Abstract
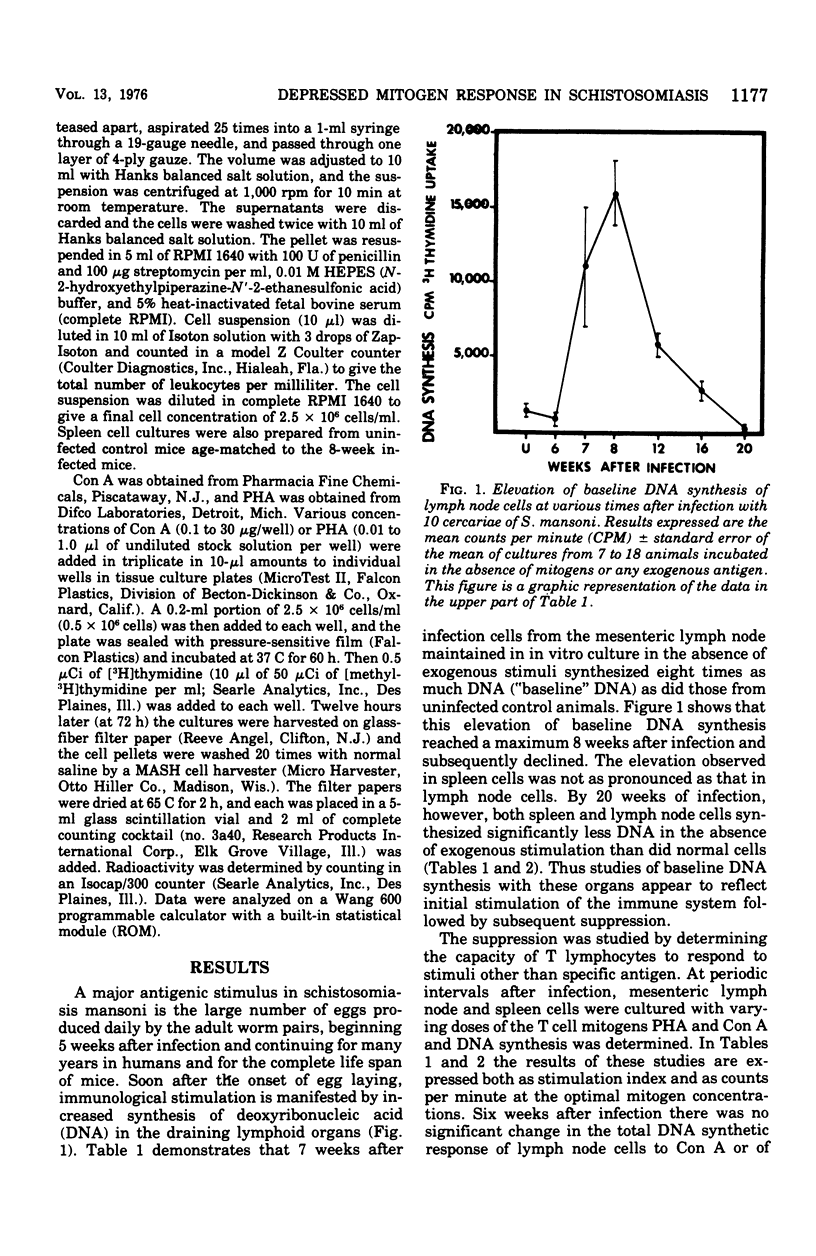
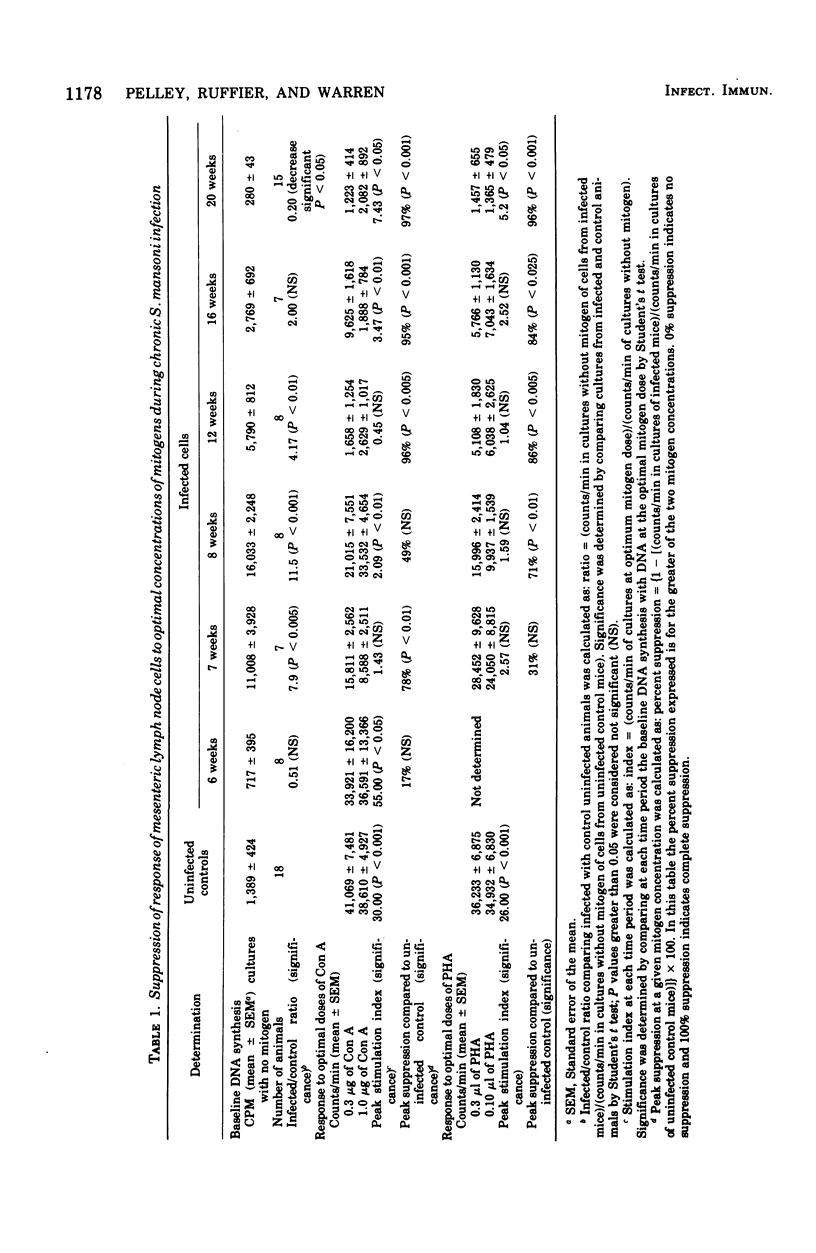
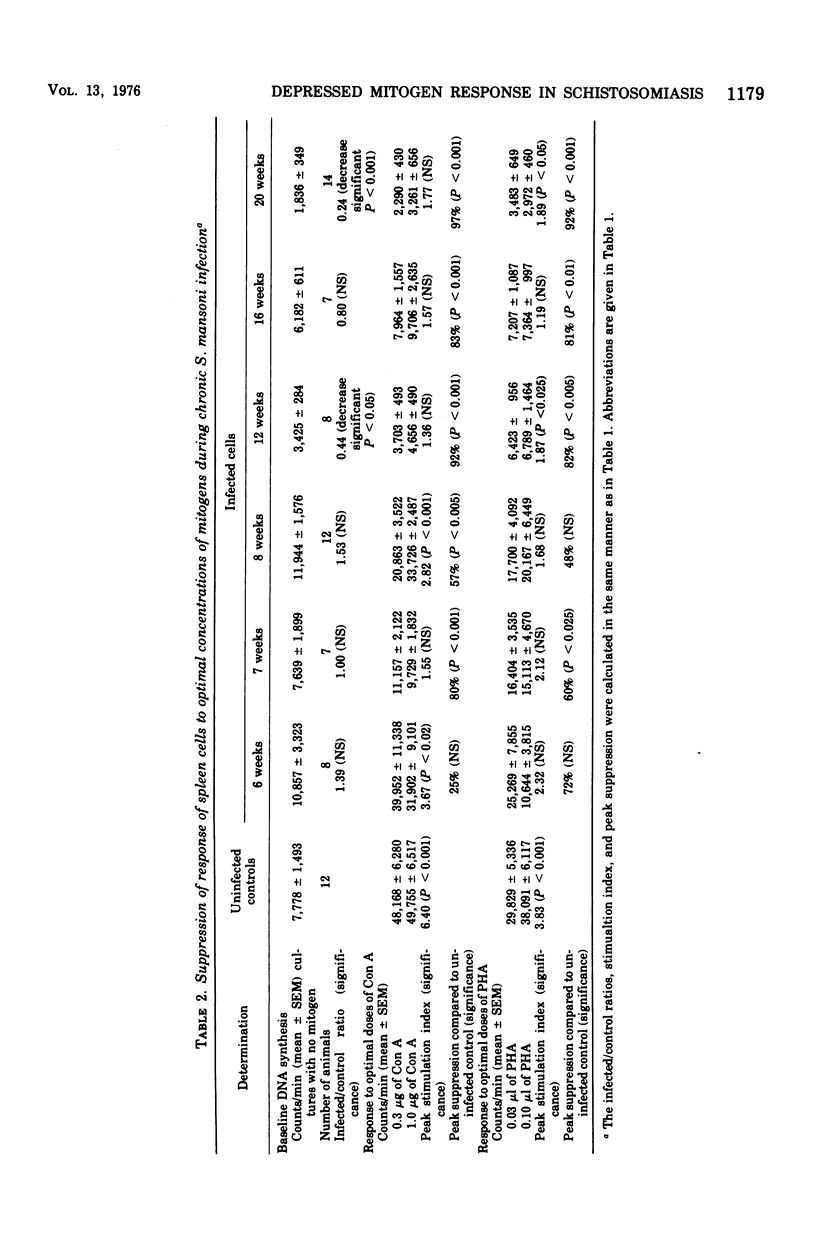
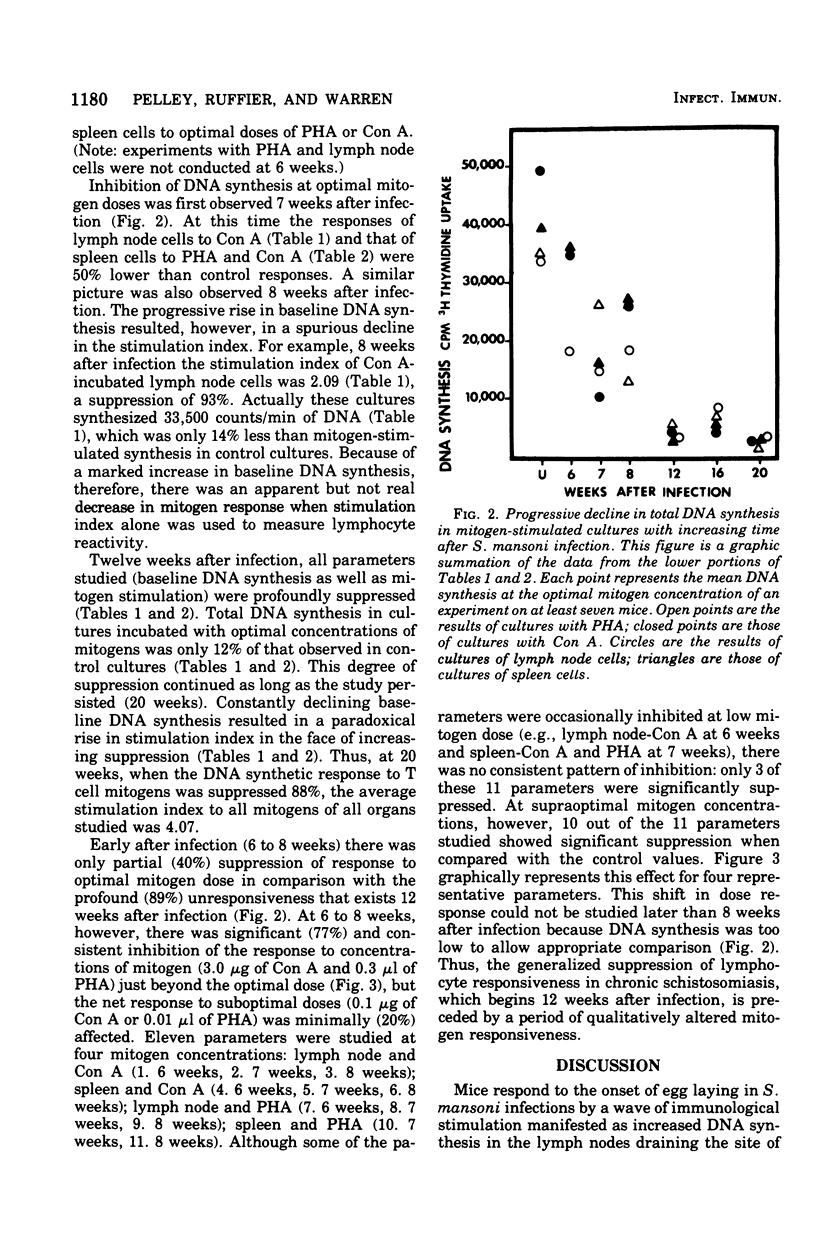
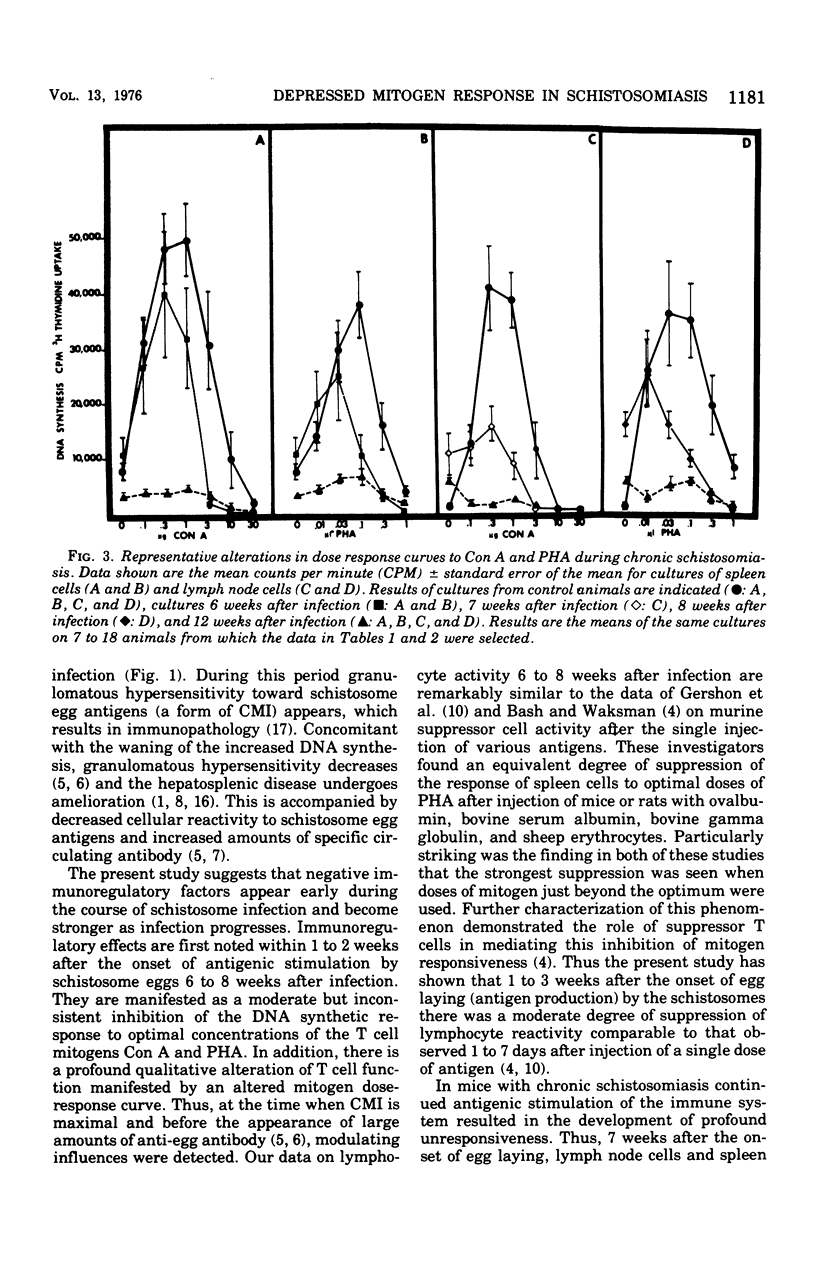
Chronic murine schistosomiasis mansoni is associated with depressed cell-mediated immune responses to Schistosoma mansoni egg antigens. The present study has examined the possibility that factors develop during infection that are capable of altering the response of lymphocytes to stimuli other than specific schistosomal antigens. Egg production begins at 5 weeks, and 1 to 3 weeks later there is a moderate degree of unresponsiveness of lymph node and spleen cells to the mitogens concanavalin A and phytohemagglutinin. This was associated with an altered dose response curve to the mitogens similar to that observed in antigenic systems. Seven weeks after the initiation of antigenic stimulation (egg prodiction lymph node and spleen cells from chronically infected