Abstract
We examined a series of selenorhodamines with amide and thioamide functionality at the 5-position of a 9-(2-thienyl) substituent on the selenorhodamine core for their potential as photosensitizers for photodynamic therapy (PDT) in P-glycoprotein (P-gp) expressing cells. These compounds were examined for their photophysical properties (absorption, fluorescence, and ability to generate singlet oxygen), for their uptake into Colo-26 cells in the absence or presence of verapamil, for their dark and phototoxicity toward Colo-26 cells, for their rates of transport in monolayers of multidrug-resistant, P-gp-overexpressing MDCKII-MDR1 cells, and for their colocalization with mitochondrial specific agents in Colo-26 cells. Thioamide derivatives 16b and 18b were more effective photosensitizers than amide derivatives 15b and 17b. Selenorhodamine thioamides 16b and 18b were useful in a combination therapy to treat Colo-26 cells in vitro: a synergistic therapeutic effect was observed when Colo-26 cells were exposed to PDT and treatment with the cancer drug doxorubicin.
Introduction
The treatment of cancer cells expressing P-glycoprotein (P-gp, also known as MDR1 or ABCB1) or other ABC transporters is often limited by the ability of the chemotherapeutic agent to penetrate the cellular membrane in the presence of the ABC transporter.1 P-gp expression and associated drug resistance can be quite rapid, with mdr gene expression commencing within an hour of treatment.3 Effective clinical intervention with multidrug-resistant (MDR) cancer will require design of mechanism-based inhibitors of P-gp and other multidrug-binding proteins. Currently, there are no approved reversal agents for use in the clinic.4−6
As a class, the rhodamines are transported rapidly by P-gp with tetramethylrosamine [1 (E = O), Chart 1] being transported roughly 5- to 10-fold faster than either rhodamine 123 (2) or rhodamine 6G (3) in isolated P-gp.7−9 In non-drug-resistant cancer, rhodamines have found therapeutic applications as anticancer agents. As delocalized lipophilic cations (DLCs), rhodamines are concentrated in the mitochondria of cancer cells because of increased mitochondrial membrane potential in the transformed cells.10,11 Rhodamine 123 (2) has also been used to treat cancers in vitro12 and in vivo.13 Other DLCs such as the thiopyrylium dye 4 are also cytotoxic to cancer cells in vitro and have antitumor activity in vivo.14
Chart 1. Structures of the Chalcogenorosamines [1 (E = O, S, Se)], Rhodamine 123 (1), Rhodamine 6G (2), Thiopyrylium 4, Rhodamines 5, and Julolidylrosamines 6 (E = S, Se).
Photodynamic therapy (PDT) is a treatment modality for a variety of cancers including cancers of the lung, gastrointestinal tract, the head and neck region, bladder, prostate, and nonmelanoma skin cancer.15 In PDT, irradiation of a cancer-targeted, light absorbing molecule (a photosensitizer) leads to phototoxicity beyond any observed dark toxicity toward the cancer.15 While in principle, the rhodamines and 4-like dye molecules have the potential to be photosensitizers for PDT of cancer,15 irradiation of cells or tumors treated with 2 or 4 gives no increase in toxicity in vitro11,14 or in vivo.13,14 Furthermore, one might ask whether rhodamine derivatives, which are excellent transport substrates for P-gp, would function as effective photosensitizers in cancers showing drug resistance.
Among the attributes of an ideal photosensitizer are (1) strong, high extinction coefficient absorbance in the 600–800 nm window, where tissue penetration of light is at a maximum and where wavelengths of light are still energetic enough to produce 1O2, (2) a high quantum yield for the photochemical event [production of 1O2 or other reactive oxygen species (ROS)], and (3) targeting of the desired tissue or cellular/subcellular site.15 While rhodamines selectively target the mitochondria of transformed cells, they are poor photosensitizers, absorbing wavelengths of light too short for effective penetration of tissue and producing 1O2 and other ROS inefficiently.16,17
Rhodamines brominated on the xanthylium core have increased quantum yields for the generation of 1O2 [Φ(1O2)] due to heavy atom effects from bromine.16 Tetrabromo derivative 5a(18) and dibromo derivative 5b(19) (Chart 1) still target mitochondria and are phototoxic to transformed cells, but wavelengths of absorption are unchanged relative to 2. Dibromorhodamine 5b has been evaluated in several clinical trials.19 Replacing the oxygen atom of the xanthylium core of 1 with the heavier chalcogen atoms S or Se (Chart 1) gives derivatives with longer wavelengths of absorption and increased values of Φ(1O2).17 These derivatives are phototoxic and target the mitochondria of cancer cells, but both the thio- [1 (E = S)] and selenorosamine [1 (E = Se)] have values of λmax < 600 nm,17,20 which will limit their utility in vivo.
When examining the role of rhodamine-derived photosensitizers in the PDT of MDR cells, one must reconcile the rapid transport of the rhodamines by P-gp out of the cell with the mitochondrial specificity of the rhodamines. The transport of 2 was used to define substrates and antagonists for P-gp in the NCI 60 set of cells with the NCI Drug Screen Database of compounds.21,22 The rhodamine binding site (the “R” site) in P-gp was first suggested by Shapiro and Ling to define that rhodamines, in general, are substrates for P-gp.23,24 With the assumption that the rhodamines have a common locus for binding, we examined several discrete libraries of rhodamine/rosamine compounds for their ability to stimulate ATPase activity leading to active transport.25,26 These studies indicated a greater than a 1000-fold variation in ATPase activities with small structural changes within the rhodamines/rosamines.25,26
With respect to rates of rhodamine transport, single atom changes can also give large differences in transport rates in a P-gp-expressing monolayer of cells in both absorptive transport (apical to basolateral transport, PAB) and secretory transport (basolateral to apical transport, PBA). The ratio of secretory to absorptive transport (PBA/PAB) is an excellent indicator of whether a compound is a substrate for P-gp transport. For chalcogenorosamines 1 (E = O, S) and the julolidylrosamine 6a (Chart 1), values of PBA/PAB are large, in the range of 149–450.27 Replacing the sulfur atom with a selenium atom in 6b (Chart 1) gave a PBA/PAB of 15, which is at least an order of magnitude smaller.27 Another single-atom change with tremendous impact on PBA is the “amide/thioamide switch” in which amide derivatives 7a, 9a, 11a, 13a, 15a, and 17a (Chart 2) have values of PBA that are 1.5- to 7-fold greater than the corresponding thioamide derivatives 8a, 10a, 12a, 14a, 16a, and 18a, respectively (Chart 2).28 Among these 12 examples, PBA for amide 17a (PBA = 230 × 10–9 m s–1) was 7-fold greater than PBA for thioamide 18a (PBA = 34 × 10–9 m s–1).28
Chart 2. Structures of Thiorhodamines 7a–18a and Selenorhodamines 15b–18b.
The amide and thioamide derivatives of thiorhodamines 7a–18a shown in Chart 2 were also micromolar inhibitors of P-gp. The tetrahydroquinoline derivatives 15a–18a display the greatest ability to inhibit P-gp in whole cell studies based on values of IC50 for the enhancement of calcein AM (CAM) uptake into MDCKII-MDR1 transfected cells.28
The tetrahydroquinoline derivatives 15a–18a have not been evaluated as photosensitizers for PDT. If these molecules were to generate ROS such as 1O2 efficiently upon irradiation, then they should be effective photosensitizers toward P-gp-expressing cells, since their inhibitory effects toward P-gp transport would allow increased photosensitizer uptake and, presumably, greater efficacy in P-gp expressing cells. Incorporation of a heavy atom into 15a–18a should give increased triplet yields and increased values of Φ(1O2), leading to better photosensitizers.29 This approach has given Se-containing analogues of both chalcogenopyrylium dyes30−32 and rhodamine dyes,18−20,33 which all show increased phototoxicity relative to the S-containing analogues. The heavy-atom analogues of these DLCs still target the mitochondria of cells in culture.30−33
Herein, we describe the synthesis of the Se-containing analogues 15b–18b (Chart 2) of the tetrahydroquinoline derivatives 15a–18a and examine the utility of these compounds as photosensitizers for PDT in a murine colon carcinoma cell line expressing P-gp. The presence of the heavy Se atom imparts more desirable photophysical properties to the 15b–18b relative to 15a–18a including values of λmax > 600 nm and values of Φ(1O2) ≥ 0.44. The thioamide analogues 16b and 18b also are useful in combination therapy involving PDT with the chemotherapeutic doxorubicin (Dox).
Chemistry
Synthesis of Selenorhodamine Derivatives
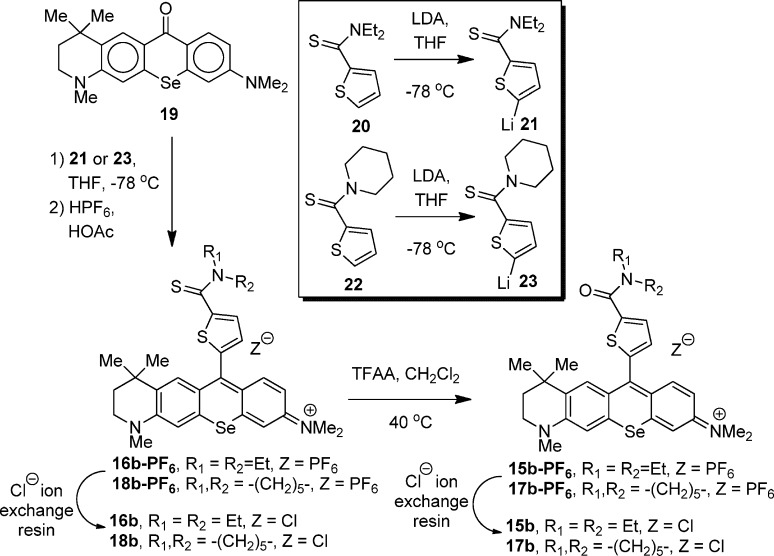
Selenoxanthone 19 (Scheme 1), whose synthesis was recently described,34 is the key intermediate for the preparation of selenorhodamine dyes 15b–18b. Willgerodt–Kindler oxidation of thiophene-2-carboxaldehyde with elemental sulfur and diethylamine gave thioamide 20 in 49% isolated yield (Scheme 1).28 Deprotonation of 20 with sterically bulky lithium diisopropylamide (LDA) gave the 2-thienyl anion 21 (Scheme 1), which was then added to a THF solution of selenoxanthone 19. Workup with aqueous HPF6 gave 16b as the PF6 salt in 81% isolated yield. Ion exchange with a chloride exchange resin converted 16b-PF6 to selenorhodamine 16b as the Cl salt in 95% isolated yield (77% overall). The 1H and 13C NMR spectra of Cl and PF6 salts were superimposable. Unlike the tertiary amide group,35 which is highly directing, the thioamide functionality does not direct lithiation in thiophenes. Only the more acidic α-proton of 20 was removed and none of the corresponding 2,3-disubstituted thiophene was detected in the product mixture.36
Scheme 1. Synthesis of Selenorhodamines 15b–18b.
Similarly, Willgerodt–Kindler oxidation of thiophene-2-carboxaldehyde with elemental sulfur and piperidine gave thioamide 22 in 94% isolated yield.28,37 Deprotonation of 22 with LDA gave 2-lithiothiophene 23 (Scheme 1), which was then added to a THF solution of 19.34 Workup with aqueous HPF6 gave 18b-PF6 in 94% yield. Ion exchange with a chloride exchange resin converted 18b-PF6 to 18b in 94% isolated yield (88% overall). The 1H and 13C NMR spectra of Cl and PF6 salts were superimposable.
The PF6 salts of thioamides 16b and 18b were converted to the PF6 salts of amides 15b and 17b with trifluoroacetic anhydride in CH2Cl2.28 Following workup, the intermediate salts were isolated as 5:1 and 3:1 mixtures, respectively, of the PF6 and CF3CO2 salts based on the results of elemental analysis. The mixtures were subjected to ion exchange with a chloride exchange resin to give 15b and 17b in 55% and 98% overall yields, respectively, as a single salt. The 1H and 13C NMR spectra of Cl and PF6 salts were superimposable.
