Abstract
Background
Multiple clinical practice guidelines exist for breast and cervical cancer screening, and differ in aggressiveness with respect to the recommended frequency and target populations for screening.
Objectives
To determine (1) US primary care physicians’ (PCPs) perceptions of the influence of different clinical practice guidelines; (2) the relationship between the number, aggressiveness, and agreement of influential guidelines and the aggressiveness of physicians’ screening recommendations; and (3) factors associated with guideline perceptions.
Research Design and Methods
A nationally representative sample of 1212 PCPs was surveyed in 2006–2007. Cross-sectional analyses examined physicians’ perceptions of the influence of different breast and cervical cancer screening guidelines, the relationship of guideline perceptions to screening recommendations in response to hypothetical vignettes, and the predictors of guideline perceptions.
Results
American Cancer Society and American College of Obstetricians and Gynecologists guidelines were perceived as more influential than other guidelines. Most physicians (62%) valued multiple guidelines, and conflicting and aggressive rather than conservative guideline combinations. The number, aggressiveness, and agreement of influential guidelines were associated with the aggressiveness of screening recommendations (P < 0.01)—which was highest for physicians valuing multiple-aggressive, lowest for physicians valuing multiple-conservative, and intermediate for physicians valuing multiple-conflicting, single, and no guidelines. Obstetrician/gynecologists specialty predicted valuation of aggressive guidelines (P < 0.001).
Conclusions
PCPs’ perceptions of cancer screening guidelines vary, relate to screening recommendations in logically-consistent ways, and are predicted by specialty and other factors. The number, aggressiveness, and agreement of valued guidelines are associated with screening recommendations, suggesting that guideline multiplicity is an important problem in clinical decision-making.
Keywords: clinical practice guidelines, perceptions, cancer screening, primary care physicians
Clinical practice guidelines (CPGs) have grown in number and influence over the nearly 2 decades following publication of the seminal 1990 Institute of Medicine report on the topic.1 Since then, more than 2000 publicly accessible CPGs have been developed by over 350 US and international organizations, including professional societies, government agencies, and healthcare payers.2,3 The continued dissemination of CPGs has received expanding support from health policymakers,3,4 and physicians’ attitudes toward guidelines have become increasingly favorable.5,6
An important consequence of the enormous growth in CPGs has been a multiplicity of guidelines addressing the same clinical problems.3,4 This is particularly true for breast and cervical cancer screening, for which dozens of CPGs currently exist.2 Although many of these guidelines are concordant, others are conflicting. Some are more aggressive—recommending screening on a more frequent basis, among more types of patients, or using more tests. However, others are more conservative, recommending more limited screening. Professional and public awareness of this diversity was recently heightened by the release of updated breast cancer screening recommendations by the US Preventive Services Task Force (USPSTF).7
This multiplicity of CPGs may be problematic. Guideline conflict creates potential confusion, and raises questions about the validity of individual CPGs.3,8–10 It may also negatively influence physicians’ attitudes toward CPGs, and because negative attitudes can reduce guideline adoption,11–13 such conflict may ultimately diminish guideline effectiveness.3,8,10 Concordant or redundant guidelines, on the other hand, are arguably unnecessary,8 although they may heighten perceptions of expert consensus, thereby promoting guideline-consistent practices.14–16 However, guideline concordance is inappropriate if individual guidelines are not evidence-based and methodologically sound, and this matter is often in dispute. Despite these theoretical concerns, surprisingly little is known about how physicians actually respond to multiple CPGs, whether concordant or conflicting. The lack of empirical evidence on this issue is an increasingly important problem for guideline developers and health policymakers.3
In this article, we report findings from a study designed to shed light on physicians’ responses to multiple CPGs for cancer screening. Our study had the following 3 main objectives: (1) to describe US primary care physicians’ (PCPs’) perceptions of the influence of multiple different breast and cervical cancer screening guidelines; (2) to examine how the number, aggressiveness, and agreement of guidelines perceived as influential relates to physicians’ screening recommendations; and (3) to identify physician and practice characteristics associated with these perceptions.
METHODS
Data Source and Study Population
We used data from the 2006–2007 National Survey of Primary Care Physicians’ Recommendations and Practices for Breast, Cervical, Colorectal, and Lung Cancer Screening, conducted by the National Cancer Institute in collaboration with the Agency for Healthcare Research and Quality and the Centers for Disease Control and Prevention. The survey assessed PCPs’ knowledge, attitudes, recommendations, and practices regarding screening for these cancers. The survey used a split-sample design: half of PCPs were randomly assigned to receive a questionnaire on breast and cervical cancer screening, and the other half a questionnaire on colorectal and lung cancer screening. Only the breast and cervical cancer survey contained detailed items on physicians’ perceptions of CPGs from a wide variety of professional organizations; the current study analyzed these data.
Respondents consisted of a nationally representative sample of PCPs identified from the American Medical Association Physician Masterfile. Eligible respondents were non-Federal, office-based family physicians (FP), general practitioners, general internists (internal medicine), and obstetrician/gynecologists (OB/GYN), aged 75 years or younger holding an active license and reporting patient care as their major activity. Physicians listed as retired, deceased, residing outside the United States, in residency training, teaching, research, or administration full-time were ineligible. Further methodological details are reported elsewhere,17,18 and complete survey instrumentation is available at http://healthservices.cancer.gov/surveys/screening_rp/. The survey was approved by the US Office of Management and Budget. The protocol was reviewed by the National Institutes of Health Office of Human Subjects Research and considered exempt by the National Institutes of Health Institutional Review Board.
Analysis Plan
Existing evidence suggests variation in physicians’ perceptions of the influence of CPGs,16,19,20 and several potential determinants of these perceptions.15,16,20–23 A conceptual framework developed by Cabana24 highlighted physician characteristics including age,16,25,26 gender,23,27–29 race,28,29 specialty,16,25,28,30 country of training,26 and practice characteristics including group versus solo,25,28,30,31 rural versus urban,31,32 academic versus nonacademic,31–33 HMO versus non-HMO,27 electronic medical record (EMR) use,6,28 CPG implementation,6 computerized decision support,34 and payfor-performance incentives.6 We explored similar variables in our analyses, hypothesizing that physician specialty would be the strongest predictor of perceptions of CPGs issued by different professional organizations, on the basis of empirical15,22,25 and theoretical35 work highlighting the influence of social reference groups on attitudes and behaviors.