animals were profoundly unresponsive to all concentrations of concanavalin A and phytohemagglutinin tested. These investigations suggest that, in addition to possible blockade by serum antibody, other suppressive factors may be involved in the spontaneous modulation of immunopathology in chronic schistosomiasis. These are detectable 1 to 3 weeks after the onset of egg production and are prominent at 12 weeks. Such findings are consistent with, but do not prove, the existence of suppressor T cells in chronic schistosomiasis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRADE Z. A. WARREN KS: MILD PROLONGED SCHISTOSOMIASIS IN MICE: ALTERATIONS IN HOST RESPONSE WITH TIME AND THE DEVELOPMENT OF PORTAL FIBROSIS. Trans R Soc Trop Med Hyg. 1964 Jan;58:53–57. doi: 10.1016/0035-9203(64)90068-9. [DOI] [PubMed] [Google Scholar]
- Asherson G. L. Antigen-mediated depression of delayed hypersensitivity. Br Med Bull. 1967 Jan;23(1):24–29. doi: 10.1093/oxfordjournals.bmb.a070510. [DOI] [PubMed] [Google Scholar]
- Asherson G. L., Zembala M. Suppression of contact sensitivity by T cells in the mouse. I. Demonstration that suppressor cells act on the effector stage of contact sensitivity; and their induction following in vitro exposure to antigen. Proc R Soc Lond B Biol Sci. 1974 Nov 5;187(1088):329–348. doi: 10.1098/rspb.1974.0078. [DOI] [PubMed] [Google Scholar]
- Bash J. A., Waksman B. H. The suppressive effect of immunization on the proliferative responses of rat T cells in vitro. J Immunol. 1975 Feb;114(2 Pt 2):782–787. [PubMed] [Google Scholar]
- Boros D. L., Pelley R. P., Warren K. S. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J Immunol. 1975 May;114(5):1437–1441. [PubMed] [Google Scholar]
- Colley D. G. Immune responses to a soluble schistosomal egg antigen preparation during chronic primary infection with Schistosoma mansoni. J Immunol. 1975 Jul;115(1):150–156. [PubMed] [Google Scholar]