Absorption Spectra
Absorption maxima (λmax) and molar extinction coefficients (ε) in CH3OH for 15a–18a28 and 15b–18b are compiled in Table 1. Thiorhodamines 15a–18a have values of λmax of 597–598 nm, while 15b–18b have values of λmax of 608–609 nm with values of ε between 7.18 × 104 and 9.78 × 104 M–1 cm–1. Values of λmax for 15b–18b are >600 nm and within the desired therapeutic window for PDT.15 The electronic absorption spectra for 15b–18b are compiled in Figure S1 (Supporting Information).
Table 1. Absorption Maxima (λmax) and Molar Extinction Coefficients (ε) in CH3OH, Fluorescence Emission Maxima (λFL) and Quantum Yields for Fluorescence (ΦFL) in CH3OH, Quantum Yields for the Generation of Singlet Oxygen [Φ(1O2)] in CH3OH, n-Octanol/Water Partition Coefficients (log P) for Thiorhodamines 15a–18a and Selenorhodamines 15b–18ba.
| compd | λmax, nm | ε, M–1 cm–1 | λFL, nm | ΦFL | Φ(1O2) | log P |
|---|---|---|---|---|---|---|
| 15ab | 597 | 6.77 × 104 | 626 | 0.09 ± 0.01 | <0.05 | 1.4 |
| 15b | 609 | 7.18 × 104 | 636 | 0.009 ± 0.001 | 0.50 ± 0.03 | 2.26 ± 0.04 |
| 16ab | 597 | 6.30 × 104 | 626 | 0.07 ± 0.01 | <0.05 | 2.7 |
| 16b | 608 | 9.78 × 104 | 635 | 0.008 ± 0.001 | 0.54 ± 0.03 | 2.41 ± 0.04 |
| 17ab | 598 | 8.31 × 104 | 626 | 0.09 ± 0.01 | <0.05 | 1.7 |
| 17b | 609 | 8.73 × 104 | 634 | 0.009 ± 0.001 | 0.48 ± 0.03 | 2.23 ± 0.04 |
| 18ab | 598 | 6.18 × 104 | 626 | 0.07 ± 0.01 | <0.05 | 2.6 |
| 18b | 608 | 8.11 × 104 | 634 | 0.008 ± 0.001 | 0.44 ± 0.03 | 1.61 ± 0.06 |
Error limits are ±SD.
Values of λmax, ε, and log P for 15a–18a are taken from ref (28).
Fluorescence Yields
Steady-state fluorescence spectra for 15a–18a and 15b–18b were acquired in CH3OH with excitation at 532 nm 17 using 3 in CH3OH as a standard (ΦFL = 0.93).38 The thiorhodamines are fluorescent with ΦFL of 0.07–0.09, while selenorhodamines 15b–18b are weakly fluorescent (ΦFL ≤ 0.009) because of the presence of Se as a heavy atom (Table 1). The fluorescence from 15b–18b is still sufficient to visualize the dyes in cells as described below.
Singlet-Oxygen Yields
Values of Φ(1O2) for 15a–18a and 15b–18b were measured using time-resolved spectroscopy of 1O2 luminescence at 1270 nm in air-saturated CH3OH. Decay traces are compiled in Figure S2 in Supporting Information. Tetramethylselenorosamine hexafluorophosphate [Φ(1O2) = 0.87]17 was used as a standard. For 15b–18b, values of Φ(1O2) fall in the range of 0.44–0.54 (Table 1). For 15a–18a, the signal from 1O2 luminescence could not be separated from background, suggesting values of Φ(1O2) of <0.05 (Table 1).
Photostability
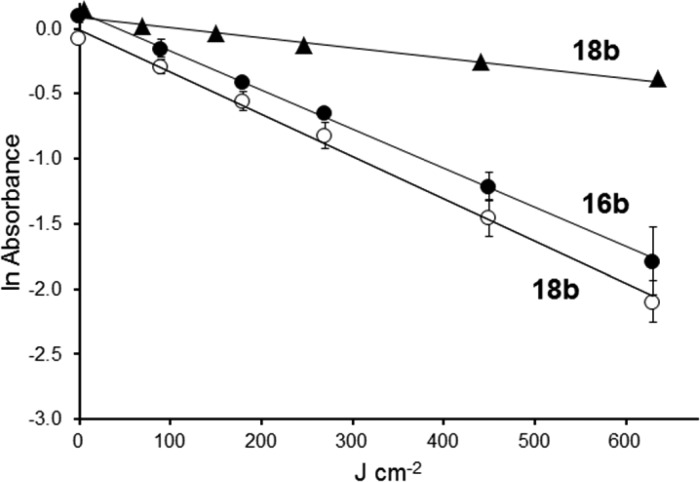
Since the selenorhodamines produce 1O2 efficiently, photobleaching of the dyes under conditions of continuous illumination could limit the utility of 15b–18b as photosensitizers. Under conditions of continuous illumination with 350–800 nm light from a tungsten source delivered at 50 mW cm–2, selenorhodamine thioamides 16b and 18b followed a first-order loss as a function of fluence with half of the dye chromophore lost after ∼230 J cm–2 in solutions of 10% CH3OH in pH 7.4 buffer as shown in Figure 1. If longer wavelengths of light were used, 18b was more stable with half of the dye chromophore lost after ∼850 J cm–2 of continuous illumination with 500–800 nm light in solutions of 10% CH3OH in pH 7.4 buffer.
Figure 1.
Photostability of 16b (filled circles) and 18b (open circles) toward 350–800 nm light delivered at 50 mW cm–2 and photostability of 18b (filled triangles) toward 500–800 nm light delivered at 50 mW cm–2. Error bars are ±SD.
n-Octanol/Water Partition Coefficients
Experimental values of the n-octanol/water partition coefficient (log P) for 15b–18b were measured using the “shake flask” method.39 A saturated n-octanol solution of selenorhodamine was shaken with an equal volume of phosphate buffered saline (PBS) at pH 7.4, and the concentrations in the two layers were determined spectrophotometrically. Values of log P are compiled in Table 1 and covered a range from 1.61 for 18b to 2.41 for 16b. For comparison purposes, values of log P for 15a–18a28 are also compiled in Table 1. On the basis of this range of values of log P, 15b–18b would have access to both aqueous and hydrophobic environments in the studies with whole cells described below.
Biology
Uptake of Rhodamines in the Presence of Verapamil in Colo-26 Cells
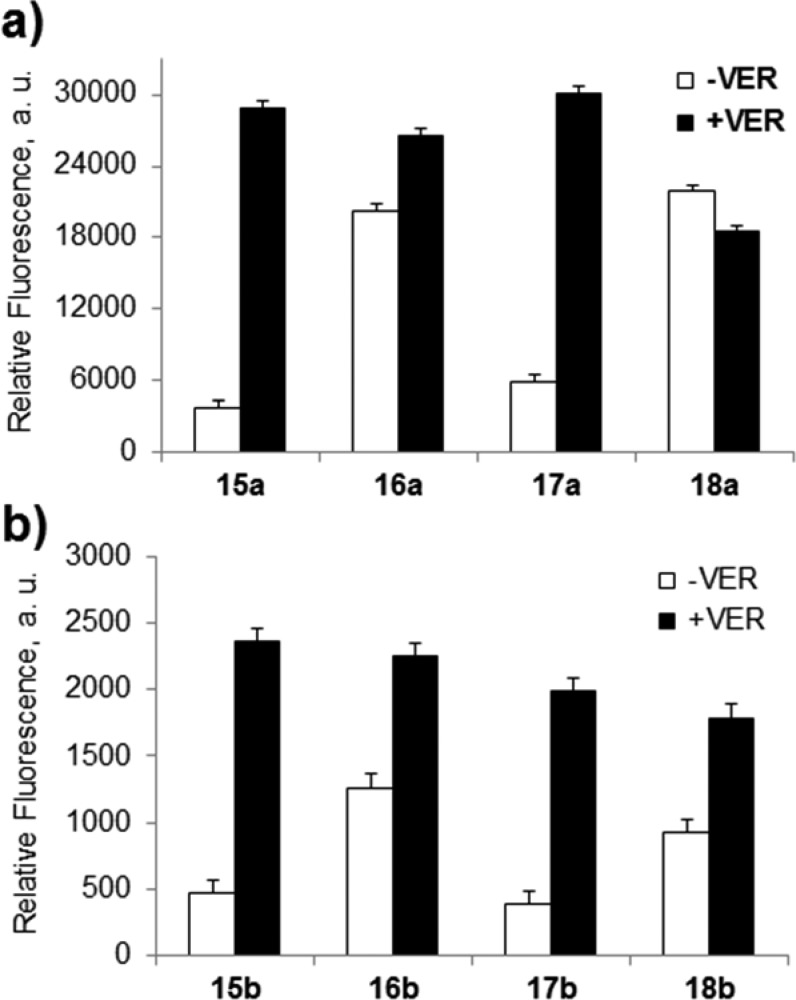
Colo-26 cells (a murine colon carcinoma cell line) express P-gp but are not deemed truly drug resistant.40 Multidrug-resistance modifiers such as verapamil (VER) have shown significant effects in Colo-26 cells with respect to daunorubicin cytotoxicity, accumulation, and efflux.41 We examined the uptake of 15a–18a and 15b–18b (0.5 μM) in Colo-26 cells incubated for 1 h with and without 100 μM VER by flow cytometry (Figures S3 and S4, Supporting Information). Results are shown in Figure 2 for mean fluorescence in the absence and presence of VER.
Figure 2.
Uptake of (a) thiorhodamines 15a–18a and (b) selenorhodamines 15b–18b in Colo-26 cells as measured by relative fluorescence in the absence and presence of 100 μM verapamil (VER). Error bars represent the SD.
In the absence of VER, uptake of thioamides 16a, 16b, 18a, and 18b was significantly greater than the corresponding amide derivatives 15a, 15b, 17a, and 17b (p < 0.02 for all pairwise comparisons with Student t test) as shown in Figure 2. Uptake of 15a increased more than 7-fold, and the uptake of 17a, 15b, and 17b increased 5-fold in the presence of VER. In contrast, the uptake of thioamide derivative 18a was essentially unchanged in the presence of VER while thioamides 16a, 16b, and 18b only showed a 1.3- to 2-fold increase in uptake in the presence of VER. These data are consistent with (1) the presence of P-gp in the Colo-26 cells and (2) increased rates of transport of amide derivatives 15a, 15b, 17a, and 17b from Colo-26 cells relative to the thioamide-containing derivatives 16a, 16b, 18a, and 18b.
Dark and Phototoxicity of 15a–18a and 15b–18b toward Colo-26 Cells
Cell cultures of Colo-26 cells were incubated for 1 h in the dark with various concentrations of 15a–18a (0.1–0.5 μM). None of the dyes displayed any dark toxicity (surviving fraction of >0.95) at these concentrations. Light-treated cells and dark controls were incubated for 48 h, and cell survival was determined using the sulforhodamine B assay.42,43 Thiorhodamines 15a–18a displayed limited phototoxicity in Colo-26 cells incubated with dye concentrations of ≤0.5 μM and up to 1.0 J cm–2 of 350–700 nm light from a tungsten–halogen source (3.7–4.1 mW cm–2) (Figure S5, Supporting Information). Compounds 15a–18a were not investigated further as photosensitizers.