Our other analytic focus was the relationship between physicians’ guideline perceptions and clinical decision-making. In theory, these phenomena should be related; favorable perceptions of a CPG should promote guideline-consistent decision-making. It is less clear what happens when physicians perceive multiple CPGs as influential. Indirect evidence suggests that when influential guidelines are concordant, physicians will increase adoption of guideline-consistent practices. Redundancy in expert opinions is known to promote perceptions of consensus and decision-making confidence,14 and physicians report greater confidence in CPGs endorsed by multiple organizations.15,21,22,36
On the other hand, when influential guidelines are conflicting, physicians might respond in various ways. Behavioral research has shown that people often synthesize conflicting expert opinions by averaging them.14 Physicians who endorse the influence of CPGs that differ in aggressiveness might therefore adopt practices at intermediate levels of aggressiveness. However, other decision-making biases might favor one extreme. Fear of malpractice litigation might promote more aggressive cancer screening,37–40 whereas a psychological tendency to avoid decision-making when conflicting opinions exist, known as “ambiguity aversion,”41,42 might promote less-aggressive screening. We explored these possibilities by examining how the number, aggressiveness, and agreement of cancer screening guidelines perceived as influential relates to the aggressiveness of physicians’ screening recommendations.
Measures
Perceived Influence of CPGs
We ascertained physicians’ perceptions of the influence of different CPGs using an item asking physicians the degree to which breast or cervical cancer screening guidelines from the following 5 organizations were influential in their practices: USPSTF, American Cancer Society (ACS), American College of Obstetricians and Gynecologists (ACOG), American Academy of Family Physicians (AAFP), and American College of Physicians (ACP). Respondents rated the influence of each guideline using a 4-point Likert scale (very influential/somewhat influential/not influential/not applicable or not familiar with).
We derived each physician's perceptions of the influence of different CPGs by ascertaining the guidelines perceived as “very influential”; on the basis of these responses, we classified physicians as endorsing the influence of either no, single, or multiple guidelines. We chose very influential as a cutpoint to maximize specificity in detecting true guideline influence, because midpoint responses such as “somewhat” often indicate ambivalence or uncertainty.43
Guideline Aggressiveness
Few physicians (≥5%) valued USPSTF, AAFP, ACP, or ACS guidelines exclusively, and because of our interest in the relative aggressiveness of different CPGs, we further categorized each guideline on the basis of the number and/or frequency of tests recommended; we categorized USPSTF, AAFP, and ACP guidelines as “conservative,” ACS and ACOG as “aggressive.” (Table 1, Supplemental Digital Content 1, online only, available at: http://links.lww.com/MLR/A151). The main characteristic distinguishing the 3 conservative guidelines from the 2 aggressive ones was that for most screening tests, they made no recommendation for or against screening, recommended individualized decision-making, or recommended against screening. For example, none of the 3 conservative but both aggressive guidelines recommended annual (vs. every 1–2 year) mammography for women ≥50 years, and clinical or self breast examination. For cervical cancer screening, none of the conservative but both aggressive guidelines recommended an initial annual Pap testing schedule for all women and more frequent subsequent testing (“every 2–3 years” vs. “at least every 3 years”), or routine HPV testing. We further grouped physicians by number, aggressiveness, and agreement of influential guidelines. Physicians perceiving conflicting guidelines—both conservative and aggressive—as influential were classified accordingly. This resulted in the following 6 discrete, mutually exclusive groups of physicians valuing different guideline combinations: none, single-conservative, single-aggressive, multiple-conservative, multiple-aggressive, and multiple-conflicting.
Cancer Screening Aggressiveness
To examine how the number, aggressiveness, and agreement of influential guidelines are related to screening decision-making, we derived an index of cancer screening aggressiveness as our main outcome variable. We based this summary measure on responses to hypothetical vignettes, describing asymptomatic patients differing in age and comorbidities, and asking physicians which screening strategies they would recommend in an ideal setting without care barriers (Figure 1, Supplemental Digital Content 2, online only, available at: http://links.lww.com/MLR/A152). Alternative screening strategies were assigned point values (Table 1) on the basis of the absolute number and/or frequency of tests involved, irrespective of their concordance with any particular guideline. We summed vignette responses to create breast and cervical cancer screening aggressiveness scores; higher scores indicate a propensity to recommend screening more frequently, among more types of patients, or using more tests. Focusing on screening aggressiveness allowed us to avoid treating any single guideline as the reference standard, and to model the net effect of valuing different guideline combinations on decision-making.
TABLE 1.
Derivation of Cancer Screening Aggressiveness Scores (2006–2007 National Primary Care Physician Cancer Screening Survey)
| Breast Cancer Screening Aggressiveness Score (Range, 0–36) |
| Based on 9 vignettes describing patients varying in age (50–80) and comorbidities (healthy, congestive heart failure, unresectable non–small-cell lung cancer) |
| 5 test options: |
| “No screening” = 0 points |
| “Other-discuss with patient” = 1 point |
| “Clinical breast exam only” = 2 points |
| “Mammography only” = 3 points |
| “Both clinical breast exam and mammography” = 4 points |
| Cervical Cancer Screening Aggressiveness Score (Range, 0–50) |
| Based on 10 vignettes describing patients varying in age (18–71), sexual history, Pap smear and hysterectomy history, and comorbidities (healthy, unresectable non–small-cell lung cancer) |
| 6 test options: |
| “No Pap” = 0 points |
| “Other-discuss with patient/refer to GYN)” = 1 point |
| “Pap > every 3 yr” = 2 points |
| “Pap every 3 yr” = 3 points |
| “Pap every 2 yr” = 4 points |
| “Pap annually (at least for the first 3 yr)” = 5 points |
Pap indicates Papanicolaou.
