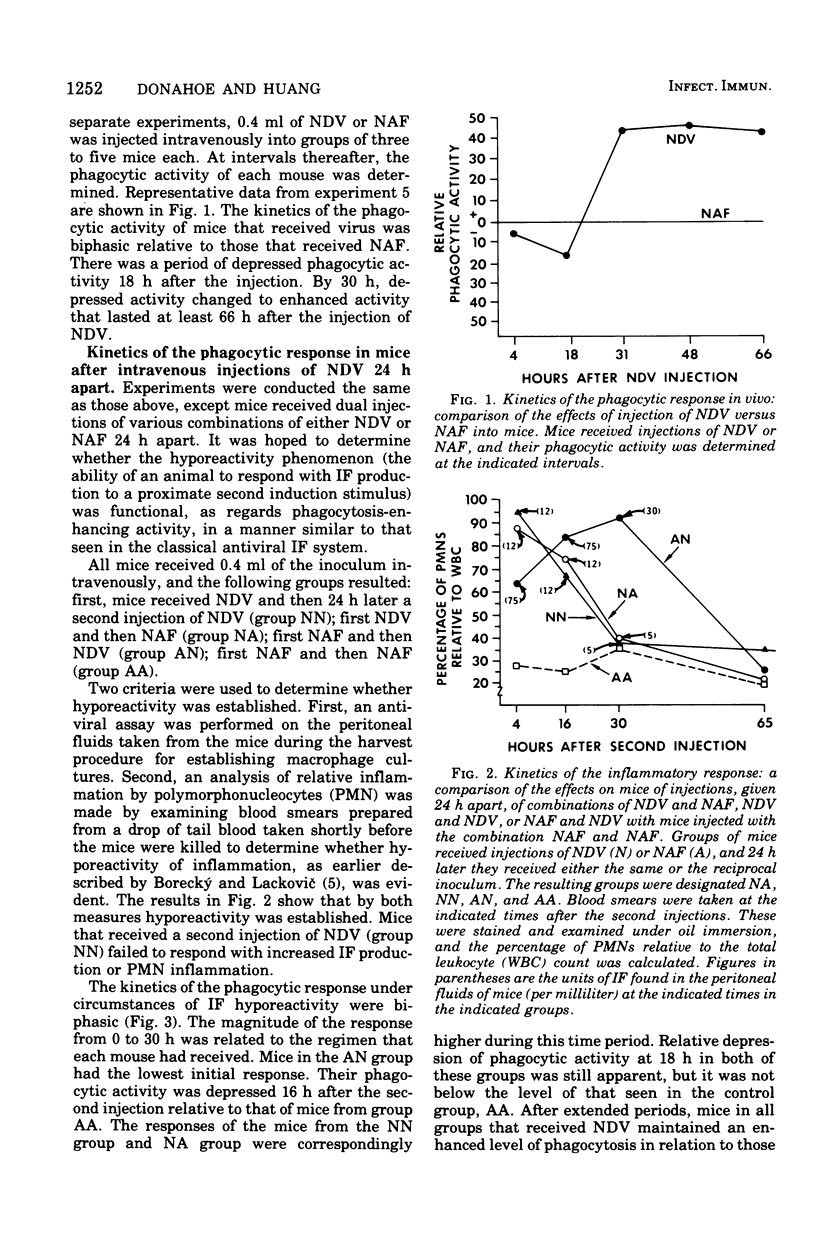
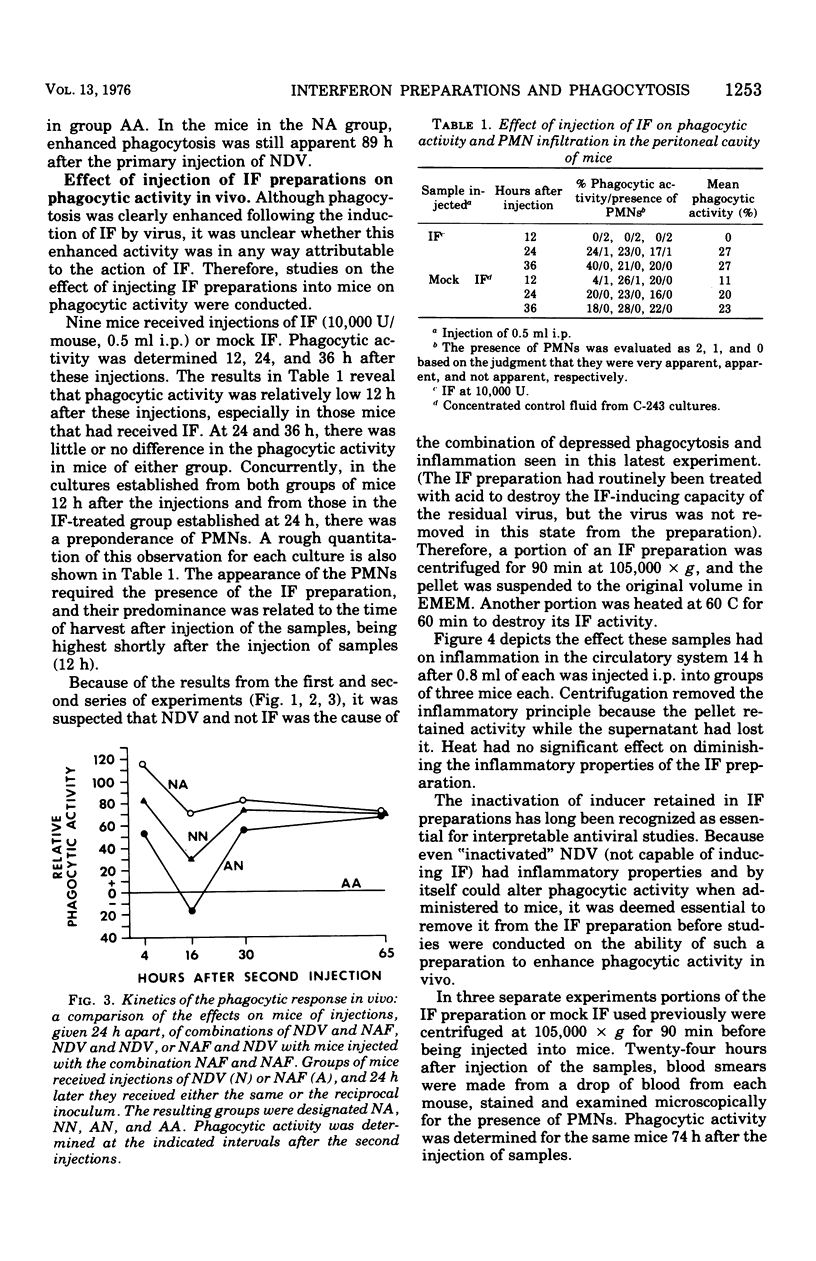
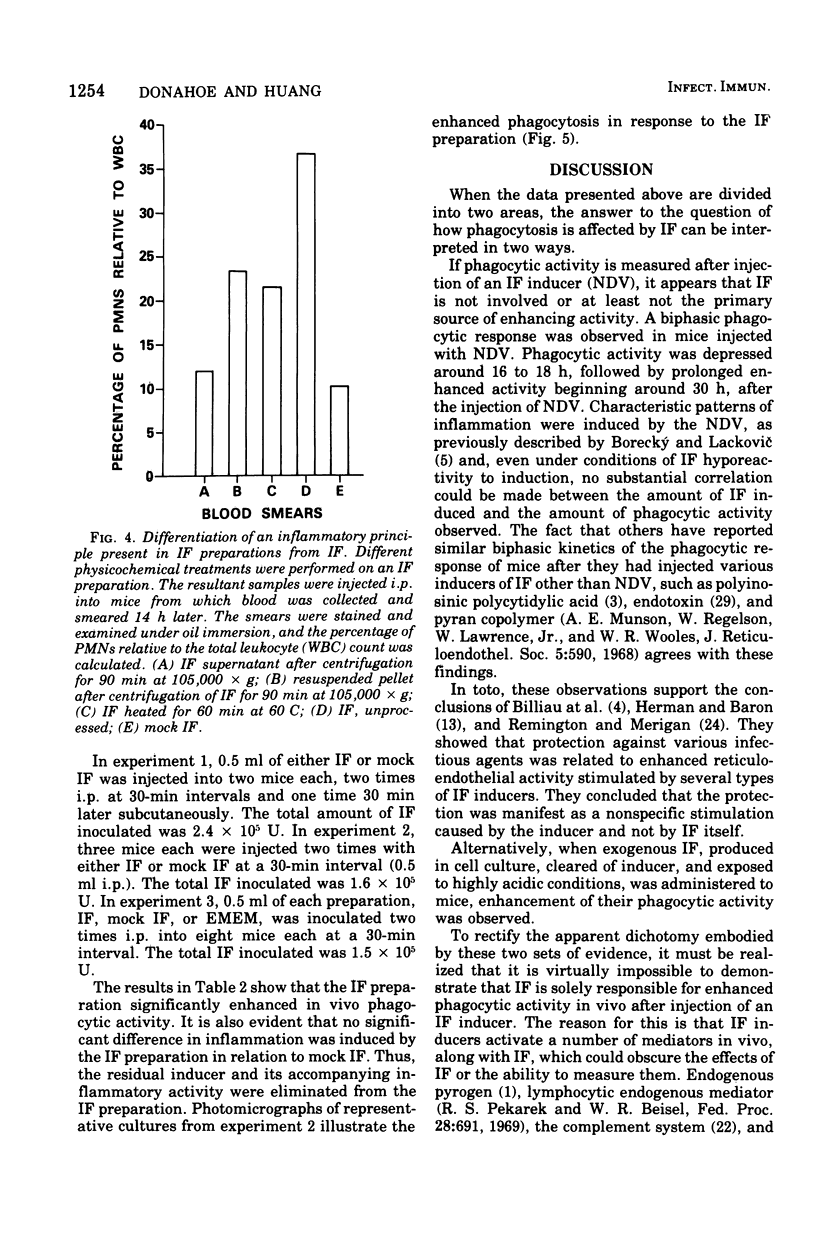
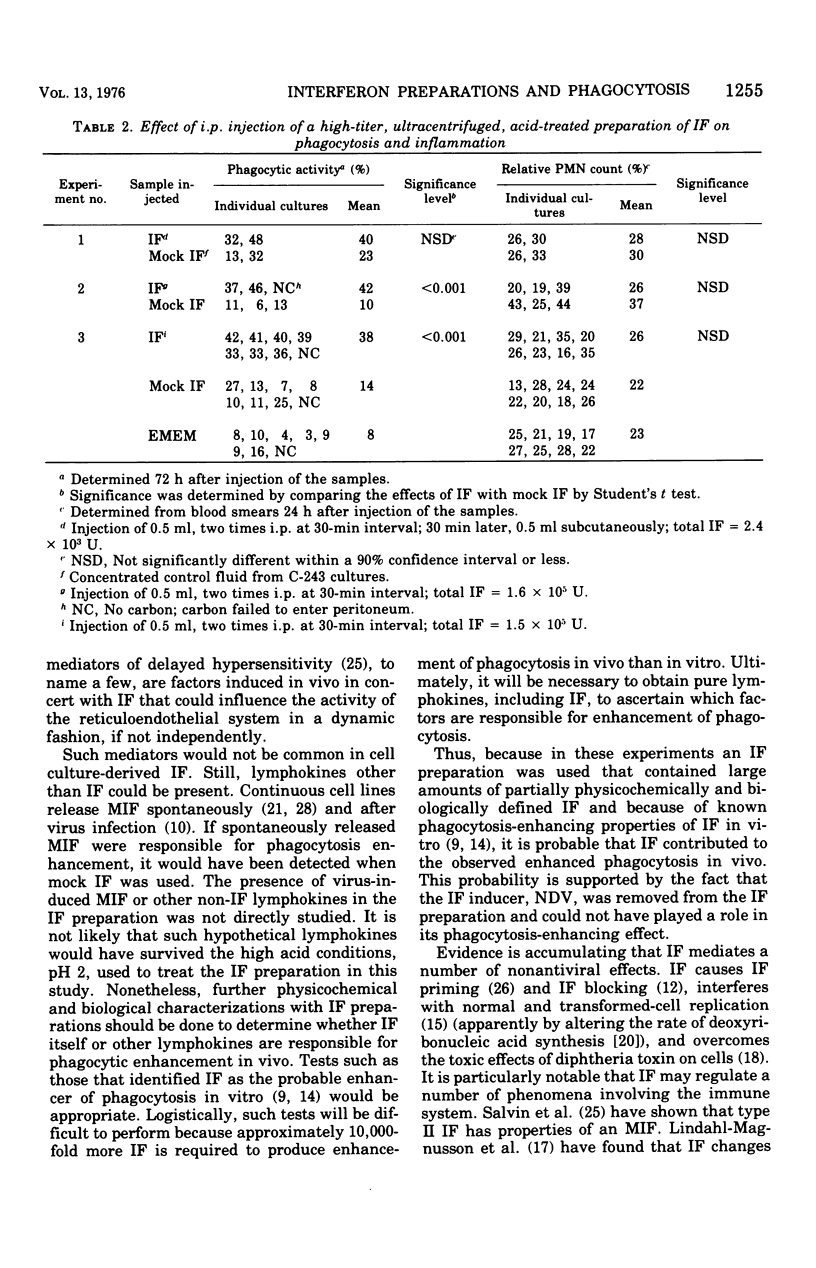
Abstract
Previous studies have shown that interferon (IF) preparations enhance phagocytic activity in cultured mouse peritoneal macrophages. It is shown here that cell culture fluids containing large amounts of IF, which had been treated with acid and clarified of the inducer, Newcastle disease virus, enhanced phagocytic activity when injected into mice. Enhanced phagocytic activity also was observed after injection of Newcastle disease virus into mice, but the contribution of IF to this event was unclear. The kinetics of the phagocytic response to inducers in vivo were biphasic. Depression of phagocytosis occurred around 16 to 18 h after injection of Newcastle disease virus. The observed enhancement began about 12 h later and lasted for at least 60 h more. It was concluded that the complexity of the response of mice to an inducer makes analysis of the role of IF in the ensuing events difficult. However, because of documented phagocytosis-enhancing effects of IF in vitro, it is very likely that the in vivo effects observed here are to some degree mediated by IF. On this basis, the concept of the activity of IF as a lymphokine is potentially expanded.
Full text
PDF



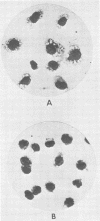