Phototoxicity of 15b–18b toward Colo-26 Cells with a Tunable Dye Laser Light Source
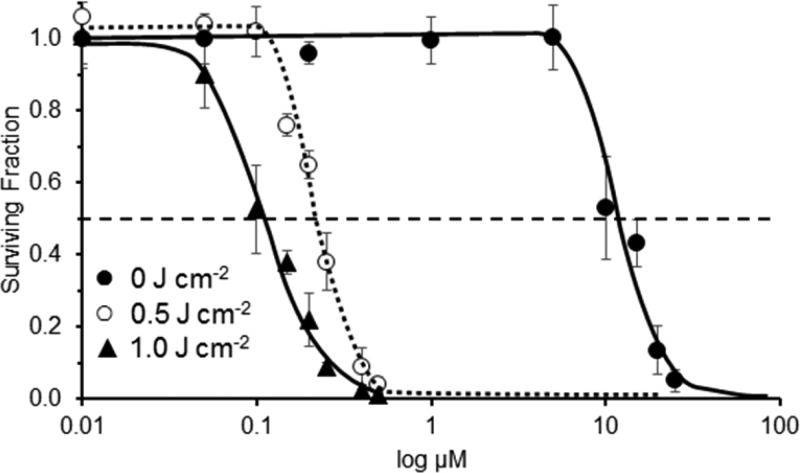
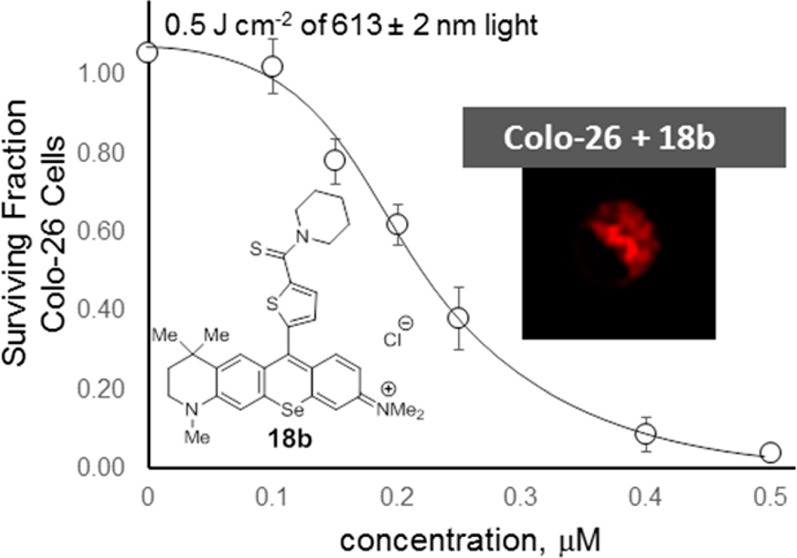
The phototoxicity of 15b–18b toward Colo-26 cells was examined using a tunable dye laser delivering light at the absorption maximum (λmax ± 2 nm). This approach allowed specific conditions to be tailored for each dye. In a solution of 17% fetal bovine serum (FBS) in PBS, values of λmax for 15b–18b were red-shifted 2–3 nm relative to values in CH3OH. Colo-26 cells in 96-well plates were treated with varying concentrations of 15b–18b (0.01–0.5 μM) and light (0.5 and 1.0 J cm–2), which was delivered at λmax (±2 nm) at a fluence rate of 3.2 mW cm–2. The light-treated cells were then incubated with fresh medium for 48 h, and cell survival was determined for various concentrations of 15b–18b and either a 0.5 or a 1.0 J cm–2 light dose, or for various light doses at 0.15 μM 15b–18b. Dose–response curves for 18b are summarized in Figure 3. Values of EC50 for 0.5 and 1.0 J cm–2 of light at λmax are compiled in Table 2 (dose–response curves for 15b–17b, Figure S6 in Supporting Information).
Figure 3.
Dark toxicity (filled circles) of 18b toward Colo-6 cells and phototoxicity of 18b toward Colo-6 cells with irradiation from a tunable dye laser. Irradiation at 613 ± 2 nm was delivered at 3.2 mW cm–2 for varying concentrations of 18b and 0.5 J cm–2 of light (open cicles) and 1.0 J cm–2 of light (filled triangles). Values of LD50 and EC50 were determined by sigmoidal dose–response (variable slope) analysis. Error bars are ±SD.
Table 2. EC50 Values for Selenorhodamines 15b–18b with Colo-26 cCells and 0.5 or 1.0 J cm–2 of Light (λmax ± 2 nm), Dark Toxicities (LD50), and Ratios of LD50/EC50 with 1.0 J cm–2 of Light.
| EC50,a ×10–7 M (λmax ± 2 nm)c |
||||
|---|---|---|---|---|
| compd | 0.5 J cm–2 | 1.0 J cm–2 | LD50,b ×10–6 M (dark) | LD50/EC50 (laser, 1.0 J cm–2) |
| 15b | 3.1 ± 0.1 (613 nm) | 3.0 ± 0.1 (613 nm) | 7.8 ± 0.4 | 26 ± 2 |
| 16b | 2.0 ± 0.1 (611 nm) | 1.7 ± 0.1 (611 nm) | 9.5 ± 0.1 | 56 ± 4 |
| 17b | >5 (613 nm) | 4.1 ± 0.1 (613 nm) | 9.0 ± 0.1 | 22 ± 1 |
| 18b | 2.2 ± 0.1 (611 nm) | 1.4 ± 0.2 (611 nm) | 8.5 ± 0.2 | 61 ± 10 |
Mean of six determinations. Error limits are ±SD.
Mean of four determinations. Error limits are ±SD.
Values in parentheses are wavelengths of irradiation ± 2 nm.
With 0.15 μM photosensitizer concentration and variable light dose as shown in Figure S6 (Supporting Information), thioamides 16b and 18b were comparably phototoxic with EC50 values of ∼1.0 J cm–2 of laser light. Amide 17b showed little if any phototoxicity with 5.0 J cm–2 of laser light while 0.15 μM 15b displayed some phototoxicity, but EC50 required >5.0 J cm–2 of light.
Dark Toxicity of 15b-18b toward Colo-26 Cells
The dark toxicity of 15b–18b toward Colo-26 cells was examined at dye concentrations of 0.01–20 μM. Colo-26 cell cultures were incubated for 1 h in the dark with 15b–18b. The medium was removed, and fresh medium was added. Cells were incubated for 48 h prior to determination of cell viability. Results are shown in Figure 3 for 18b and in Figure S7 (Supporting Information) for 15b–17b with sigmoidal dose–response (variable slope) analysis to allow values of LD50 (the concentration to give a surviving fraction of 0.50) with respect to dark toxicity to be determined for each dye. Values of LD50 are compiled in Table 2 as the mean of four replicates. The rank ordering of dark toxicity is 15b > 18b > 17b > 16b within the range of 7.8–9.5 μM. All pairwise comparisons were significantly different from one another (p < 0.05).
The ratio of dark toxicity to phototoxicity (as an approximation of the therapeutic ratio for the photosensitizers) could be a better measure of photosensitizer effectiveness. Values of LD50/EC50 with 1.0 J cm–2 of laser light as a measure of therapeutic ratio are compiled in Table 2. Among 15b–18b, amides 15b and 17b have lower LD50/EC50 ratios of 22 and 26, respectively, relative to thioamides 16b and 18b with LD50/EC50 ratios of 56 and 61, respectively.
P-gp Transport Studies of 11-Se–12-Se in Monolayers of MDCKII-MDR1 Cells
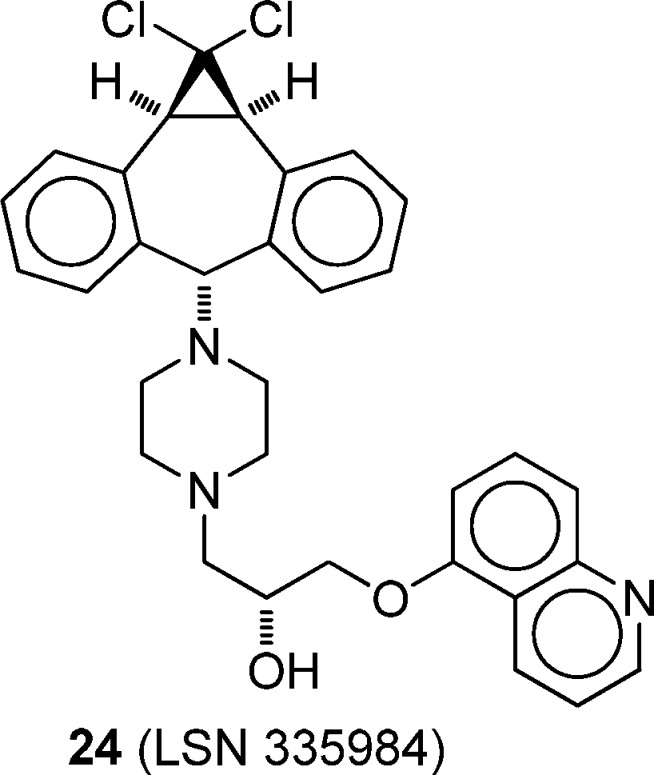
The interactions of the amide/thioamide pair 17b/18b with P-gp were examined in monolayers of MDCKII-MDR1 cells, which overexpress P-gp.44 Transport in this model approximates near-physiological conditions for studying P-gp–photosensitizer interactions.44 The monolayers display apical and basolateral polarized membranes with P-gp solely present at the apical membrane. For 17b and 18b, transport was measured in the absorptive (PAB) and secretory (PBA) direction of the cell monolayer. Bovine serum albumin (BSA) addition to the buffer was required because a marked fraction of mass added to the donor equilibrated with the cell monolayer for 17b and 18b, resulting in gross underestimation of the permeability coefficient.44 The assay was repeated with 5 μM 24 (LSN 335984, IC50 = 0.4 μM, Chart 3),46 which completely inhibits P-gp. Compound 24 is related to the P-gp-specific inhibitor (R)-4-[(1a,6,10b)-1,1-difluoro-1,1a,6,10b-tetrahydrodibenzo[a,e]cyclopropa[c]cyclohepten-6-yl][(5-quinolinyloxy)methyl]-1-piperazineethanol (LSN 335979, Chart 3).4,46 Values of PAB and PBA in the absence of inhibitor, passive transport (PPassive) in the fully inhibited system, and the % cell-associated dye in the AB direction in the absence or presence of inhibitor are compiled in Table 3. For comparison purposes, the same values are included in Table 3 for 17a and 18a.28
Chart 3. Structure of P-gp Inhibitor Used in Transport Studies.
Table 3. Transport and Cell Association Studies of Amide/Thioamide Pair 17b and 18b with MDCK-MDR1 Cellsa and for Thiorhodamine Analogues 17a and 18a.
| compd | PAB, ×10–9 m s–1 | PBA, ×10–9 m s–1 | PBA/PAB | PPassive,b ×10–9 m s–1 | % cell associatedc | ratio (±inh)d |
|---|---|---|---|---|---|---|
| 17ae (+inh) | ≤1 | 230 ± 24 | 230 | 8.6 ± 0.1 | 5.2 | |
| ≤1 | 7.5 ± 0.1 | ∼4 | 45 ± 1 | |||
| 17b (+inh) | 0.9 ± 0.3 | 164 ± 4 | 182 | 16 ± 1 | 2.4 | |
| 1.0 ± 0.1 | 11.1 ± 0.2 | ∼6 | 39 ± 1 | |||
| 18ae (+inh) | ≤1 | 34 ± 22 | 34 | 34 ± 3 | 1.8 | |
| ≤1 | 0.2 ± 0.1 | <1 | 62 ± 1 | |||
| 18b (+inh) | 1.9 ± 0.5 | 29 ± 3 | 15 | 45 ± 1 | 1.2 | |
| 1.7 ± 0.2 | 1.8 ± 0.1 | ∼2 | 55 ± 1 |
Experiments were run with 5 μM dye and 4.3 mg mL–1 BSA. Values of transport in the absorptive (PAB) and secretory (PBA) mode in the absence or presence of inhibitor, the ratio PBA/PAB, the % cell associated rhodamine analogue in the absence or presence of inhibitor, and the ratio of cell associated rhodamine in the presence or absence of inhibitor are reported. Details for methods are provided in Experimental Section. Error limits are ± SD.
PPassive represents the mean of PAB and PBA in the fully inhibited system.
% cell associated is the fraction of mass extracted from the cell monolayer by methanol wash after a 1 h flux in the AB direction.
For % cell associated dye.
Values from ref (28).
Selenorhodamines 17b and 18b, as well as 17a and 18a, are P-gp substrates with PBA/PAB ratios of >3.2 This ratio is much larger for amide derivatives 17a and 17b (PBA/PAB of 228 and 182, respectively) relative to thioamide derivatives 18a and 18b (PBA/PAB of 38 and 15, respectively). For all seven compounds, PPassive is very slow: ≤6 × 10–9 m s–1.