Physician and Practice Variables
Physician variables included years since medical school graduation, gender, race, specialty, and US versus international medical school training. Practice variables included rural versus urban, size, employment status, medical school affiliation, patient volume, proportion of uninsured patients, EMR, patient or physician cancer screening reminder systems, cancer screening guideline implementation, screening performance data availability, and pay-for-performance incentives.
Data Analysis
We used descriptive statistics to examine the distribution of physician and practice characteristics and the perceived influence of different CPGs. We further compared screening aggressiveness scores of physicians in the 6 guideline perception groups using general linear models. Aggressiveness scores were transformed into standardized T-scores (M = 50, SD = 10), to facilitate comparisons across cancer types. Model-adjusted means (predicted marginals44), controlling for all physician and practice characteristics, were calculated to further evaluate differences between aggressiveness scores, and to estimate the variance attributable to guideline perceptions. Pairwise contrasts were performed to compare score differences between guideline perception groups; statistical significance was determined using the Wald F test. Physicians who reported not performing Pap screening (n = 97) were excluded from analyses.
Finally, we explored the relationship between physician and practice characteristics and physicians’ perceptions of different CPGs. Separate multivariate logistic regression models, including all physician and practice characteristics, were fitted to identify predictors of guideline perceptions; physicians’ membership (yes vs. no) in each of the 6 mutually exclusive guideline perception groups was treated as a dichotomous outcome.
The statistical program SUDAAN45 was used to adjust for the survey's complex sampling design, utilizing survey weights to account for selection probability and nonresponse.
RESULTS
A total of 1212 physicians responded to the breast or cervical cancer survey. The absolute response rate, calculated according to a standard formula (RR3) approved by the American Association for Public Opinion Research,46 was 67.5%; the cooperation rate—excluding physicians without valid contact information—was 73.4%. There were no significant differences in response rates by specialty. The highest nonresponse rates were seen in the earliest medical school graduates reporting non-Black minority race, and in more recent graduates reporting no or few offices and in female respondents. Table 2, Supplemental Digital Content 3, online only, available at: http://links.lww.com/MLR/A153, summarizes the distribution of physician and practice characteristics.
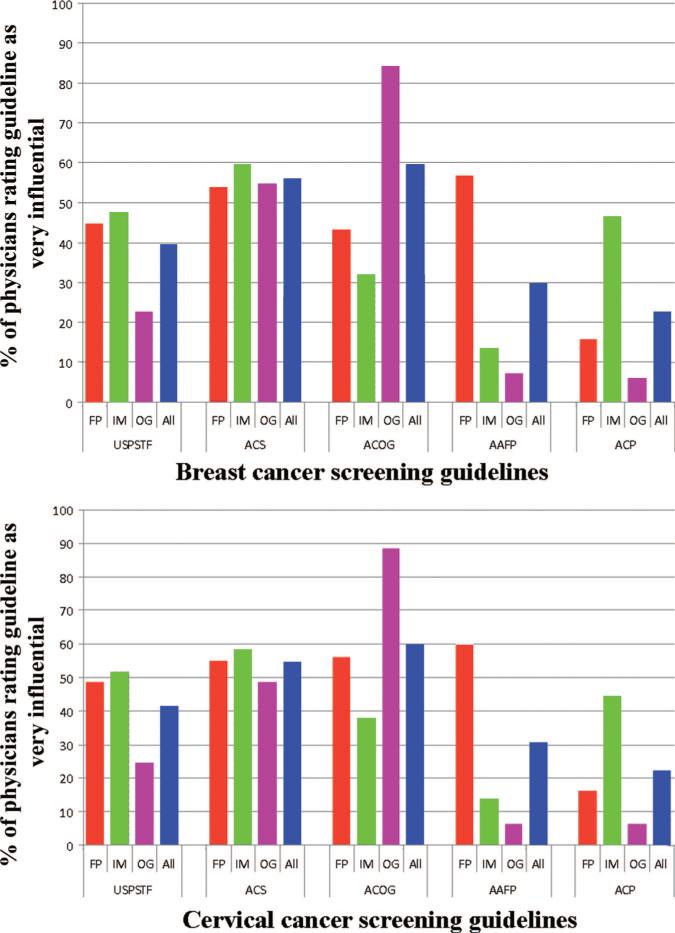
Figure 1 shows the proportion of different physicians who rated individual breast and cervical cancer screening guidelines as very influential. For OB/GYN and FP physicians, the highest proportions of very influential ratings were for CPGs from their own professional societies. OB/GYN physicians showed the greatest variability in ratings for different CPGs, ranging from >80% for ACOG guidelines, to <10% for AAFP and ACP guidelines. Internal medicine physicians demonstrated the least variability, rating guidelines from their own specialty society (ACP) no higher than guidelines from other organizations. Physicians from all specialties rated ACS guidelines as very influential more often than they did USPSTF guidelines.
FIGURE 1.
Proportion of physicians rating individual breast and cervical cancer screening guidelines as very influential, by physician specialty (2006–2007 National Primary Care Physician Cancer Screening Survey). Bars represent the proportion of physicians of a given specialty group rating CPGs as a very influential. Total N = 1212 for breast cancer screening group, 1115 for cervical cancer screening group. Within-specialty differences in perceived influence of different clinical practice guidelines significant at P < 0.0001, for both breast and cervical cancer screening. USPSTF indicates US Preventive Service Task Force; ACP, American College of Physicians; AAFP, American Academy of Family Physicians; ACS, American Cancer Society; ACOG, American College of Obstetrics and Gynecology; FP, family practice/general practice; IM, internal medicine; OG, obstetrics/gynecology; All, all physicians combined.