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINS E., HUANG W. C. Studies on the pathogenesis of fever with influenzal viruses. I. The appearance of an endogenous pyrogen in the blood following intravenous injection of virus. J Exp Med. 1958 Mar 1;107(3):383–401. doi: 10.1084/jem.107.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron S. The biological significance of the interferon system. Arch Intern Med. 1970 Jul;126(1):84–93. [PubMed] [Google Scholar]
- Berry L. J., Smythe D. S., Colwell L. S., Schoengold R. J., Actor P. Comparison of the effects of a synthetic polyribonucleotide with the effects of endotoxin on selected host responses. Infect Immun. 1971 Mar;3(3):444–448. doi: 10.1128/iai.3.3.444-448.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiau A., Muyembe J. J., De Somer P. Mechanism of antiviral activity in vivo of polycarboxylases which induce interferon production. Nat New Biol. 1971 Aug 11;232(2):183–186. doi: 10.1038/newbio232183a0. [DOI] [PubMed] [Google Scholar]
- Braun W., Levy H. B. Interferon preparations as modifiers of immune responses. Proc Soc Exp Biol Med. 1972 Dec;141(3):769–773. doi: 10.3181/00379727-141-36868. [DOI] [PubMed] [Google Scholar]
- Brodeur B. R., Merigan T. C. Suppressive effect of interferon on the humoral immune response to sheep red blood cells in mice. J Immunol. 1974 Oct;113(4):1319–1325. [PubMed] [Google Scholar]
- David J. R. Mediators produced by sensitized lymphocytes. Fed Proc. 1971 Nov-Dec;30(6):1730–1735. [PubMed] [Google Scholar]