The % cell-associated dye was determined by a methanol wash of the cells in the monolayer following 1 h efflux in the AB direction. The trends observed in the % cell-associated dye indicate that the amide derivatives 17a and 17b show a much higher % cell-associated dye in the inhibited system relative to the uninhibited system (ratio of % cell ± inhibitor of 5.2 and 2.4, respectively, Table 3) relative to thioamide derivatives 18a and 18b (ratio of % cell ± inhibitor of 1.8 and 1.2, Table 3). These are the same trends as observed in the cellular uptake by flow cytometry of Colo-26 cells in the absence or presence of VER for 17a/18a and 17b/18b.28
Combination PDT and Chemotherapy
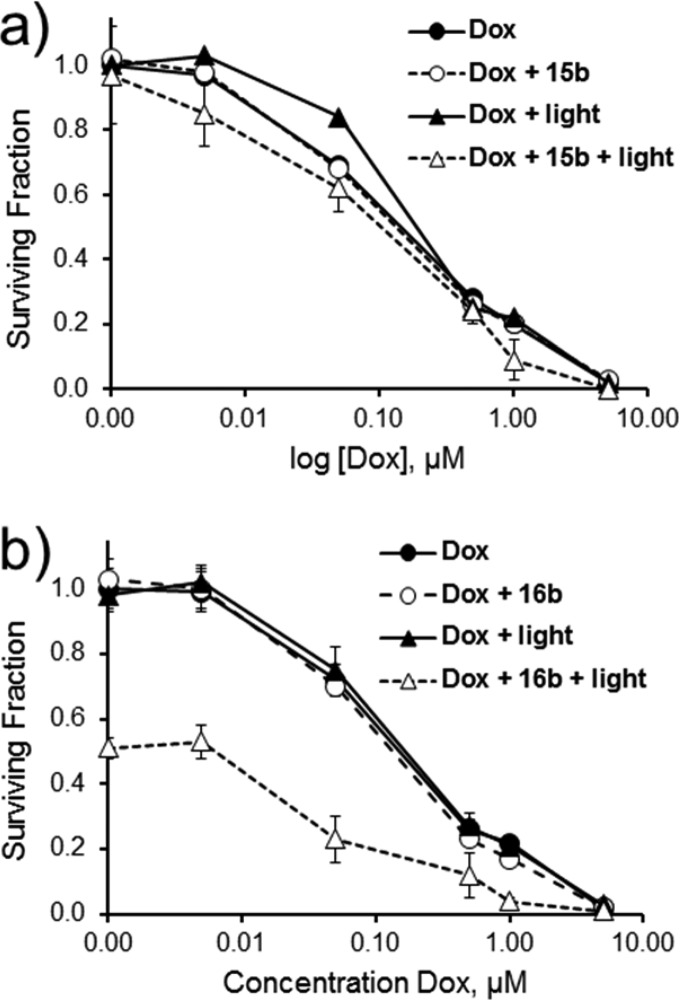
The ability of the selenorhodamines 15b–18b to interact with P-gp in the Colo-26 cells should make it possible to do combination therapy involving PDT and a chemotherapeutic agent. Colo-26 cells were treated with combinations of Dox and 15b–18b with and without light as shown in Figure 4 for 15b and 16b and in Figure S8 (Supporting Information) for 17b and 18b. In the dark, no synergy was observed between 0.15 μM 15b–18b and various concentrations of Dox (0.005, 0.05, 0.5, 1.0, and 5.0 μM) and the surviving fraction was determined by the Dox concentration, with or without 0.15 μM photosensitizer (p > 0.05 for all pairwise comparisons). Irradiation of Colo-26 cells treated with 0.15 μM 15b or 17b with 1.0 J cm–2 of 613 nm laser light gave no significant phototoxicity (p > 0.05) relative to dark controls. No synergy was observed upon irradiation of Colo-26 cells with various concentrations of Dox in the presence of 0.15 μM 15b or 17b. All curves were essentially superimposable on Dox-only curves in the dark (Figures 4a and S8c).
Figure 4.
Combination treatment of Colo-26 cells with various concentrations of Dox alone or in combination with (a) 15b (0.15 μM) and (b) 16b (0.15 μM) in the dark or with 1.0 J cm–2 of 613 nm light (for 15b) or 611 nm light (for 16b). Values are the mean of six replicates. Error bars are ±SD.
In contrast, cells treated with 0.15 μM 16b or 18b and 1.0 J cm–2 of 611 nm light displayed some phototoxicity upon irradiation with 1.0 J cm–2 of 611 nm light in the absence of Dox (Figures 4b and S8d, respectively). Colo-26 cells treated with 0.15 μM photosensitizer, 1.0 J cm–2 of 611 nm light, and 0.5 μM Dox showed a statistically significant decrease in pairwise comparisons to Dox-only treatment with and without light, photosensitizer-only treatment with and without light, and photosensitizer and Dox treatment in the dark (p ≤ 0.0079 for 18b in pairwise comparisons and p ≤ 0.0064 for 16b in pairwise comparisons). Statistically significant differences in surviving fraction were also noted with 1.0 μM Dox and 16b or 18b, but the surviving fraction was ≤0.20 for these combinations, which was very similar to the Dox-only treatment (minimizing the impact of PDT).
Localization of 15b–18b in the Mitochondria of Colo-26 Cells
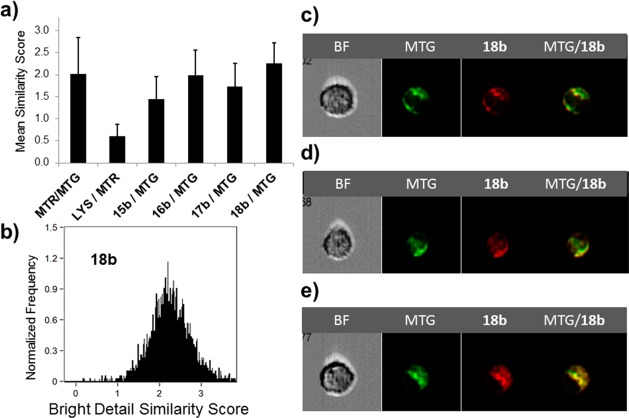
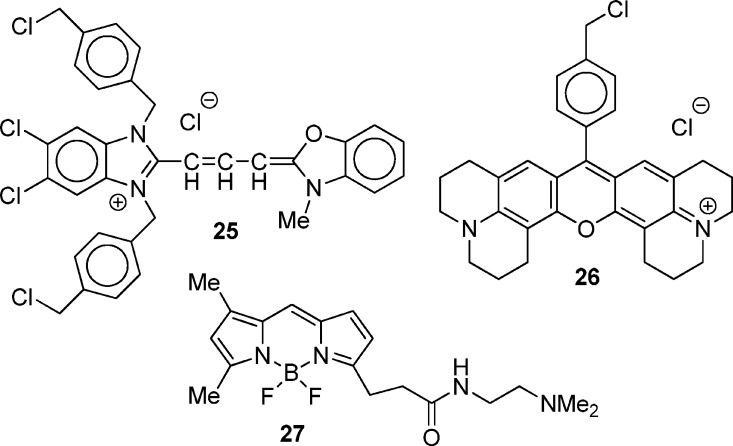
Rhodamine dyes such as 2 and 3 (Chart 1) are concentrated in the mitochondria of cancer cells because of the increased mitochondrial membrane potential in the transformed cells.10,11 While one would expect a similar pattern with selenorhodamines 15b–18b, ImageStream flow cytometry demonstrated mitochondrial targeting in Colo-26 cells by these agents as shown in Figure 5. A statistical analysis of the similarity of localization of the mitochondrial specific agents 9-[4-(chloromethyl)phenyl]-2,3,6,7,12,13,16,17-octahydro[1H,5H,11H,15H]xantheno[2,3,4-ij:5,6,7-i′j′]diquinolizin-18-ium chloride (25 or MTG, Chart 4) and 9-[4-(chloromethyl)phenyl]-2,3,6,7,12,13,16,17-octahydro[1H,5H,11H,15H]xantheno[2,3,4-ij:5,6,7-i′j′]diquinolizin-18-ium chloride (26 or MTR) in Colo-26 cells incubated with both agents gave a mean bright detail similarity score of 2.0 ± 0.8 for 1300 cells, indicating a high degree of colocalization47 of these two agents (Figure 5a). In contrast, Colo-26 cells incubated with the lysosome-specific 3-(5,5-difluoro-7,9-dimethyl-5H-4λ4,5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-3-yl)-N-(2-(dimethylamino)ethyl)propanamide (27 or LYS, Chart 4) and MTR gave a much lower bright detail similarity score of 0.6 ± 0.3 for 2000 cells, indicating that MTR and LYS do not localize to the same places in the cell (Figure 5a).
Figure 5.
(a) The average similarity coefficient determined by ImageStream flow cytometry of all cells for each pair of agents (MTR, MTG, LYS, 15b–18b) is shown; error bars represent SD. (b) Histogram of the pixel-by-pixel statistical analysis of each cell (n = 3900) analyzed, in which the y-axis is number of cells and the x-axis is the similarity coefficient between MitoTracker Green and 18b. Shown are representative examples of 18b/MTG-stained Colo-26 cells as a bright field image (BF), MTG fluorescence, 18b fluorescence, and a merged image of MTG/18b fluorescence for cells with (c) low similarity, (d) intermediate similarity, and (e) high similarity.
Chart 4. Structures of 25 (MTG), 26 (MTR), and 27 (LYS).
Colo-26 cells were next incubated for 15 min with a dye solution consisting of MTG and 0.2 μM 15b–18b. ImageStream flow cytometry gave the 15b–18b/MTG bright detail similarity scores shown in Figure 5a alongside a comparison with the MTR/MTG and LYS/MTR similarity scores. Similarity scores in the range 1.4–2.3 suggest that 15b–18b colocalize with MTG.
The histogram of Figure 5b provides an analysis of the similarity of localization of MTG and 18b. The individual cells shown in Figure 5c–e represent examples of low similarity, intermediate similarity, and high similarity, respectively, for localization of 18b and MTG. Similar data (cell images, histograms of similarity scores) for selenorhodamines 15b and 16b are compiled in Figure S9of Supporting Information and for selenorhodamine 17b in Figure S10.
Discussion
Our initial interest in heavy-chalcogen analogues of the various rhodamines/rosamines was as photosensitizers for use in PDT20,33 and for the photodynamic inactivation of viral and bacterial pathogens in blood.48,49 We found that chalcogenorhodamines with a 2-thienyl substituent in the 9-position gave values of λmax ≥ 20 nm longer than chalcogenorhodamines with a 9-phenyl substituent.50 Another exciting observation was the efficacy of the Se-analogue of 6 (Chart 1) as a photosensitizer toward MDR cells.33 The added lipophilicity from the julolidyl fragment was thought to be important with respect to efficacy in MDR cells. This observation led to the screening of numerous chalcogenorhodamines as modulators/inhibitors of P-gp with the intent of developing efficient photosensitizers for use with MDR cells. Among the rhodamine libraries that we examined, several structures emerged as modulators of P-gp ATPase activity and have increased the cellular uptake of agents such as CAM and vinblastine into MDR cells.27,28
Impact of Structure on Physical and Photophysical Properties
The structural characteristics of the best rhodamine modulators include the incorporation of the julolidine fragment (11a–14a, Chart 2) or the tetrahydroquinoline fragment (15a–18a, Chart 2). A second structural feature is the presence of the thioamide functionality (12a, 14a, 16a, and 18a, Chart 2) which decreases or inhibits P-gp ATPase activity relative to the amide structures (11a, 13a, 15a, and 17a). Those incorporating the tetrahydroquinoline fragment were better modulators than those incorporating the julolidine fragment based on values of IC50 for uptake of CAM.28
A structural feature with little impact on P-gp modulation is the chalcogen atom in the rhodamine core. Thioamide 10a and its Se-analogue have comparable values of IC50 for CAM uptake as do thioamide 14a and its Se-analogue.28 Thus, photosensitizer photophysical properties can be optimized via the chalcogen atom without major consequence to P-gp modulation.
Selenorhodamines 15b–18b were designed to have improved physical and photophysical properties as photosensitizers (Table 1) relative to thiorhodamines 15a–18a. Because of the incorporation of a 2-thienyl substituent at the 9-position and the incorporation of the Se atom in the xanthylium core, selenorhodamines 15b–18b have values of λmax > 600 nm, absorb light strongly at λmax (ε = 7.18 × 104 to 9.78 × 104 M–1 cm–1), and generate 1O2 efficiently [Φ(1O2) = 0.44–0.54]. The thioamides 16b and 18b are reasonably photostable at pH 7.4 with half-lives of ∼230 J cm–2 for exposure to 350–800 nm light and, for 18b, ∼850 J cm–2 for exposure to 500–800 nm light (Figure 1). The range of values of log P (1.61–2.41) for 15b–18b suggests that these molecules should have access to both aqueous and hydrophobic environments in the cell.