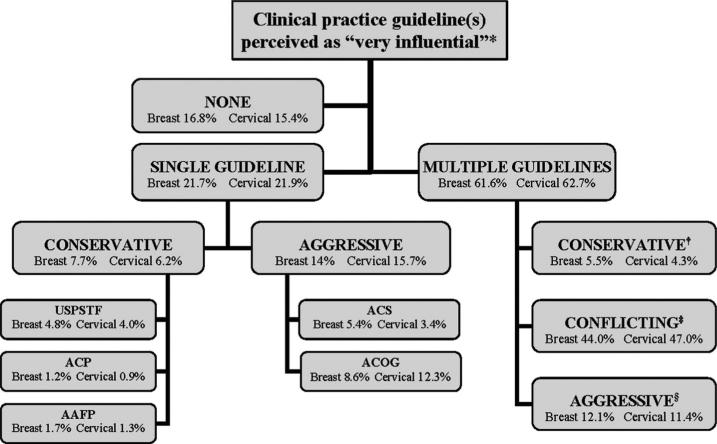
Figure 2 shows the proportion of physicians who perceived various individual or combinations of breast and cervical cancer screening guidelines as very influential. Most physicians (62%) endorsed multiple, rather than no or single guidelines. For physicians perceiving multiple influential guidelines, guideline perceptions were more often conflicting or aggressive than conservative. Overall, physicians more often perceived aggressive rather than conservative guidelines or guideline combinations as influential.
FIGURE 2.
Classification of primary care physicians according to breast and cervical cancer screening guideline(s) perceived as very influential (2006–2007 National Primary Care Physician Cancer Screening Survey). *Each box displays the proportion of physicians who perceived the designated guidelines or guideline combination as very influential. Separate percentages are displayed for physicians in the breast cancer (N = 1212) and cervical cancer (N = 1115) groups. †Multiple-conservative group included any of the following guideline combinations: USPSTF + ACP, USPSTF + AAFP, ACP + AAFP, USPSTF + ACP + AAFP. ‡Multiple-conflicting group included any of the following guideline combinations: USPSTF + ACS, USPSTF + ACOG, ACP + ACS, ACP + ACOG, AAFP + ACS, AAFP + ACOG, ≥3 guidelines (not including USPSTF + ACP + AAFP). §Multiple-aggressive group consisted of ACS + ACOG. USPSTF indicates US Preventive Service Task Force; ACP, American College of Physicians; AAFP, American Academy of Family Physicians; ACS, American Cancer Society; ACOG, American College of Obstetrics and Gynecology.
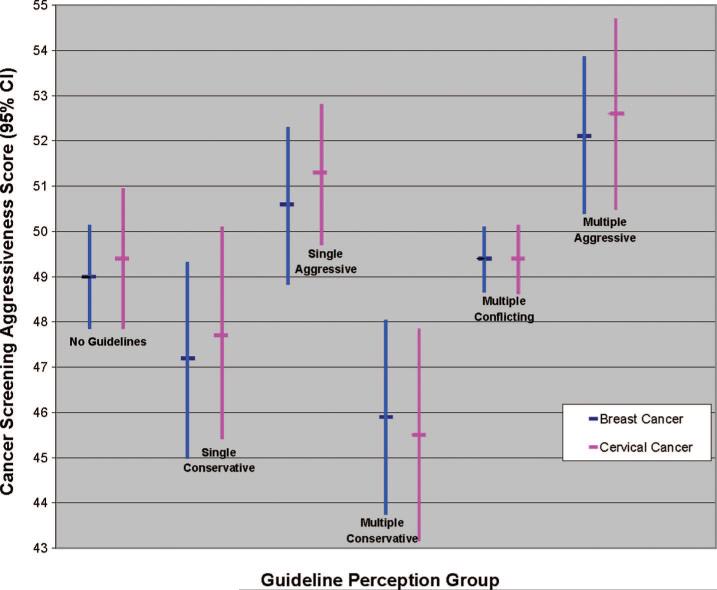
Figure 3 shows the model-adjusted breast and cervical cancer screening aggressiveness scores of physicians in the 6 guideline perception groups (complete adjusted and unadjusted scores are in Table 3, Supplemental Digital Content 4, online only, available at: http://links.lww.com/MLR/A154). Scores were lowest for the single- and multiple-conservative groups, and highest for the single- and multiple-aggressive groups. The no-guideline and multiple-conflicting groups demonstrated intermediate scores. Pairwise contrasts revealed significant differences (P < 0.05) between the 3 multiple-guideline groups, but not between the 3 single-guideline groups. The conservative and aggressive multiple-guideline groups did not differ significantly from the corresponding single-guideline groups, but did differ from the single-guideline groups at the opposite extremes.
FIGURE 3.
Cancer screening aggressiveness scores of primary care physicians in different guideline perception groups (2006–2007 National Primary Care Physician Cancer Screening Survey). Model-adjusted means, representing predicted marginals from general linear model of cancer screening aggressiveness, controlling for physician and practice characteristics. A 10-point difference in aggressiveness score represents 1 standard deviation. Total N = 1212 for breast cancer screening group, 1115 for cervical cancer screening group. Multiple-conservative group included any of the following guideline combinations: USPSTF + ACP, USPSTF + AAFP, ACP + AAFP, USPSTF + ACP + AAFP. Multiple-conflicting group included any of the following guideline combinations: USPSTF + ACS, USPSTF + ACOG, ACP + ACS, ACP + ACOG, AAFP + ACS, AAFP + ACOG, ≥3 guidelines (not including USPSTF + ACP + AAFP). Multiple-aggressive group consisted of ACS + ACOG. CI indicates confidence interval; USPSTF, US Preventive Service Task Force; ACP, American College of Physicians; AAFP, American Academy of Family Physicians; ACS, American Cancer Society; ACOG, American College of Obstetrics and Gynecology.
Tables 2 and 3 summarizes the physician and practice characteristics significantly associated (P < 0.05) in multivariate analyses with the perceived influence of different breast and cervical cancer CPGs; complete findings are in Table 4, Supplemental Digital Content 5, online only, available at: http://links.lww.com/MLR/A155 and Table 5, Supplemental Digital Content 6, online only, available at: http://links.lww.com/MLR/A156. Specialty was the most strongly associated factor overall. For both breast and cervical cancer screening, OB/GYN physicians were most likely to perceive single- and multiple-aggressive guidelines, and least likely to perceive single- and multiple-conservative, multiple-conflicting, or no guidelines as influential. FP physicians were more likely to rate multiple-conflicting cervical screening guidelines as influential, whereas international medical school training was associated with lower likelihood of perceiving no guidelines as influential. Remaining physician variables demonstrated differing cancer-specific associations, and include time since medical school graduation and race. Several practice variables were also associated with guideline perceptions, and include practice ownership, rural setting, patient volume, EMR use, and CPG implementation in practice.
