London: Allen & Unwin; 1957. [Google Scholar]
- Walbot V, Evans MMS. Unique features of the plant life cycle and their consequences. Nat. Rev. Genet. 2003;4:369–379. doi: 10.1038/nrg1064. [DOI] [PubMed] [Google Scholar]
- Wang H, Schauer N, Usadel B, Frasse P, Zouine M, Hernould M, Latche A, Pech J-C, Fernie AR, Bouzayen M. Regulatory features underlying pollination-dependent and -independent tomato fruit set revealed by transcript and primary metabolite profiling. Plant Cell. 2009;21:1428–1452. doi: 10.1105/tpc.108.060830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hopkins CJ, Hou JN, Zou XX, Wang CN, Long Y, Kurup S, King GJ, Meng JL. Promoter variation and transcript divergence in brassicaceae lineages of FLOWERING LOCUS T. PLoS ONE. 2012;7:1–11. doi: 10.1371/journal.pone.0047127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hu J, Qian Q, Xue HW. LC2 and OsVIL2 promote rice flowering by photoperoid-induced epigenetic silencing of OsLF. Mol. Plant. 2013;6:514–527. doi: 10.1093/mp/sss096. [DOI] [PubMed] [Google Scholar]
- Wang JW, Park MY, Wang LJ, Koo Y, Chen XY, Weigel D, Poethig RS. miRNA Control of Vegetative Phase Change in Trees. PLoS Genet. 2011;7:1–8. doi: 10.1371/journal.pgen.1002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Broadley MR. Biofortification of crops with seven mineral elements often lacking in human diets—iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009;182:49–84. doi: 10.1111/j.1469-8137.2008.02738.x. [DOI] [PubMed] [Google Scholar]
- Wigge PA, Kim MC, Jaeger KE, Busch W, Lohmann JU, Weigel D. Integration of spatial and temporal information during floral induction in Arabidopsis. Science. 2005;309:1056–1059. doi: 10.1126/science.1114358. [DOI] [PubMed] [Google Scholar]
- Wiltshire RJE, Potts BM, Reid JB. Genetic control of reproductive and vegetative phase change in the Eucalyptus risdonii E-tenuiramis complex. Aust. J. Bot. 1998;46:45–63. [Google Scholar]
- Wollenberg AC, Amasino RM. Natural variation in the temperature range permissive for vernalization in accessions of Arabidopsis thaliana. Plant Cell Environ. 2012;35:2181–2191. doi: 10.1111/j.1365-3040.2012.02548.x. [DOI] [PubMed] [Google Scholar]
- Wollmann H, Berger F. Epigenetic reprogramming during plant reproduction and seed development. Curr. Opin. Plant Biol. 2012;15:63–69. doi: 10.1016/j.pbi.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Wurr DCE, Fellows JR, Fuller MP. Simulated effects of climate change on the production pattern of winter cauliflower in the UK. Sci. Hortic. 2004;101:359–372. [Google Scholar]
- Xiao WY, Custard KD, Brown RC, Lemmon BE, Harada JJ, Goldberg RB, Fischer RL. DNA methylation is critical for Arabidopsis embryogenesis and seed viability. Plant Cell. 2006;18:805–814. doi: 10.1105/tpc.105.038836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Zhang H, Xing LJ, Xu SJ, Liu HH, Chong K, Xu YY. Requirement of histone acetyltransferases HAM1 and HAM2 for epigenetic modification of FLC in regulating flowering in Arabidopsis. J. Plant Physiol. 2013;170:444–451. doi: 10.1016/j.jplph.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Xu ZY, Kim DH, Hwang I. ABA homeostasis and signaling involving multiple subcellular compartments and multiple receptors. Plant Cell Rep. 2013;32:807–813. doi: 10.1007/s00299-013-1396-3. [DOI] [PubMed] [Google Scholar]
- Yaish M, Guevara D, El-Kereamy A, Rothstein S. Plant Developmental Biology—Biotechnological Perspectives. 1 & 2. Berlin, Heidelberg: Springer-Verlag; 2010. [Google Scholar]
- Yaish MW, Colasanti J, Rothstein SJ. The role of epigenetic processes in controlling flowering time in plants exposed to stress. J. Exp. Bot. 2011;62:3727–3735. doi: 10.1093/jxb/err177. [DOI] [PubMed] [Google Scholar]