Interactions with P-gp and the Amide/Thioamide Switch
The biological properties of 15b–18b also suggest that thioamides 16b and 18b should be excellent photosensitizer candidates. The amide/thioamide switch appears to be operative in this series of compounds with respect to stimulation/inhibition of P-gp ATPase activity. In Colo-26 cells, the uptake of amide derivatives 15b and 17b is increased 5-fold in the presence of 100 μM VER while uptake of thioamides 16b and 18b increased only 2-fold (Figure 2). Higher accumulation of the 16b and 18b is likely a consequence of partial inhibition of P-gp, while amides 15b and 17b stimulate ATPase activity.
Increased uptake of thioamide 18b relative to amide 17b was also demonstrated in monolayers of MDCKII-MDR1 cells. The % cell-associated dye in cells treated with thioamide 18b (45%, Table 3) was more than 2-fold higher relative to amide 17b-treated cells (16%, Table 3). In the presence of inhibitor, % cell-associated dye was essentially unchanged with thioamide 18b (45–55% with inhibitor; ratio ± inhibitor of 1.2, Table 3) but increased by a factor of 2.4 in the presence of inhibitor for cells treated with amide 17b (16–39%, Table 3). These results again suggest that higher accumulation in the thioamide-treated cells is likely due to partial inhibition of P-gp by thioamide 18b. If one examines rates of absorptive (PAB) and secretory (PBA) transport, values of PAB for 17b and 18b, as well as their thiorhodamine counterparts 17a and 18a, are nearly identical: ∼(1–2) × 10–9 m s–1. However, values of PBA are much higher for amides 17a and 17b (230 × 10–9 and 182 × 10–9 m s–1, respectively) relative to thioamides 18a and 18b (34 × 10–9 and 15 × 10–9 m s–1, respectively), which is again consistent with exclusion of the amides to a greater extent than the thioamides.
Efficacy of 15b–18b as Photosensitizers
With higher accumulation of 16b and 18b in P-gp-expressing cells, thioamides 16b and 18b appear to be better photosensitizers than their amide counterparts 15b and 17b. The thioamides 16b and 18b have lower values of EC50 than amides 15b and 17b, and thioamide-treated cells also show a lower surviving fraction than amide-treated cells for a given photosensitizer concentration and light dose (Table 2). With 1.0 J cm–2 of laser light (λmax ± 2 nm), thioamides 16b and 18b have values of EC50 of 0.17 and 0.14 μM, respectively. To put these values in perspective, the selenopyrylium analogue of 4 (Chart 1) and closely related structures give values of EC50 of 0.07 M to 0.37 μM toward Colo-26 cells with 15 J cm–2 of 360–800-nm light.31 Toward different cell lines, the selenium analogue of 6 (Chart 1) required 5 J cm–2 of 350–800-nm light to give an EC50 of 0.1 μM.
The dark toxicity of 15b–18b toward Colo-26 cells gave values of LD50 of 7.8 to 9.5 μM, which is a much lower dark toxicity than observed with related cationic photosensitizers. Values of LD50 for thiopyrylium dye 4 (Chart 1) and closely related structures toward Colo-26 cells are 0.1–2.6 μM.32 When the dark toxicity of 16b and 18b is combined with the very low values of EC50, the therapeutic ratio between dark and phototoxicity is >55 for these two dyes (Table 2).
Like other rhodamines, 15b–18b appear to target the mitochondria of cells. As shown in Figure 5, the average similarity coefficients determined by image streamflow cytometry are in the range 1.4–2.3 in comparison of selenorhodamine localization with MTG localization. Similarity scores of >1.0 are indicative of colocalization of agents.47 The overlay of emission from MTG with emission from 18b is also readily apparent in Figure 5c–e.
The amide derivatives 15b and 17b had low dark toxicity and also target mitochondria in the Colo-26 cells but were less efficient as photosensitizers than the thioamide derivatives. As shown in Figure 2, the uptake of 15b and 17b was roughly 40–50% of the corresponding thioamide derivatives. The reduced phototoxicity is easily understood. However, the amide derivatives are still interacting with P-gp in the Colo-26 cells and irradiation of 15b or 17b-treated cells may damage P-gp even though cellular phototoxicity is reduced.
Potential for Combination Therapy
The anthracycline anticancer drug Dox, while useful for the treatment of many malignancies,51,52 suffers from the side effect of cardiotoxicity, which may limit its clinical use.52,53 The onset of cardiomyopathy can be quite rapid, occurring within 2–3 days following Dox administration.53
The combination therapy of PDT with thioamides 16b and 18b along with coadministration of Dox shows synergistic effects as illustrated in Figures 4 and S8 (Supporting Information). The combination of 0.15 μM photosensitizer, 0.05 μM Dox, and 1.0 J cm–2 of light gives a surviving fraction that is equivalent to 0.15 μM photosensitizer and 2 J cm–2 of light in the absence of Dox (Figure S6c) or that is equivalent to 0.5 μM Dox in the absence of 16b or 0.3 μM Dox in the absence of 18b (Figures 3b and S8d, respectively). These encouraging results suggest that animal studies to test the combination therapy would be appropriate.
The amide analogues 15b and 17b do not show significant synergistic effects. The active transport of these photosensitizers by P-gp from the Colo-26 cells is likely responsible for the difference in results with amide and thioamide subsets.
Conclusions
The incorporation of a selenium atom in the xanthylium core of the rhodamines and a 2-thienyl substituent at the 9-position of the rhodamines and the locking of one nitrogen atom into conjugation with the xanthylium core provide selenorhodamines with values of λmax > 600 nm and with values of Φ(1O2) ≥ 0.44. Both of these attributes are desirable characteristics for photosensitizers for the photodynamic therapy of cancer. The family of selenorhodamines 15b–18b targets the mitochondria of Colo-26 cells as determined by colocalization studies with MTG. The mitochondria are cellular targets of DLCs used as photosensitizers for PDT.15 Within this family, thioamide analogues 16b and 18b modulate/inhibit P-gp expressed by the Colo-26 cells, allowing increased uptake of the thioamides relative to amide analogues 15b and 17b. The thioamides are effective photosensitizers against P-gp-expressing cells and have the potential to be used in combination therapy with other chemotherapeutic agents. Subsequent animal studies to examine efficacy in vivo are ongoing.
Experimental Section
General Methods
Selenoxanthone 19 was prepared by literature methods.34 Thiorhodamines 15a–18a were prepared by literature methods.28 Reactions were run under Ar. Tetrahydrofuran was distilled from sodium benzophenone ketyl prior to use. Concentration in vacuo was performed on a Büchi rotary evaporator. NMR spectra were recorded on an Inova 500 instrument (500 MHz for 1H, 125 MHz for 13C) with residual solvent signal as internal standard. Infrared spectra were recorded on a PerkinElmer FTIR instrument. UV–vis–near-IR spectra were recorded on a PerkinElmer Lambda 12 spectrophotometer or on a Shimadzu UV-3600 spectrophotometer in quartz cuvettes with a 1 cm path length. Melting points were determined with a Büchi capillary melting point apparatus and are uncorrected. All compounds tested have a purity of at least 95%, which was determined from NMR spectra (Supporting Information) or by elemental analyses for C, H, and N (Atlantic Microlab, Inc., Norcross, GA). Experimental values of C, H, and N are within 0.4% of theoretical values.
Preparation of 9-(5-(Diethylcarbamothioyl)thiophen-2-yl) Selenorhodamine 16b
n-Butyllithium (1.38 M in hexanes, 1.92 mL, 2.93 mmol) was added dropwise to a stirred solution of N,N-diisopropylamine (0.500 mL, 3.53 mmol) in THF (10 mL) at −78 °C. The resulting mixture was stirred for 10 min before it was transferred to a stirred solution of N,N-diethylthiophene-2-carbothioamide (599 mg, 3.00 mmol) in THF (60 mL) at −78 °C. The resulting solution was stirred at −78 °C for 2 min before it was transferred via cannula to a stirred solution of selenoxanthone 19 (300 mg, 0.751 mmol, 1.0 equiv) in THF (30 mL) at room temperature. The resulting solution was heated to 45 °C for 0.5 h before it was cooled to ambient temperature. Glacial acetic acid (2 mL) was added, and the resulting mixture was poured into 10% aqueous HPF6 at 0 °C. The resulting mixture was stirred 12 h, and the precipitate was collected via filtration and then washed with water (50 mL) and diethyl ether (100 mL). The product was purified via column chromatography (SiO2, 6% MeOH/CH2Cl2, Rf = 0.4), followed by recrystallization from ether/CH2Cl2 to yield 441 mg (81%) of 16b-PF6 as a purple solid, mp 226–229 °C. 1H NMR (500 MHz, CD2Cl2) δ 7.80 (d, 1 H, J = 10.0 Hz), 7.59 (s, 1 H), 7.26–7.20 (m, 2 H), 7.19 (s, 1 H), 7.05 (d, 1 H, J = 4.0 Hz), 6.93 (dd, 1 H, J = 2.0, 10.0 Hz), 4.12 (br s, 2 H), 3.86 (br s, 2 H), 3.60 (t, 2 H, J = 6.0 Hz), 3.27 (s, 3 H), 3.25 (s, 6 H), 1.79 (t, 2 H, J = 6.0 Hz), 1.39 (t, 6 H, J = 6.5 Hz), 1.67 (s, 6 H); 13C NMR (500 MHz, CD2Cl2) δ 189.0, 153.1, 152.2, 150.8, 148.9, 145.1, 144.7, 139.6, 137.9, 135.4, 132.4, 130.0, 124.6, 121.2, 120.6, 115.2, 109.0, 108.4, 49.1, 48.0 (br), 40.9, 40.4, 34.6, 32.3, 28.6; HRMS (ESI, HRDFMagSec) m/z 582.1511 (calcd for C30H36N3S280Se+, 582.1510). Anal. Calcd for C30H36N3S2Se·PF6: C, 49.59; H, 4.99; N, 5.78. Found: C, 49.95; H, 5.10; N, 5.84.
The hexafluorophosphate salt 16b-PF6 (25.0 mg, 0.0344 mmol) was dissolved in CH2Cl2 (10 mL), and Amberlite IRA-400 chloride ion-exchange resin (3.0 g) was added. The mixture was stirred at ambient temperature for 24 h. The Amberlite exchange resin was removed via filtration, and the filtrate was concentrated under reduced pressure. The process was repeated two additional times, yielding 20.1 mg (95%, 77% overall) of 16b as the chloride salt. 1H NMR (500 MHz, CD2Cl2) δ 7.80 (d, 1 H, J = 10.0 Hz), 7.59 (s, 1 H), 7.26–7.20 (m, 2 H), 7.19 (s, 1 H), 7.05 (d, 1 H, J = 4.0 Hz), 6.93 (dd, 1 H, J = 2.0, 10.0 Hz), 4.12 (br s, 2 H), 3.86 (br s, 2 H), 3.60 (t, 2 H, J = 6.0 Hz), 3.27 (s, 3 H), 3.25 (s, 6 H), 1.79 (t, 2 H, J = 6.0 Hz), 1.39 (t, 6 H, J = 6.5 Hz), 1.67 (s, 6 H); 13C NMR (500 MHz, CD2Cl2) δ 189.0, 153.1, 152.2, 150.8, 148.9, 145.1, 144.7, 139.6, 137.9, 135.4, 132.4, 130.0, 124.6, 121.2, 120.6, 115.2, 109.0, 108.4, 49.1, 48.0 (br), 40.9, 40.4, 34.6, 32.3, 28.6; IR (film on NaCl) 1592, 1506, 1472, 1446, 1407, 1386, 1356, 1329, 1254, 1212 cm–1; λmax (MeOH) 608 nm (ε = 8.63 × 104 M–1 cm–1); HRMS (ESI, HRDFMagSec) m/z 582.1511 (calcd for C30H36N3S280Se+, 582.1510). Anal. Calcd for C30H36N3S2SeCl·4H2O: C, 52.28; H, 6.44; N, 6.10. Found: C, 52.33; H, 6.41; N, 6.18.