TABLE 2.
Multivariate Associations Between Physician and Practice Characteristics and Guideline(s) Rated as “Very Influential”: Breast Cancer Screening (2006–2007 National Primary Care Physician Cancer Screening Survey)
| Physician and Practice Characteristics | No Guidelines (n = 1104) OR (95% CI) | Single Conservative (n = 1104) OR (95% CI) | Single Aggressive (n = 1104) OR (95% CI) | Multiple Conservative (n = 798) OR (95% CI) | Multiple Conflicting (n = 1104) OR (95% CI) | Multiple Aggressive (n = 1104) OR (95% CI) |
|---|---|---|---|---|---|---|
| Race | ||||||
| White | 1.00* | |||||
| Black | 0.31 (0.11–0.87) | |||||
| Other | 1.47 (0.77–2.80) | |||||
| Years since medical school graduation | ||||||
| <10 | 1.00* | |||||
| 10–19 | 0.55 (0.28–1.10) | |||||
| 20–29 | 0.36 (0.18–0.73) | |||||
| 30 + | 0.60 (0.27–1.32) | |||||
| Medical school training | ||||||
| US | 1.00† | 1.00* | ||||
| International | 0.48 (0.30–0.77) | 1.80 (1.01–3.21) | ||||
| Specialty | ||||||
| IM | 1.00* | 1.00‡ | 1.00‡ | 1.00‡ | 1.00‡ | 1.00‡ |
| FP/GP | 0.95 (0.63–1.43) | 0.74 (0.42–1.28) | 0.67 (0.40–1.13) | 1.61 (0.85–3.04) | 1.23 (0.85–1.78) | 0.71 (0.37–1.39) |
| OB-GYN | 0.61 (0.39–0.94) | 0.05 (0.02–0.16) | 4.40 (2.76–7.03) | — | 0.33 (0.23–0.46) | 8.12 (4.46–14.77) |
| Employment status | ||||||
| Employee | 1.00* | |||||
| Full- or part-time owner | 1.49 (1.00–2.21) | |||||
| Electronic medical record system (EMR) | ||||||
| None | 1.00* | |||||
| Partial or full EMR | 0.65 (0.43–0.98) |
Multivariate logistic regression models adjusted for physician and practice characteristics: age, gender, race, medical school training, specialty, practice setting, type, and size, medical school affiliation, employment status, patient volume, uninsured patients, EMR, patient and physician screening reminder systems, CPG implementation, screening performance data, screening pay-for-performance.
Decreased and unequal N for individual models due to missing data; only respondents with non-missing values for physician and practice characteristics variables are included in the model.
P values for Wald F test of significance:
P < 0.05.
P < 0.005.
Only 1 nonzero cell for the dependent variable, observations excluded from the model.
FP/GP indicates family practice/general practice; IM, internal medicine; OB-GYN, obstetrics-gynecology; CPG, clinical practice guideline.
TABLE 3.
Multivariate Associations Between Physician and Practice Characteristics and Guideline(s) Rated as “Very Influential”: Cervical Cancer Screening (2006–2007 National Primary Care Physician Cancer Screening Survey)
| Physician and Practice Characteristics | No Guidelines (n = 1014) OR (95% CI) | Single Conservative (n = 710) OR (95% CI) | Single Aggressive (n = 1014) OR (95% CI) | Multiple Conservative (n = 710) OR (95% CI) | Multiple Conflicting (n = 1014) OR (95% CI) | Multiple Aggressive (n = 1014) OR (95% CI) |
|---|---|---|---|---|---|---|
| Race | ||||||
| White | 1.00† | 1.00* | 1.00‡ | |||
| Black | 0.51 (0.29–0.90) | 1.05 (0.45–2.45) | 1.04 (0.53–2.06) | |||
| Other | 1.42 (0.71–2.83) | 0.50 (0.29–0.86) | 1.71 (1.23–2.38) | |||
| Medical school training | ||||||
| US | 1.00§ | |||||
| International | 0.38 (0.22–0.68) | |||||
| Specialty | ||||||
| IM | 1.00* | 1.00¶ | 1.00¶ | 1.00¶ | 1.00¶ | 1.00¶ |
| FP/GP | 0.75 (0.47–1.20) | 0.66 (0.36–1.21) | 0.74 (0.42–1.30) | 0.61 (0.31–1.20) | 1.69 (1.17–2.45) | 0.74 (0.35–1.57) |
| OB-GYN | 0.47 (0.29–0.79) | — | 6.33 (3.63–11.06) | — | 0.35 (0.25–0.49) | 5.84 (2.72–12.54) |
| Practice setting | ||||||
| Urban | 1.00* | 1.00§ | ||||
| Rural—large city | 0.80 (0.32–2.04) | 0.80 (0.36–1.77) | ||||
| Rural—small town | 2.50 (1.07–5.84) | 0.11 (0.06–0.20) | ||||
| Patient volume (patients/wk) | ||||||
| ≤100 | 1.00* | 1.00* | ||||
| >100 | 1.36 (1.03–1.80) | 0.57 (0.34–0.95) | ||||
| CPG implementation | ||||||
| Not implemented | 1.00† | |||||
| Implemented, at POC | 0.82 (0.46–1.46) | |||||
| Implemented, N/A at POC | 0.50 (0.31–0.79) |
Multivariate logistic regression models adjusted for physician and practice characteristics: age, gender, race, medical school training, specialty, practice setting, type, and size, medical school affiliation, employment status, patient volume, uninsured patients, EMR, patient and physician screening reminder systems, CPG implementation, screening performance data, screening pay-for-performance.
Decreased and unequal N for individual models due to missing data; only respondents with non-missing values for physician and practice characteristics variables are included in the model.
P values for Wald F test of significance:
P < 0.05.
P < 0.01.
P < 0.005.
P < 0.001.
Only 1 nonzero cell for the dependent variable, observations excluded from the model.
FP/GP indicates family practice/general practice; IM, internal medicine; OB-GYN, obstetrics-gynecology; CPG, clinical practice guideline; N/A, not applicable; POC, point of care.