- Donahoe R. M., Huang K. Y. Neutralization of the phagocytosis-enhancing activity of interferon preparations by anti-interferon serum. Infect Immun. 1973 Mar;7(3):501–503. doi: 10.1128/iai.7.3.501-503.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan T. D., Yoshida T., Cohen S. Production of macrophage migration inhibition factors by virus-infected cell cultures. Infect Immun. 1973 Aug;8(2):145–150. doi: 10.1128/iai.8.2.145-150.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisler R. H., Lindahl P., Gresser I. Effects of interferon on antibody synthesis in vitro. J Immunol. 1974 Aug;113(2):438–444. [PubMed] [Google Scholar]
- Golgher R. R., Paucker K. Blocking of interferon production by chromatographically purified L cell interferon. Proc Soc Exp Biol Med. 1973 Jan;142(1):167–174. doi: 10.3181/00379727-142-36981. [DOI] [PubMed] [Google Scholar]
- Herman R., Baron S. Effects of interferon inducers on the intracellular growth of the protozoan parasite, Leishmania donovani. Nature. 1970 Apr 11;226(5241):168–170. doi: 10.1038/226168a0. [DOI] [PubMed] [Google Scholar]
- Huang K. Y., Donahoe R. M., Gordon F. B., Dressler H. R. Enhancement of phagocytosis by interferon-containing preparations. Infect Immun. 1971 Nov;4(5):581–588. doi: 10.1128/iai.4.5.581-588.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl-Magnusson P., Leary P., Gresser I. Interferon and cell division. VI. Inhibitory effect of interferon on the multiplication of mouse embryo and mouse kidney cells in primary cultures. Proc Soc Exp Biol Med. 1971 Dec;138(3):1044–1050. doi: 10.3181/00379727-138-36047. [DOI] [PubMed] [Google Scholar]