Preparation of 9-(5-(Diethylcarbamoyl)thiophen-2-yl) Selenorhodamine 15b
Trifluoroacetic anhydride (0.308 mL, 2.22 mmol) was slowly added to a stirred solution of hexafluorophosphate salt of 16b (161 mg, 0.222 mmol) in CH2Cl2 (30 mL). The resulting mixture was heated at reflux for 12 h and then cooled to ambient temperature. A solution of 10% aqueous Na2CO3 (20 mL) was added, and the mixture was extracted with CH2Cl2 (3 × 25 mL). The combined organic extracts were dried over anhydrous MgSO4, filtered, and concentrated. The resulting product was purified via recrystallization in ether/CH2Cl2 to give 15b-PF6 as a 5:1 mixture of presumably the hexafluorophosphate and trifluoroacetate salts as a blue solid. 1H NMR (500 MHz, CD3CN) δ 7.63 (d, 1 H, J = 9.5 Hz), 7.52–7.46 (m, 2 H), 7.38 (d, 1 H, J = 2.5 Hz), 7.35 (s, 1 H), 7.17 (d, 1 H, J = 3.5 Hz), 6.96 (dd, 1 H, J = 2.5, 9.5 Hz), 3.56 (t, 6 H, J = 6.0 Hz), 3.21 (s, 3 H), 3.19 (s, 6 H), 1.74 (t, 2 H, J = 6.0 Hz), 1.25 (t, 6 H, J = 7.0 Hz), 1.10 (s, 6 H); 13C NMR (300 MHz, CDCl3) δ 162.5, 152.4, 151.1, 150.2, 145.2, 144.7, 141.3, 139.7, 137.2, 135.0, 131.6, 129.9, 127.9, 120.7, 120.0, 114.7, 108.8, 108.4, 48.5, 42.5 (br), 40.6, 40.3, 34.2, 31.8, 28.5; HRMS (ESI, HRDFMagSec) m/z 566.1745 (calcd for C30H36N3OS80Se+, 566.1739). Anal. Calcd for C30H36N3OSSe·(5/6PF6 + 1/6CF3CO2): C, 51.66; H, 5.15; N, 5.96. Found: C, 51.36; H, 5.27; N, 5.96.
15b-PF6 was converted to the chloride salt as described for the preparation of 16b to give 15b (68.6 mg, 44% overall) as a blue solid, mp 144–147 °C. 1H NMR (500 MHz, CD3CN) δ 7.63 (d, 1 H, J = 9.5 Hz), 7.52–7.46 (m, 2 H), 7.38 (d, 1 H, J = 2.5 Hz), 7.35 (s, 1 H), 7.17 (d, 1 H, J = 3.5 Hz), 6.96 (dd, 1 H, J = 2.5, 9.5 Hz), 3.56 (t, 6 H, J = 6.0 Hz), 3.21 (s, 3 H), 3.19 (s, 6 H), 1.74 (t, 2 H, J = 6.0 Hz), 1.25 (t, 6 H, J = 7.0 Hz), 1.10 (s, 6 H); 13C NMR (300 MHz, CDCl3) δ 162.5, 152.4, 151.1, 150.2, 145.2, 144.7, 141.3, 139.7, 137.2, 135.0, 131.6, 129.9, 127.9, 120.7, 120.0, 114.7, 108.8, 108.4, 48.5, 42.5 (br), 40.6, 40.3, 34.2, 31.8, 28.5; IR (film on NaCl) 1591, 1447, 1386, 1328, 1254 cm–1; λmax (MeOH) 609 nm (ε = 1.04 × 105 M–1 cm–1); HRMS (ESI, HRDFMagSec) m/z 566.1745 (calcd for C30H36N3OS80Se+, 566.1739). Anal. Calcd for C30H36N3OSSeCl·4H2O: C, 53.53; H, 6.59; N, 6.24. Found: C, 53.52; H, 6.47; N, 6.27.
Preparation of 9-(5-(Piperidylcarbamothioyl)thiophen-2-yl) Selenorhodamine 18b
n-Butyllithium (1.38 M in hexanes, 2.34 mL, 2.93 mmol), N,N-diisopropylamine (0.490 mL, 3.53 mmol), piperidin-1-yl(thiophen-2-yl)methanethione (635 mg, 3.00 mmol), and selenoxanthone 19 (300 mg, 0.751 mmol) in THF (10 and 60 mL) were treated as described for the preparation of 16b to give 0.521 g (94%) of 18b-PF6, mp 233–236 °C. 1H NMR (500 MHz, CD2Cl2) δ 7.82 (d, 1 H, J = 9.5 Hz), 7.56 (s, 1 H), 7.23 (d, 1 H, J = 2.0 Hz), 7.22–7.17 (m, 2 H), 7.06 (d, 1 H, J = 3.5 Hz), 6.93 (dd, 1 H, J = 9.5, 2.0 Hz), 4.30 (broad s, 2 H), 3.99 (broad s, 2 H), 3.60 (t, 2 H, J = 6.0 Hz), 3.27 (s, 3 H), 3.25 (s, 6 H), 1.79 (t, 8 H, J = 6.0 Hz), 1.16 (s, 6 H); 13C NMR (300 MHz, CDCl3) δ 188.5, 162.2, 152.7, 151.3, 150.4, 148.1, 145.1, 145.0, 144.6, 140.6, 139.6, 139.4, 137.6, 137.4, 135.1, 131.8, 129.9, 129.7, 125.1, 120.7, 120.1, 115.0, 108.7, 108.3, 48.6, 40.5, 40.2, 34.3, 31.9, 28.5, 26.2, 24.5, 24.1, with splitting due to isomerization; HRMS (ESI, HRDFMagSec) m/z 594.1505 (calcd for C31H36N3S280Se+, 594.1510). Anal. Calcd for C31H36N3S2Se·PF6: C, 50.41; H, 4.91; N, 5.69. Found: C, 50.58; H, 5.04; N, 5.64.
The 18b-PF6 (0.521 g, 0.706 mmol) was treated with Amberlite IRA-400 chloride as described for the preparation of 16b to yield the chloride salt 18b (418 mg, 94%) as a blue solid, mp 233–236 °C. 1H NMR (500 MHz, CD2Cl2) δ 7.82 (d, 1 H, J = 9.5 Hz), 7.56 (s, 1 H), 7.23 (d, 1 H, J = 2.0 Hz), 7.22–7.17 (m, 2 H), 7.06 (d, 1 H, J = 3.5 Hz), 6.93 (dd, 1 H, J = 9.5, 2.0 Hz), 4.30 (broad s, 2 H), 3.99 (broad s, 2 H), 3.60 (t, 2 H, J = 6.0 Hz), 3.27 (s, 3 H), 3.25 (s, 6 H), 1.79 (t, 8 H, J = 6.0 Hz), 1.16 (s, 6 H); 13C NMR (300 MHz, CDCl3) δ 188.5, 162.2, 152.7, 151.3, 150.4, 148.1, 145.1, 145.0, 144.6, 140.6, 139.6, 139.4, 137.6, 137.4, 135.1, 131.8, 129.9, 129.7, 125.1, 120.7, 120.1, 115.0, 108.7, 108.3, 48.6, 40.5, 40.2, 34.3, 31.9, 28.5, 26.2, 24.5, 24.1, with splitting due to isomerization; IR (film on NaCl) 2936, 2360, 1592, 1508, 1474, 1445, 1407, 1386, 1328, 1254, 1213 cm–1; λmax (MeOH) 608 nm (ε = 1.16 × 105 M–1 cm–1); HRMS (ESI, HRDFMagSec) m/z 594.1505 (calcd for C31H36N3S280Se+, 594.1510). Anal. Calcd for C31H36N3S2SeCl·4H2O: C, 53.10; H, 6.32; N, 5.99. Found: C, 53.35; H, 6.17; N, 6.04.
Preparation of 9-(5-(Piperidylcarbamoyl)thiophen-2-yl) Selenorhodamine 17b
Trifluoroacetic anhydride (0.380 mL, 2.71 mmol) and the PF6 salt of 18b (200 mg, 0.271 mmol) in CH2Cl2 (30 mL) were treated as described for the preparation of 15b to give the PF6 salt, mp 194–197 °C. 1H NMR (500 MHz, CD2Cl2) δ 7.72 (d, 1 H, J = 10.0 Hz), 7.52 (s, 1 H), 7.41 (d, 1 H, J = 3.5 Hz), 7.35–7.24 (m, 2 H), 7.13 (d, 1 H, J = 3.5 Hz), 6.89 (d, 1 H, J = 9.0 Hz), 3.72 (t, 4 H, J = 5.0 Hz), 3.60 (t, 2 H, J = 5.0 Hz), 3.29 (s, 3 H), 3.25 (s, 6 H), 1.82–1.72 (m, 4 H), 1.71–1.64 (m, 4 H), 1.14 (s, 6 H); 13C NMR (300 MHz, CDCl3) δ 162.1, 152.5, 151.1, 150.2, 145.1, 144.7, 140.4, 139.4, 137.3, 135.0, 131.6, 129.8, 128.2, 120.7, 120.0, 114.8, 108.7, 108.3, 48.5, 40.6, 40.2, 34.2, 31.8, 28.5, 26.1, 24.5; IR (film on NaCl) 2936, 2859, 1592, 1536, 1508, 1473, 1446, 1408, 1387, 1329, 1255, 1214 cm–1; HRMS (ESI, HRDFMagSec) m/z 578.1739 (calcd for C31H36N3OS80Se+, 578.1739). Anal. Calcd for C31H36N3OSSe·(2/3PF6 + 1/3CF3CO2): C, 52.63; H, 5.02; N, 5.81. Found: C, 52.55; H, 5.18; N, 5.75.
The PF6 salt 17b-PF6 was treated with Amberlite IRA-400 chloride as described for the preparation of 16b to yield 192 mg (98%) of chloride salt 17b as a blue solid, mp 194–197 °C. 1H NMR (500 MHz, CD2Cl2) δ 7.72 (d, 1 H, J = 10.0 Hz), 7.52 (s, 1 H), 7.41 (d, 1 H, J = 3.5 Hz), 7.35–7.24 (m, 2 H), 7.13 (d, 1 H, J = 3.5 Hz), 6.89 (d, 1 H, J = 9.0 Hz), 3.72 (t, 4 H, J = 5.0 Hz), 3.60 (t, 2 H, J = 5.0 Hz), 3.29 (s, 3 H), 3.25 (s, 6 H), 1.82–1.72 (m, 4 H), 1.71–1.64 (m, 4 H), 1.14 (s, 6 H); 13C NMR (300 MHz, CDCl3) δ 162.1, 152.5, 151.1, 150.2, 145.1, 144.7, 140.4, 139.4, 137.3, 135.0, 131.6, 129.8, 128.2, 120.7, 120.0, 114.8, 108.7, 108.3, 48.5, 40.6, 40.2, 34.2, 31.8, 28.5, 26.1, 24.5; IR (film on NaCl) 2936, 2859, 1592, 1536, 1508, 1473, 1446, 1408, 1387, 1329, 1255, 1214 cm–1; λmax (MeOH) 609 nm (ε = 7.44 × 104 M–1 cm–1); HRMS (ESI, HRDFMagSec) m/z 578.1739 (calcd for C31H36N3OS80Se+, 578.1739). Anal. Calcd for C31H36N3OSSeCl·3.25H2O: C, 55.44; H, 6.38; N, 6.26. Found: C, 55.20; H, 6.11; N, 6.18.
Determination of n-Octanol/Water Partition Coefficients
The octanol/water partition coefficients were all measured at pH 7.4 (PBS) at 23 °C using UV–visible spectrophotometry. The measurements were done using a shake flask direct measurement.40 Mixing for 3–5 min was followed by 1 h of settling time. Liquid chromatography grade 1-octanol was used.
Determination of Singlet Oxygen Yields from Singlet Oxygen Luminescence Spectroscopy
Generation of 1O2 was assessed at 1270 nm where its luminescence peaked. A spectrometer equipped with a NIR photodetector was used for acquisition of the emission spectra in NIR spectral range. A diode-pumped solid-state laser at 532 nm was the excitation source. The emission signal was collected at 90° relative to the exciting laser beam with the use of a 950 nm long-pass filter to attenuate the scattered light and fluorescence from the samples. A second harmonic (532 nm) from the nanosecond-pulsed Nd:YAG laser operating at 20 Hz was used as the excitation source for time-resolved measurements. The samples (CH3OH solutions of the compounds in quartz cuvettes) were placed in front of the spectrometer entrance slit.