DISCUSSION
Our study sheds new light on several aspects of PCPs’ responses to the multiplicity of breast and cervical cancer screening guidelines. Perceptions of individual CPGs vary; ACS and ACOG guidelines are perceived as influential by more physicians than other organizations’ guidelines. These data corroborate findings of previous studies16,19–21,47,48; however, our study is the first to show, using a nationally representative sample, that most physicians do not endorse the influence of any single guideline exclusively, but rather multiple CPGs simultaneously. Furthermore, guideline combinations perceived as influential are more often conflicting than concordant, differing in aggressiveness, and the minority of physicians endorsing concordant guidelines favor aggressive ones.
Our study did not ascertain physicians’ awareness, knowledge, or attitudes regarding individual guidelines; therefore, we do not know the extent to which the perceived influence of different guidelines was based on content-related factors such as the aggressiveness of recommended screening, versus other factors including the guidelines’ clarity, the reputation of the CPG developer,10,16 or organizational incentives favoring guideline adoption.24 The preponderance of physicians perceiving conflicting guidelines as influential suggests that judgments are based on factors other than content, however, and our exploratory analyses identified several possibilities. Specialty was the strongest correlate of guideline perceptions. OB/GYNs were less likely than other physicians to perceive conflicting and more likely to perceive aggressive guideline combinations as influential. Further research is needed to determine whether these differences reflect specialty-specific variation in physicians’ allegiance to their own specialty's CPGs, versus a true bias toward aggressiveness reflecting other factors—eg, tolerance of ambiguity,49 exposure to different CPGs, fear of malpractice.
However, even after adjusting for specialty, other factors showed significant associations. Physician race and recency and location of training were associated with differences in the perceived influence of different guideline combinations, and suggest the influence of cultural and historical factors on guideline exposure.16 Associated practice characteristics including setting, ownership, and patient volume suggest the additional influence of structural factors, and further research is needed to confirm and elucidate these findings.
Such work is particularly important given the strong relationship between guideline perceptions and the aggressiveness of physicians’ screening recommendations—a significant finding, since some studies suggest that responses to hypothetical vignettes reflect physicians’ actual clinical decisions.50–52 We cannot project the influence of guideline perceptions on actual screening utilization because our measure of aggressiveness used a limited range of clinical scenarios. However, we can reasonably infer that physicians endorsing different guidelines will demonstrate systematic differences in their overall propensity to recommend screening.
More importantly, our study suggests that guideline multiplicity matters, that the number, aggressiveness, and agreement of influential CPGs may influence decision-making. When multiple concordant guidelines are perceived as influential, physicians’ cancer screening recommendations are correspondingly biased toward extremes of aggressiveness or conservativeness—suggesting an additive influence of redundant expert opinions on decision-making.15,21,22,36 However, when multiple conflicting guidelines are perceived as influential, physicians’ recommendations fall at intermediate levels of aggressiveness—suggesting that when guidelines conflict, physicians individualize screening decisions or use an “averaging heuristic.”14,53
The potential polarizing and neutralizing effects of guideline concordance and conflict, respectively, highlight the need to make the sources of guideline variation explicit and to minimize variation originating from differences in the methods used to judge scientific evidence and to develop guidelines. This is the goal of recent major efforts to develop uniform standards to evaluate the quality of evidence and the strength of guideline recommendations.54–56 Some experts have also advocated centralizing or coordinating CPG development, to further reduce their number and enhance their quality.3,4,8,9
The potential neutralizing influence of guideline conflict also demonstrates how guideline multiplicity ultimately relegates decision-making to individual cases.3,4,8,10 Although this outcome is ethically appropriate when scientific uncertainty exists, it prompts a rethinking of the goals, content, and development of CPGs. The question is whether the goal of individualized decision-making is best achieved by the current pluralistic guideline system, which does so by default—through the neutralizing influence of conflict among multiple guidelines. A more efficient approach may be a centralized process that acknowledges uncertainty, directly advocates individualized decision-making, and avoids the creation of multiple conflicting guidelines.
However, a centralized process may impede development of guidelines specific to unique conditions or populations, and is unlikely to reduce guideline diversity, given that it often reflects disagreement about standards of evidence and the extent to which scientific uncertainty exists in the first place.10 In this respect, guideline diversity may be desirable, because it expresses the range of expert opinions and alternative choices available to individuals. However, it places a burden on physicians to use CPGs in a way that promotes informed and shared decision-making—adequately communicating scientific uncertainty, and respecting patients’ values and choices.
Our study had several limitations. Its cross-sectional nature makes it impossible to determine whether prior decision-making tendencies influenced physicians’ expressed guideline perceptions, or vice versa, and its limitation to a single time point precludes examination of the influence of changes in CPGs. Physicians’ guideline knowledge was not assessed, and guideline-related perceptions and recommendations were ascertained indirectly through physicians’ perceptions of the influence of individual guidelines and responses to hypothetical vignettes. Their artificial nature—eg, stipulating an absence of barriers to care—might have limited their validity. Multiple statistical comparisons were also performed, and some observed associations may have occurred by chance.
Despite these limitations, our study provides valuable, seminal empirical evidence on the potential effects and implications of the multiplicity of CPGs for cancer screening. In cancer screening and other health care domains, the problem of multiple CPGs will continue to demand both greater insight into why physicians vary in their responses to different guidelines in particular and to scientific uncertainty more generally, and a rethinking of the goals, content, development, and use of CPGs. Our study represents a preliminary step in this direction.
Supplementary Material
Acknowledgments
Supported by the Agency for Healthcare Research and Quality (inter-agency agreement numbers Y3-PC-5019-01 and Y3-PC-5019-02); the Centers for Disease Control and Prevention (inter-agency agreement number Y3-PC-6017-01); and by intramural research funds from the National Cancer Institute at the National Institutes of Health (contract number N02-PC-51308).
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Cancer Institute, the Agency for Healthcare Research and Quality, or the Centers for Disease Control and Prevention.
Presented at the American Public Health Association Annual Meeting; November 10, 2009; Philadelphia, PA.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.lww-medicalcare.com).