- Lindahl-Magnusson P., Leary P., Gresser I. Interferon inhibits DNA synthesis induced in mouse lymphocyte suspensions by phytohaemagglutinin or by allogeneic cells. Nat New Biol. 1972 May 24;237(73):120–121. doi: 10.1038/newbio237120a0. [DOI] [PubMed] [Google Scholar]
- Lindahl P., Leary P., Gresser I. Enhancement by interferon of the expression of surface antigens on murine leukemia L 1210 cells. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2785–2788. doi: 10.1073/pnas.70.10.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring T. J., Moehring J. M., Stinebring W. R. Response of interferon-treated cells to diphtheria toxin. Infect Immun. 1971 Dec;4(6):747–752. doi: 10.1128/iai.4.6.747-752.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shaughnessy M. V., Lee S. H., Rozee K. R. Interferon inhibition of DNA synthesis and cell division. Can J Microbiol. 1972 Feb;18(2):145–151. doi: 10.1139/m72-024. [DOI] [PubMed] [Google Scholar]
- Oie H. K., Gazdar A. F., Buckler C. E., Baron S. High interferon producing line of transformed murine cells. J Gen Virol. 1972 Oct;17(1):107–109. doi: 10.1099/0022-1317-17-1-107. [DOI] [PubMed] [Google Scholar]
- Papageorgiou P. S., Henley W. L., Glade P. R. Production and characterization of migration inhibitory factor(s) (MIF) of established lymphoid and non-lymphoid cell lines. J Immunol. 1972 Feb;108(2):494–504. [PubMed] [Google Scholar]