Fluorescence Experiments
Measurements of fluorescence quantum yield were performed on a spectrofluorometer using fluorescent dye 3 with known ΦFL = 0.93 38 in CH3OH as standard.
Phototoxicity and Dark Toxicity Studies with Colo-26 Cells
All cells were grown in RPMI 1640, 1× with l-glutamine medium. The medium was supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin. Cells were harvested, plated 10 000 to a well in a 96-well plate (0.32 cm2 on a flat bottom plate), and incubated for 24 h. Dyes were added from stock solutions of known concentration. All plates were incubated 1 h in the dark after dye addition and then either kept in the dark or irradiated with a tunable dye laser at λmax (±2 nm) at a fluence rate of 3.2 mW cm–2 to various light doses. New medium was added to each well before they were placed in the incubator (37 °C, 5% CO2). After a 48 h incubation, a sulforhodamine B assay41,42 was performed on the plates. The absorbance of each well was read on an EL800 BioTek plate reader at 570 nm to give fraction cell viability after data manipulation.
Combination Therapy with PDT and Doxorubicin in Colo-26 Cells
Colo-26 cells were harvested and plated 10 000 to a well, in 90 μL of medium per well, in a 96-well plate, and incubated for 24 h. Doxorubicin solutions in RPMI 1640 medium were made from a 20 mM Dox stock solution. Selenorhodamine dye solutions were made in RPMI 1640 medium from ethanol stock solutions of known concentration. Each dye was combined with each Dox concentration. To the selenorhodamine only wells, 10 μL of the 10× selenorhodamine solution was added. To the remaining wells, 10 μL of the 10× Dox-only solution or 10 μL of the combined 10× selenorhodamine + Dox solution was added to achieve the desired concentrations. The plates were incubated in the dark for 1 h and were then irradiated with a tunable dye laser at λmax (±2 nm) at a fluence rate of 3.2 mW cm–2 to a light dose of 1 J cm–2. The medium was flicked from the plates, and 100 μL of fresh medium was added to each well before they were placed in the incubator (37 °C, 5% CO2). After a 48 h incubation, a sulforhodamine B assay42,43 was performed on the plates. The absorbance of each well was read on an EL800 BioTek plate reader at 570 nm to give fraction cell viability after data manipulation.
Flow Cytometry Studies
Colo-26 cells were harvested, and flow cytometry was run on an LSR II A UV-Normal Flow instrument with an excitation wavelength of 561 nm (50 mW cm–2) and an emission of 710 nm (50 PE-Cy 5.5). Image flow cytometry was run on an ImageStream Mark II instrument. The channels used were channels 2 (480–560 nm detection) and 5 (642–745 nm detection) with MTG excitation at 488 nm and selenorhodamine excitation at 561 nm. Samples were made using 5 × 105 Colo-26 cells in 0.5 mL of medium. Each photosensitizer had a total of six samples: the photosensitizer alone at three different concentrations (0.1, 0.2, and 0.4 μM), the photosensitizer at three different concentrations plus Mito-Tracker Green (MTG, 0.5 μL of 1 mM MTG stock in DMSO), and MTG alone (0.5 μL of 1 mM MTG stock in DMSO). All samples were incubated 15 min, centrifuged, and flicked. Hanks PBS (60 μL) was added to each sample to replace the medium. The samples were resuspended, put on ice, and analyzed. Colocalization was determined in each individual cell using the IDEAS similarity feature, which is a log-transformed Pearson’s correlation coefficient of the intensities of the spatially correlated pixels within the whole cell, of the MTG and 15b–18b images, MTG and Mito-Tracker Red (MTR) images, or LysoTracker Green (LYS) and MTR images, respectively. The similarity score is a measure of the degree to which two images are linearly correlated.47
Pgp-Transport Studies across MDCK-MDR1 Monolayers
MDCK-MDR1 cells were seeded at 50 000 cells cm–2 onto 12-well (1.13 cm2 surface area) Transwell polycarbonate filters (Costar), were fed on days 3 and 5, and used on day 6. The upper and lower chamber volumes were 0.5 and 1.0 mL, respectively. Cells were rinsed 10 min in DPBSH at 37 °C with mixing on a nutator (Clay Adams). Cells were preincubated with 4.3 mg mL–1 bovine serum albumin (BSA) in DPBSH alone or containing 5 μM 24. After 30 min, 5 μM test compound (17b or 18b) in BSA/DPBSH with or without inhibitor was added to the donor chamber (0.5 mL upper or apical, 1.0 mL lower or basolateral). Initial donor samples were taken at t = 0. For apical-to-basolateral (AB) flux, D0 was taken from the mixing tube before addition to the cell monolayer. For basolateral-to-apical (BA) flux this sample was taken from the 12-well plate 10 min after transfer but before cell wells were added. Samples were taken from both the donor and receiver chambers following a 1 h incubation at 37 °C with constant mixing by nutation. Cell monolayers were rinsed briefly two times using cold DPBS and extracted with 500 μL of CH3OH for 3 min. In a 96-deep well assay plate, 50 μL samples were combined into n = 3 cassettes and protein was precipitated by adding 450 μL of CH3CN and shaken to mix. Plates were centrifuged 5 min at 5000 rpm. Compound concentrations were determined with an LC–MS/MS assay. Chromatography was performed using a Betasil C18 2 mm × 20 mm, 5 μm Javelin column (Thermo Scientific, Waltham, MA) and one of two mobile phase systems. System 1 consisted of 5 mM ammonium bicarbonate in water (mobile phase A) and 5 mM NH4HCO3 in CH3OH (mobile phase B), with elution accomplished by a CH3OH gradient at 1.5 mL/min. System 2 consisted of 0.4% trifluoracetic acid (TFA), 1 mM NH4HCO3 in H2O (mobile phase A), and 0.4% TFA/1 mM NH4HCO3 in CH3CN (mobile phase B), with elution accomplished by an CH3CN gradient at 1.5 mL/min. Mass spectrometric detection was performed with an API4000 mass spectrometer (Applied Biosystems, Foster City, CA) equipped with a turbo ion spray source, using selected reaction monitoring in positive ion mode with precursor and product ion transitions specific to each analyte.
Statistical Analyses
All statistical analyses were performed using the Student’s t-test for pairwise comparisons. A p value of <0.05 was considered significant.
Acknowledgments
We thank Dr. Piet Borst at The Netherlands Cancer Institute for supplying the MDCKII-MDR1 cells. This research was supported in part by the NIH Grant GM-94367 to M.R.D.
Glossary
Abbreviations Used
- ABC
ATP-binding cassette
- BSA
bovine serum albumin
- DLC
delocalized lipophilic cation
- DMSO
dimethylsulfoxide
- Dox
doxorubicin
- DPBSH
Dulbecco’s HEPES-containing phosphate buffered saline
- FBS
fetal bovine serum
- LDA
lithium diisopropylamide
- LYS
3-(5,5-difluoro-7,9-dimethyl-5H-4λ4,5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-3-yl)-N-(2-(dimethylamino)ethyl)propanamide
- MDCK
Madin–Darby canine kidney
- MDR
multidrug resistant
- MRP
multidrug resistance protein
- MTG
2-[3-[5,6-dichloro-1,3-bis[[4-(chloromethyl)phenyl]methyl]-1,3-dihydro-2H-benzimidazol-2-ylidene]-1-propenyl]-3-methylbenzoxazolium chloride
- MTR
9-[4-(chloromethyl)phenyl]-2,3,6,7,12,13,16,17-octahydro[1H,5H,11H,15H]xantheno[2,3,4-ij:5,6,7-i′j′]diquinolizin-18-ium chloride
- NIR
near-infrared
- PDT
photodynamic therapy
- P-gp
P-glycoprotein
- PBS
phosphate buffered saline
- THF
tetrahydrofuran
- VER
verapamil
- VIN
vinblastine
Supporting Information Available
Figures of electronic absorption spectra, singlet-oxygen decay traces, flow cytometry, dose–response curves for dark toxicity, with broadband light for 15a–18a, with laser-irradiation for 15b–18b, combination PDT and Dox treatment, and ImageStream flow cytometry showing mitochondrial localization. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Author Status
⊥ J.M.: Deceased June 5, 2014.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Gottesman M. M.; Fojo T.; Bates S. E. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [DOI] [PubMed] [Google Scholar]
- Szakacs G.; Paterson J. K.; Ludwig J. A.; Booth-Genthe C.; Gottesman M. M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discovery 2006, 5, 219–234. [DOI] [PubMed] [Google Scholar]
- Abolhoda A.; Wilson A. I.; Ross H.; Danenberg P. V.; Burt M.; Scotto K. W. Rapid activiation of MDR1 gene expression in human metastatic sarcoma after in vivo exposure to doxorubicin. Clin. Cancer Res. 1999, 5, 3352–3356. [PubMed] [Google Scholar]
- Raub T. J. P-Glycoprotein recognition of substrates and circumvention through rational drug design. Mol. Pharmaceutics 2006, 3, 3–25. [DOI] [PubMed] [Google Scholar]
- Seelig A.; Gatlik-Landwojtowicz E. Inhibitors of multidrug efflux transporters: their membrane and protein interactions. Mini-Rev. Med. Chem. 2005, 5, 135–151. [DOI] [PubMed] [Google Scholar]
- Dantzig A. H.; de Alwis D. P.; Burgess M. Considerations in the design and development of transport inhibitors as adjuncts to drug therapy. Adv. Drug Delivery Rev. 2003, 55, 133–150. [DOI] [PubMed] [Google Scholar]
- Loetchutinat C.; Saengkhae C.; Marbeuf-Gueye C.; Garnier-Suillerot A. New insights into the P-glycoprotein-mediated effluxes of rhodamines. Eur. J. Biochem. 2003, 270, 476–485. [DOI] [PubMed] [Google Scholar]
- Eytan G. D.; Regev R.; Hurwitz C. D.; Assaraf Y. G. Efficiency of P-glycoprotein-mediated exclusion of rhodamine dyes from multidrug-resistant cells is determined by their passive transmembrane movement rate. Eur. J. Biochem. 1997, 248, 104–112. [DOI] [PubMed] [Google Scholar]
- Lu P.; Liu R.; Sharom F. J. Drug transport by reconstituted P-glycoprotein in proteoliposomes. Effect of substrates and modulators, and dependence on bilayer phase state. Eur. J. Biochem. 2001, 268, 1687–1695. [PubMed] [Google Scholar]
- Johnson L. V.; Walsh M. L.; Bockus B. J.; Chen L. B. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J. Cell Biol. 1981, 88, 526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S.; Weiss M. J.; Wong J. R.; Lampidis T. J.; Chen L. B. Mitochondrial and plasma membrane potentials cause unusual accumulation and retention of rhodamine 123 by breast adenocarcinoma-derived MCF-7 cells. J. Biol. Chem. 1985, 260, 13844–13850. [PubMed] [Google Scholar]
- Lampidis T. J.; Bernal S. D.; Summerhayes I. C.; Chen L. B. Selective toxicity of rhodamine-123 in carcinoma cells in vitro. Cancer Res. 1983, 43, 716–720. [PubMed] [Google Scholar]
- Bernal S. D.; Lampidis T. J.; McIsaac R. M.; Chen L. B. Anticarcinoma activity in vivo of rhodamine 123, a mitochondrial-specific dye. Science 1986, 222, 169–172. [DOI] [PubMed] [Google Scholar]