REFERENCES
- 1.Institute of Medicine . Clinical Practice Guidelines: Directions for a New Program. National Academy Press; Washington, DC: 1990. [Google Scholar]
- 2.Agency for Healthcare Research and Quality [July 12, 2010];National Guidelines Clear-inghouse: Agency for Healthcare Research and Quality. 2008 http://www.guideline.gov/index.aspx.
- 3.Institute of Medicine . Knowing What Works in Health Care: A Roadmap for the Nation. National Academy Press; Washington, DC: 2008. [Google Scholar]
- 4.Institute of Medicine . Guidelines for Clinical Practice: From Development to Use. National Academy Press; Washington, DC: 1992. [Google Scholar]
- 5.Farquhar CM, Kofa EW, Slutsky JR. Clinicians’ attitudes to clinical practice guidelines: a systematic review. Med J Aust. 2002;177:502–506. doi: 10.5694/j.1326-5377.2002.tb04920.x. [DOI] [PubMed] [Google Scholar]
- 6.O'Malley AS, Pham HH, Reschovsky JD. Predictors of the growing influence of clinical practice guidelines. J Gen Intern Med. 2007;22:742–748. doi: 10.1007/s11606-007-0155-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Preventive Services Task Force Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716–726. W–236. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 8.Shaneyfelt TM, Centor RM. Reassessment of clinical practice guidelines: go gently into that good night. JAMA. 2009;301:868–869. doi: 10.1001/jama.2009.225. [DOI] [PubMed] [Google Scholar]
- 9.Shaneyfelt TM, Mayo-Smith MF, Rothwangl J. Are guidelines following guidelines? The methodological quality of clinical practice guidelines in the peer-reviewed medical literature. JAMA. 1999;281:1900–1905. doi: 10.1001/jama.281.20.1900. [DOI] [PubMed] [Google Scholar]
- 10.Sniderman AD, Furberg CD. Why guideline-making requires reform. JAMA. 2009;301:429–431. doi: 10.1001/jama.2009.15. [DOI] [PubMed] [Google Scholar]
- 11.Brouwers MC, Graham ID, Hanna SE, et al. Clinicians’ assessments of practice guidelines in oncology: the CAPGO survey. Int J Technol Assess Health Care. 2004;20:421–426. doi: 10.1017/s0266462304001308. [DOI] [PubMed] [Google Scholar]
- 12.Grol R, Dalhuijsen J, Thomas S, et al. Attributes of clinical guidelines that influence use of guidelines in general practice: observational study. BMJ. 1998;317:858–861. doi: 10.1136/bmj.317.7162.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimshaw J, Eccles M, Thomas R, et al. Toward evidence-based quality improvement. Evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966–1998. J Gen Intern Med. 2006;21(suppl 2):S14–S20. doi: 10.1111/j.1525-1497.2006.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Budescu DV, Rantilla AK. Confidence in aggregation of expert opinions. Acta Psychol. 2000;104:371–398. doi: 10.1016/s0001-6918(00)00037-8. [DOI] [PubMed] [Google Scholar]
- 15.Hayward RS, Guyatt GH, Moore KA, et al. Canadian physicians’ attitudes about and preferences regarding clinical practice guidelines. CMAJ. 1997;156:1715–1723. [PMC free article] [PubMed] [Google Scholar]
- 16.Tunis SR, Hayward RS, Wilson MC, et al. Internists’ attitudes about clinical practice guidelines. Ann Intern Med. 1994;120:956–963. doi: 10.7326/0003-4819-120-11-199406010-00008. [DOI] [PubMed] [Google Scholar]
- 17.Klabunde CN, Lanier D, Nadel MR, et al. Colorectal cancer screening by primary care physicians: recommendations and practices, 2006–2007. Am J Prev Med. 2009;37:8–16. doi: 10.1016/j.amepre.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yabroff KR, Saraiya M, Meissner HI, et al. Specialty differences in primary care physician reports of papanicolaou test screening practices: a national survey, 2006 to 2007. Ann Intern Med. 2009;151:602–611. doi: 10.7326/0003-4819-151-9-200911030-00005. [DOI] [PubMed] [Google Scholar]
- 19.Hamblin J, Connor PD. An overview of the American Cancer Society screening guidelines. J Tenn Med Assoc. 1995;88:10–16. [PubMed] [Google Scholar]
- 20.James PA, Cowan TM, Graham RP, et al. Family physicians’ attitudes about and use of clinical practice guidelines. J Fam Pract. 1997;45:341–347. [PubMed] [Google Scholar]
- 21.Weingarten S, Stone E, Hayward R, et al. The adoption of preventive care practice guidelines by primary care physicians: do actions match intentions? J Gen Intern Med. 1995;10:138–144. doi: 10.1007/BF02599668. [DOI] [PubMed] [Google Scholar]
- 22.Hayward RS, Wilson MC, Tunis SR, et al. Practice guidelines. What are internists looking for? J Gen Intern Med. 1996;11:176–178. doi: 10.1007/BF02600272. [DOI] [PubMed] [Google Scholar]
- 23.Stange KC, Kelly R, Chao J, et al. Physician agreement with US Preventive Services Task Force recommendations. J Fam Pract. 1992;34:409–416. [PubMed] [Google Scholar]
- 24.Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 25.Czaja R, McFall SL, Warnecke RB, et al. Preferences of community physicians for cancer screening guidelines. Ann Intern Med. 1994;120:602–608. doi: 10.7326/0003-4819-120-7-199404010-00012. [DOI] [PubMed] [Google Scholar]
- 26.Ferrier BM, Woodward CA, Cohen M, et al. Clinical practice guidelines. New-to-practice family physicians’ attitudes. Can Fam Physician. 1996;42:463–468. [PMC free article] [PubMed] [Google Scholar]
- 27.Salem-Schatz SR, Gottlieb LK, Karp MA, et al. Attitudes about clinical practice guidelines in a mixed model HMO: the influence of physician and organizational characteristics. HMO Pract. 1997;11:111–117. [PubMed] [Google Scholar]
- 28.Sammer CE, Lykens K, Singh KP. Physician characteristics and the reported effect of evidence-based practice guidelines. Health Serv Res. 2008;43:569–581. doi: 10.1111/j.1475-6773.2007.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zyzanski SJ, Stange KC, Kelly R, et al. Family physicians’ disagreements with the US Preventive Services Task Force recommendations. J Fam Pract. 1994;39:140–147. [PubMed] [Google Scholar]