- Yakovlev I, Fossdal C, Johnsen Ø. MicroRNAs, the epigenetic memory and climatic adaptation in Norway spruce. New Phytol. 2010;187:1154–1169. doi: 10.1111/j.1469-8137.2010.03341.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A, Abe M. Regulation of reproductive development by non-coding RNA in Arabidopsis: to flower or not to flower. J. Plant. Res. 2012;125:693–704. doi: 10.1007/s10265-012-0513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao XZ, Feng HY, Yu Y, Dong AW, Shen WH. SDG2-mediated H3K4 methylation is required for proper Arabidopsis root growth and development. PLoS ONE. 2013;8:1–11. doi: 10.1371/journal.pone.0056537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You WH, Lorkovic ZJ, Matzke AJM, Matzke M. Interplay among RNA polymerases II, IV and V in RNA-directed DNA methylation at a low copy transgene locus in Arabidopsis thaliana. Plant Mol. Biol. 2013;82:85–96. doi: 10.1007/s11103-013-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemach A, Kim MY, Silva P, Rodrigues JA, Dotson B, Brooks MD, Zilberman D. Local DNA hypomethylation activates genes in rice endosperm. Proc. Natl Acad. Sci. USA. 2010;107:18729–18734. doi: 10.1073/pnas.1009695107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y. Principles of micro-RNA production and maturation. Oncogene. 2006;25:6156–6162. doi: 10.1038/sj.onc.1209908. [DOI] [PubMed] [Google Scholar]
- Zeng HQ, Wang GP, Hu XY, Wang HZ, Du LQ, Zhu YY. Role of microRNAs in plant responses to nutrient stress. Plant Soil. 2014;374:1005–1021. [Google Scholar]
- Zhang JJ, Xue HW. OsLEC1/OsHAP3E participates in the determination of meristem identity in both vegetative and reproductive developments of rice. J. Integr. Plant Biol. 2013;55:232–249. doi: 10.1111/jipb.12025. [DOI] [PubMed] [Google Scholar]
- Zhang QF, Shen BZ, Dai XK, Mei MH, Maroof MAS, Li ZB. Using bulked extremes and recessive class to map genes for photoperiod-sensitive genic male-sterility in rice. Proc. Natl Acad. Sci. USA. 1994;91:8675–8679. doi: 10.1073/pnas.91.18.8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Yazaki J, Sundaresan A, Cokus S, Chan SWL, Chen HM, Henderson IR, Shinn P, Pellegrini M, Jacobsen SE, Ecker JR. Genome-wide high-resolution mapping and functional analysis of DNA methylation in Arabidopsis. Cell. 2006;126:1189–1201. doi: 10.1016/j.cell.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Zhang H, Chaudhury A, Wu X. Imprinting in plants and its underlying mechanisms. J. Genet. Genomics. 2013a;40:239–247. doi: 10.1016/j.jgg.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Fischer M, Colot V, Bossdorf O. Epigenetic variation creates potential for evolution of plant phenotypic plasticity. New Phytol. 2013b;197:314–322. doi: 10.1111/nph.12010. [DOI] [PubMed] [Google Scholar]
- Zhao XY, Yu XH, Foo E, Symons GM, Lopez J, Bendehakkalu KT, Xiang J, Weller JL, Liu XM, Reid JB, Lin CT. A study of gibberellin homeostasis and cryptochrome-mediated blue light inhibition of hypocotyl elongation. Plant Physiol. 2007;145:106–118. doi: 10.1104/pp.107.099838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Zhu B, Li M, Wang L, Xu L, Zhang H, Zheng S, Qi B, Han F, Liu B. Extensive and heritable epigenetic remodeling and genetic stability accompany allohexaploidization of wheat. Genetics. 2011;188:499–510. doi: 10.1534/genetics.111.127688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong SL, Fei ZJ, Chen YR, Zheng Y, Huang MY, Vrebalov J, McQuinn R, Gapper N, Liu B, Xiang J, Shao Y, Giovannoni JJ. Single-base resolution methylomes of tomato fruit development reveal epigenome modifications associated with ripening. Nat. Biotechnol. 2013;31:154–159. doi: 10.1038/nbt.2462. [DOI] [PubMed] [Google Scholar]
- Zhou M, Luo H. MicroRNA-mediated gene regulation: potential applications for plant genetic engineering. Plant Mol. Biol. 2013;83:59–75. doi: 10.1007/s11103-013-0089-1. [DOI] [PubMed] [Google Scholar]
- Zhou H, Liu QJ, Li J, Jiang DG, Zhou LY, Wu P, Lu S, Li F, Zhu LY, Liu ZL, Chen LT, Liu YG, Zhuang CX. Photoperiod- and thermo-sensitive genic male sterility in rice are caused by a point mutation in a novel noncoding RNA that produces a small RNA. Cell Res. 2012;22:649–660. doi: 10.1038/cr.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Reinberg D. Epigenetic inheritance: uncontested? Cell Res. 2011;21:435–441. doi: 10.1038/cr.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo JH, Zhu BZ, Fu DQ, Zhu Y, Ma YZ, Chi LH, Ju Z, Wang YX, Zhai BQ, Luo YB. Sculpting the maturation, softening and ethylene pathway: the influences of microRNAs on tomato fruits. BMC Genomics. 2012;13:1–12. doi: 10.1186/1471-2164-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