- Sun X.; Wong J. R.; Song K.; Hu J.; Garlid K. D.; Chen L. B. AA1, a newly synthesized monovalent lipophilic cation, expresses potent in vivo antitumor activity. Cancer Res. 1994, 54, 1465–1471. [PubMed] [Google Scholar]
- Detty M. R.; Gibson S. L.; Wagner S. J. Current clinical and preclinical photosensitizers for use in photodynamic therapy. J. Med. Chem. 2004, 47, 3987–3915. [DOI] [PubMed] [Google Scholar]
- Pal P.; Zeng H.; Durocher G.; Girard D.; Li T. C.; Gupta A. K.; Giasson R.; Blanchard L.; Gaboury L.; Balassy A.; Turmel C.; Laperriere A.; Villeneuve L. Phototoxicity of some bromine-substituted rhodamine dyes: synthesis, photophysical properties and application as photosensitizers. Photochem. Photobiol. 1996, 63, 161–168. [DOI] [PubMed] [Google Scholar]
- Ohulchanskyy T.; Donnelly D. J.; Detty M. R.; Prasad P. N. Heteroatom substitution changes in excited-state photophysics and singlet oxygen generation in chalcogenoxanthylium dyes: effect of sulfur and selenium substitutions. J. Phys. Chem. B 2004, 108, 8668–8672. [Google Scholar]
- a Kessel D.; Woodburn K. Selective photodynamic inactivation of a multidrug transporter by a cationic photosensitizing agent. Br. J. Cancer 1995, 71, 306–310. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ogata M.; Inanami O.; Nakajima M.; Nakajima T.; Hiraoka W.; Kuwabara M. Ca2+-dependent and caspace-3-independent apoptosis caused by damage in Golgi apparatus due to 2,4,5,7-tetrabromorhodamine 123 bromide-induced photodynamic effects. Photochem. Photobiol. 2003, 78, 241–247. [DOI] [PubMed] [Google Scholar]
- a Solomon S. R.; Mielke S.; Savani B. N.; Montero A.; Wisch L.; Childs R.; Hensel N.; Schindler J.; Ghetie V.; Leitman S. F.; Mai T.; Carter C. S.; Kurlander R.; Read E. J.; Vitetta E. S.; Barrett A. J. Selective depletion of alloreactive donor lymphocytes: a novel method to reduce the severity of graft-versus-host disease in older patients undergoing matched sibling donor stem cell transplantation. Blood 2005, 106, 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Mielke S.; Nunes R.; Rezvani K.; Fellowes V. S.; Venne A.; Solomon S. R.; Fan Y.; Gostick E.; Price D. A.; Scotto C.; Read E. J.; Barrett A. J. A clinical-scale selective allodepletion approach for the treatment of HLA-mismatched and matched donor-recipient pairs using expanded T lymphocytes as antigen-presenting cells and a TH9402-based photodepletion technique. Blood 2008, 111, 4392–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detty M. R.; Prasad P. N.; Donnelly D. J.; Ohulchanskyy T.; Gibson S. L.; Hilf R. Synthesis, properties, and photodynamic properties in vitro of heavy-chalcogen analogues of tetramethylrosamine. Bioorg. Med. Chem. 2004, 12, 2537–2544. [DOI] [PubMed] [Google Scholar]
- Scala S.; Akhmed N.; Rao U. S.; Paull K.; Lan L.-B.; Dickstein B.; Lee J.-S.; Elgemeie G. H.; Stein W. D.; Bates S. E. P-glycoprotein substrates and antagonists cluster into two distinct groups. Mol. Pharmacol. 1997, 51, 1024–1033. [DOI] [PubMed] [Google Scholar]
- Lee J. S.; Paull K.; Alvarez M.; Hose C.; Monks A.; Grever M.; Fojo A. T.; Bates S. E. Rhodamine efflux patterns predict P-glycoprotein substrates in the National Cancer Institute drug screen. Mol. Pharmacol. 1994, 46, 627–638. [PubMed] [Google Scholar]
- Shapiro A. B.; Ling V. Stoichiometry of rhodamine 123 transport to ATP hydrolysis by P-glycoprotein. Eur. J. Biochem. 1998, 254, 189–193. [DOI] [PubMed] [Google Scholar]
- Shapiro A. B.; Ling V. Positively cooperative sites for drug transport by P-glycoprotein with distinct drug specificities. Eur. J. Biochem. 1997, 250, 130–137. [DOI] [PubMed] [Google Scholar]
- Tombline G.; Donnelly D. J.; Holt J. J.; You Y.; Ye M.; Gannon M. K.; Nygren C. L.; Detty M. R. Stimulation of P-glycoprotein ATPase by analogues of tetramethylrosamine: coupling of drug binding at the “R” site to the ATP hydrolysis transition state. Biochemistry 2006, 45, 8034–8047. [DOI] [PubMed] [Google Scholar]
- Tombline G.; Holt J. J.; Gannon M. K. II; Donnelly D. J.; Wetzel B.; Sawada G. A.; Raub T. J.; Detty M. R. ATP occlusion as a surrogate measure for drug coupling. Biochemistry 2008, 47, 3294–3307. [DOI] [PubMed] [Google Scholar]
- Gannon M. K. II; Holt J. J.; Bennett S. M.; Wetzel B. R.; Loo T. W.; Bartlett M. C.; Clarke D. M.; Sawada G. A.; Higgins J. W.; Tombline G.; Raub T. J.; Detty M. R. Rhodamine inhibitors of P-glycoprotein: an amide/thioamide “switch” for ATPase activity. J. Med. Chem. 2009, 52, 3328–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard A.; Schamerhorn G. A.; Calitree B. D.; Sawada G. A.; Loo T. W.; Bartlett M. C.; Clarke D. M.; Detty M. R. Thiorhodamines containing amide and thioamide functionality as inhibitors of the ATP-binding cassette drug transporter P-glycoprotein (ABCB1). Bioorg. Med. Chem. 2012, 20, 4290–4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A.; Killoran J.; O’Shea C.; Kenna T.; Gallagher W. M.; O’Shea D. F. In vitro demonstration of the heavy-atom effect for photodynamic therapy. J. Am. Chem. Soc. 2004, 126, 10619–10631. [DOI] [PubMed] [Google Scholar]
- Leonard K. A.; Hall J. P.; Nelen M. I.; Davies S. R.; Gollnick S. O.; Camacho S.; Oseroff A. R.; Gibson S. L.; Hilf R.; Detty M. R. A selenopyrylium photosensitizer for photodynamic therapy related in structure to the antitumor agent AA1 with potent in vivo activity and no long-term skin photosensitization. J. Med. Chem. 2000, 43, 4488–4498. [DOI] [PubMed] [Google Scholar]
- Brennan N. K.; Hall J. P.; Davies S. R.; Gollnick S. O.; Oseroff A. R.; Gibson S. L.; Hilf R.; Detty M. R. In vitro photodynamic properties of chalcogenopyrylium analogues of the thiopyrylium antitumor agent AA1. J. Med. Chem. 2002, 45, 5123–5135. [DOI] [PubMed] [Google Scholar]
- Detty M. R.; Gibson S. L.; Hilf R. Comparison of the dark and light-induced toxicity of thio and seleno analogues of the thiopyrylium dye AA1. Bioorg. Med. Chem. 2004, 12, 2589–2596. [DOI] [PubMed] [Google Scholar]
- Holt J. J.; Gannon M. K.; Tombline G.; McCarty T. A.; Page P. M.; Bright F. V.; Detty M. R. A cationic chalcogenoxanthylium photosensitizer effective in vitro in chemosensitive and multidrug-resistant cells. Biorg. Med. Chem. 2006, 14, 8635–8643. [DOI] [PubMed] [Google Scholar]
- Kryman M. W.; Schamerhorn G. A.; Hill J. E.; Calitree B. D.; Davies K. S.; Linder M. K.; Ohulchanskyy T. Y.; Detty M. R. Synthesis and properties of heavy chalcogen analogues of the Texas reds and related rhodamines. Organometallics 2014, 33, 2628–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beak P.; Brown R. A. The tertiary amide as an effective director of ortho lithiation. J. Org. Chem. 1982, 47, 34–36. [Google Scholar]
- Carpenter A. J.; Chadwick D. J. High yield syntheses of 2,3-disubstituted furans and thiophenes. Tetrahedron Lett. 1985, 26, 1777–1980. [Google Scholar]
- Katritzky A. R.; Witek R. M.; Rodriguez-Garcia V.; Mohapatra P. P.; Rogers J. W.; Cusido J.; Abdel-Fattah A. A. A.; Steel P. J. Benzotriazole-assisted thioacylation. J. Org. Chem. 2005, 70, 7866–7881. [DOI] [PubMed] [Google Scholar]
- Magde D. R.; Wong R.; Seybold P. G. Fluorescence quantum yields and their relation to lifetimes of rhodamine 6G and fluorescein in nine solvents: improved absolute standards for quantum yields. Photochem. Photobiol. 2002, 75, 327–334. [DOI] [PubMed] [Google Scholar]
- Partition Coefficients:Sangster J. In Octanol–Water Partition Coefficients: Fundamentals and Physical Chemistry; Fogg P. G. T., Ed.; Wiley Series in Solution Chemistry, Vol. 2; John Wiley and Sons: New York, 1997. [Google Scholar]
- Spoelstra E. C.; Dekker H.; Schuurhuis G. J.; Broxterman H. J.; Lankelma J. P-glycoprotein drug efflux pump involved in the mechanisms of intrinsic drug resistance in various colon cancer cell lines. Evidence for a saturation of active daunorubicin transport. Biochem. Pharmacol. 1991, 41, 349–359. [DOI] [PubMed] [Google Scholar]
- Evers R.; Kool M.; Smith A. J.; van Deemter L.; de Haas M.; Borst P. Inhibitory effect of the reversal agents V-104, GF120918 and Pluronic L61 on MDR1Pgp-, MRP1- and MRP2-mediated transport. Br. J. Cancer 2000, 83, 366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton P.; Fang R.; Techatanawat I.; Steventon G.; Hylands P. J.; Lee C. C. The sulphorhodamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activities related to reputed anticancer activity. Methods 2007, 42, 377–387. [DOI] [PubMed] [Google Scholar]
- Vichai V.; Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [DOI] [PubMed] [Google Scholar]
- Sawada G. A.; Barsuhn C. L.; Lutzke B. S.; Houghton M. E.; Padbury G. E.; Ho N. F. H.; Raub T. J. Increased lipophilicity and subsequent cell partitioning decrease passive transcellular diffusion of novel, highly lipophilic antioxidants. J. Pharmacol. Exp. Ther. 1999, 288, 1317–1326. [PubMed] [Google Scholar]
- Dantzig A. H.; Shepard R. L.; Law K. L.; Tabas L.; Pratt S.; Gillespie J. S.; Binkley S. N.; Kuhfeld M. T.; Starling J. J.; Wrighton S. A. Selectivity of the multidrug resistance modulator, LY335979, for P-glycoprotein and effect on cytochrome P450 activities. J. Pharmacol. Exp. Ther. 1999, 290, 854–890. [PubMed] [Google Scholar]
- Riddell J. R.; Wang X.-Y.; Hans Minderman H.; Gollnick S. O. Peroxiredoxin 1 stimulates secretion of proinflammatory cytokines by binding to TLR4. J. Immunol. 2010, 184, 1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S. J.; Skripchenko A.; Donnelly D. J.; Ramaswamy K.; Detty M. R. Chalcogenoxanthylium photosensitizers for the photodynamic purging of blood-borne viral and bacterial pathogens. Bioorg. Med. Chem. 2005, 13, 5927–5935. [DOI] [PubMed] [Google Scholar]
- Wagner S. J.; Skripchenko A.; Thompson-Montgomery D.; Awatefe H.; Donnelly D. J.; Detty M. R. Use of a red cell band 3-ligand/antioxidant to improve red cell storage properties following virucidal phototreatment with chalcogenoxanthylium photosensitizers for pathogen reduction. Photochem. Photobiol. 2006, 76, 514–517. [DOI] [PubMed] [Google Scholar]
- Calitree B. D.; Donnelly D. J.; Holt J. J.; Gannon M. K. II; Nygren C.; Sukumaran D. K.; Autschbach J.; Detty M. R. Tellurium analogues of rosamine and rhodamine dyes: synthesis, structure, 125Te NMR, and heteroatom contributions to excitation energies. Organometallics 2007, 26, 6248–6257. [Google Scholar]
- Weiss R. B. The anthracyclines: Will we ever find a better doxorubicin?. Semin. Oncol. 1992, 19, 670–686. [PubMed] [Google Scholar]
- Jordon M. A. Anti-cancer agents. Cur. Med. Chem. 2002, 2, 1–17. [DOI] [PubMed] [Google Scholar]
- Chatterjee K.; Zhang J.; Honbo N.; Karliner J. S. Doxorubicin cardiomyopathy. Cardiology 2010, 115, 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.