- 30.Butzlaff M, Kempkens D, Schnee M, et al. German ambulatory care physicians’ perspectives on clinical guidelines—a national survey. BMC Fam Pract. 2006;7:47. doi: 10.1186/1471-2296-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolfe RM, Sharp LK, Wang RM. Family physicians’ opinions and attitudes to three clinical practice guidelines. J Am Board Fam Pract. 2004;17:150–157. doi: 10.3122/jabfm.17.2.150. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Malek N, Chiarelli AM, Sloan M, et al. Influence of physician and patient characteristics on adherence to breast cancer screening recommendations. Eur J Cancer Prev. 2008;17:48–53. doi: 10.1097/CEJ.0b013e32809b4cef. [DOI] [PubMed] [Google Scholar]
- 33.Greving JP, Denig P, de Zeeuw D, et al. Physicians’ attitudes towards treatment guidelines: differences between teaching and nonteaching hospitals. Eur J Clin Pharmacol. 2006;62:129–133. doi: 10.1007/s00228-005-0062-2. [DOI] [PubMed] [Google Scholar]
- 34.Lobach DF, Hammond WE. Computerized decision support based on a clinical practice guideline improves compliance with care standards. Am J Med. 1997;102:89–98. doi: 10.1016/s0002-9343(96)00382-8. [DOI] [PubMed] [Google Scholar]
- 35.Connor M, Norman P, editors. Predicting Health Behavior: Research and Practice With Social Cognition Models. Open University Press; Buckingham, United Kingdom: 2001. [Google Scholar]
- 36.Zitzelsberger L, Grunfeld E, Graham ID. Family physicians’ perspectives on practice guidelines related to cancer control. BMC Fam Pract. 2004;5:25. doi: 10.1186/1471-2296-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berlin L. Breast cancer, mammography, and malpractice litigation: the controversies continue. Am J Roentgenol. 2003;180:1229–1237. doi: 10.2214/ajr.180.5.1801229. [DOI] [PubMed] [Google Scholar]
- 38.McNaughton Collins M, Stafford RS, Barry MJ. Age-specific patterns of prostate-specific antigen testing among primary care physician visits. J Fam Pract. 2000;49:169–172. [PubMed] [Google Scholar]
- 39.Nguyen TT, Gildengorin G, Truong A, et al. Factors influencing physicians’ screening behavior for liver cancer among high-risk patients. J Gen Intern Med. 2007;22:523–526. doi: 10.1007/s11606-007-0128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merenstein D. A piece of my mind. Winners and losers. JAMA. 2004;291:15–16. doi: 10.1001/jama.291.1.15. [DOI] [PubMed] [Google Scholar]
- 41.Camerer C, Weber M. Recent developments in modeling preferences: uncertainty and ambiguity. J Risk Uncertain. 1992;5:325–370. [Google Scholar]
- 42.Han PK, Kobrin SC, Klein WM, et al. Perceived ambiguity about screening mammography recommendations: association with future mammography uptake and perceptions. Cancer Epidemiol Biomarkers Prev. 2007;16:458–466. doi: 10.1158/1055-9965.EPI-06-0533. [DOI] [PubMed] [Google Scholar]
- 43.Klopfer FJ, Madden TM. The middlemost choice on attitude items: ambivalence, neutrality, or uncertainty? Pers Soc Psychol Bull. 1980;6:97–101. [Google Scholar]
- 44.Korn EL, Graubard BI. Analysis of Health Surveys. John Wiley & Sons; New York, NY: 1999. [Google Scholar]
- 45.Institute RT. SUDAAN Language Manual. Release 9.0. Research Triangle Institute; Research Triangle Park, NC: 2004. [Google Scholar]
- 46.Research AAFPO . Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys. 5th ed. American Association for Public Opinion Research; Lenexa, KS: 2008. [Google Scholar]
- 47.Holland-Barkis P, Forjuoh SN, Couchman GR, et al. Primary care physicians’ awareness and adherence to cervical cancer screening guidelines in Texas. Prev Med. 2006;42:140–145. doi: 10.1016/j.ypmed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Tudiver F, Guibert R, Haggerty J, et al. What influences family physicians’ cancer screening decisions when practice guidelines are unclear or conflicting? J Fam Pract. 2002;51:760. [PubMed] [Google Scholar]
- 49.Geller G, Tambor ES, Chase GA, et al. Measuring physicians’ tolerance for ambiguity and its relationship to their reported practices regarding genetic testing. Med Care. 1993;31:989–1001. doi: 10.1097/00005650-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Peabody JW, Luck J, Glassman P, et al. Measuring the quality of physician practice by using clinical vignettes: a prospective validation study. Ann Intern Med. 2004;141:771–780. doi: 10.7326/0003-4819-141-10-200411160-00008. [DOI] [PubMed] [Google Scholar]
- 51.Peabody JW, Luck J, Glassman P, et al. Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. JAMA. 2000;283:1715–1722. doi: 10.1001/jama.283.13.1715. [DOI] [PubMed] [Google Scholar]
- 52.Dresselhaus TR, Peabody JW, Luck J, et al. An evaluation of vignettes for predicting variation in the quality of preventive care. J Gen Intern Med. 2004;19:1013–1018. doi: 10.1007/s11606-004-0003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clemen RT, Winkler RL. Combining probability distributions from experts in risk analysis. Risk Anal. 1999;19:187–203. doi: 10.1111/0272-4332.202015. [DOI] [PubMed] [Google Scholar]
- 54.Shiffman RN, Shekelle P, Overhage JM, et al. Standardized reporting of clinical practice guidelines: a proposal from the Conference on Guideline Standardization. Ann Intern Med. 2003;139:493–498. doi: 10.7326/0003-4819-139-6-200309160-00013. [DOI] [PubMed] [Google Scholar]
- 55.AGREE Collaboration Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12:18–23. doi: 10.1136/qhc.12.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.