- Domingo E. O., Warren K. S. Endogenous desensitization: changing host granulomatou response to schistosome eggs at different stages of infection with schistosoma mansoni. Am J Pathol. 1968 Feb;52(2):369–379. [PMC free article] [PubMed] [Google Scholar]
- Faubert G., Tanner C. E. Trichinella spiralis: inhibition of sheep hemagglutinins in mice. Exp Parasitol. 1971 Aug;30(1):120–123. doi: 10.1016/0014-4894(71)90077-4. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Gery I., Waksman B. H. Suppressive effects of in vivo immunization on PHA responses in vitro. J Immunol. 1974 Jan;112(1):215–221. [PubMed] [Google Scholar]
- Hellström K. E., Hellström I. Lymphocyte-mediated cytotoxicity and blocking serum activity to tumor antigens. Adv Immunol. 1974;18:209–277. doi: 10.1016/s0065-2776(08)60311-9. [DOI] [PubMed] [Google Scholar]
- Krettli A. U., Nussenzweig R. Depletion of T and B lymphocytes during malarial infections. Cell Immunol. 1974 Sep;13(3):440–446. doi: 10.1016/0008-8749(74)90263-9. [DOI] [PubMed] [Google Scholar]
- Mansfield J. M., Wallace J. H. Suppression of cell-mediated immunity in experimental African trypanosomiasis. Infect Immun. 1974 Aug;10(2):335–339. doi: 10.1128/iai.10.2.335-339.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx J. L. Suppressor T cells: role in immune regulation. Science. 1975 Apr 18;188(4185):245–247. doi: 10.1126/science.188.4185.245. [DOI] [PubMed] [Google Scholar]
- Schwab J. H. Suppression of the immune response by microorganisms. Bacteriol Rev. 1975 Jun;39(2):121–143. doi: 10.1128/br.39.2.121-143.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren K. S. Hepatosplenic schistosomiasis mansoni: an immunologic disease. Bull N Y Acad Med. 1975 Apr;51(4):545–550. [PMC free article] [PubMed] [Google Scholar]
- Warren K. S. The pathogenesis of "clay-pipe stem cirrhosis" in mice with chronic schistosomiasis mansoni, with a note on the longevity of the schistosomes. Am J Pathol. 1966 Sep;49(3):477–489. [PMC free article] [PubMed] [Google Scholar]