- Rathová V., Kocisková D., Borecký L. Activation of the complement system by interferon inducers. Acta Virol. 1972 Nov;16(6):508–508. [PubMed] [Google Scholar]
- Remington J. S., Merigan T. C. Synthetic polyanions protect mice against intracellular bacterial infection. Nature. 1970 Apr 25;226(5243):361–363. doi: 10.1038/226361a0. [DOI] [PubMed] [Google Scholar]
- Salvin S. B., Youngner J. S., Lederer W. H. Migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. Infect Immun. 1973 Jan;7(1):68–75. doi: 10.1128/iai.7.1.68-75.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Gosser L. B., Lockart R. Z., Jr Priming: a nonantiviral function of interferon. J Virol. 1971 Jun;7(6):792–801. doi: 10.1128/jvi.7.6.792-801.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubergen D. G., Feldman J. D., Pollock E. M., Lerner R. A. Production of macrophage migration inhibition factor by continuous cell lines. J Exp Med. 1972 Feb 1;135(2):255–266. doi: 10.1084/jem.135.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIENER E., SHILO M., BECK A. EFFECT OF BACTERIAL LIPOPOLYSACCHARIDES ON MOUSE PERITONEAL LEUKOCYTES. Lab Invest. 1965 May;14:475–487. [PubMed] [Google Scholar]
- Wheelock E. F. Interferon-like virus-inhibitor induced in human leukocytes by phytohemagglutinin. Science. 1965 Jul 16;149(3681):310–311. [PubMed] [Google Scholar]