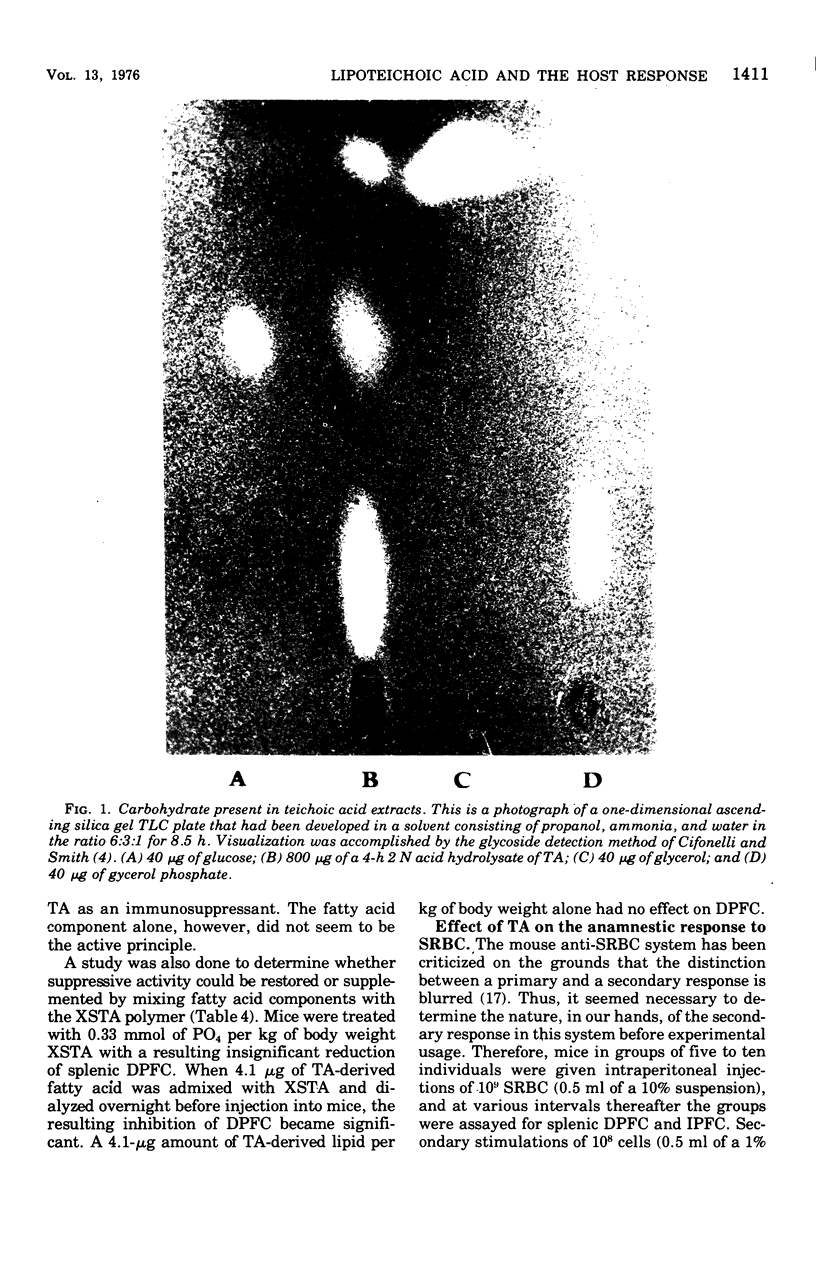
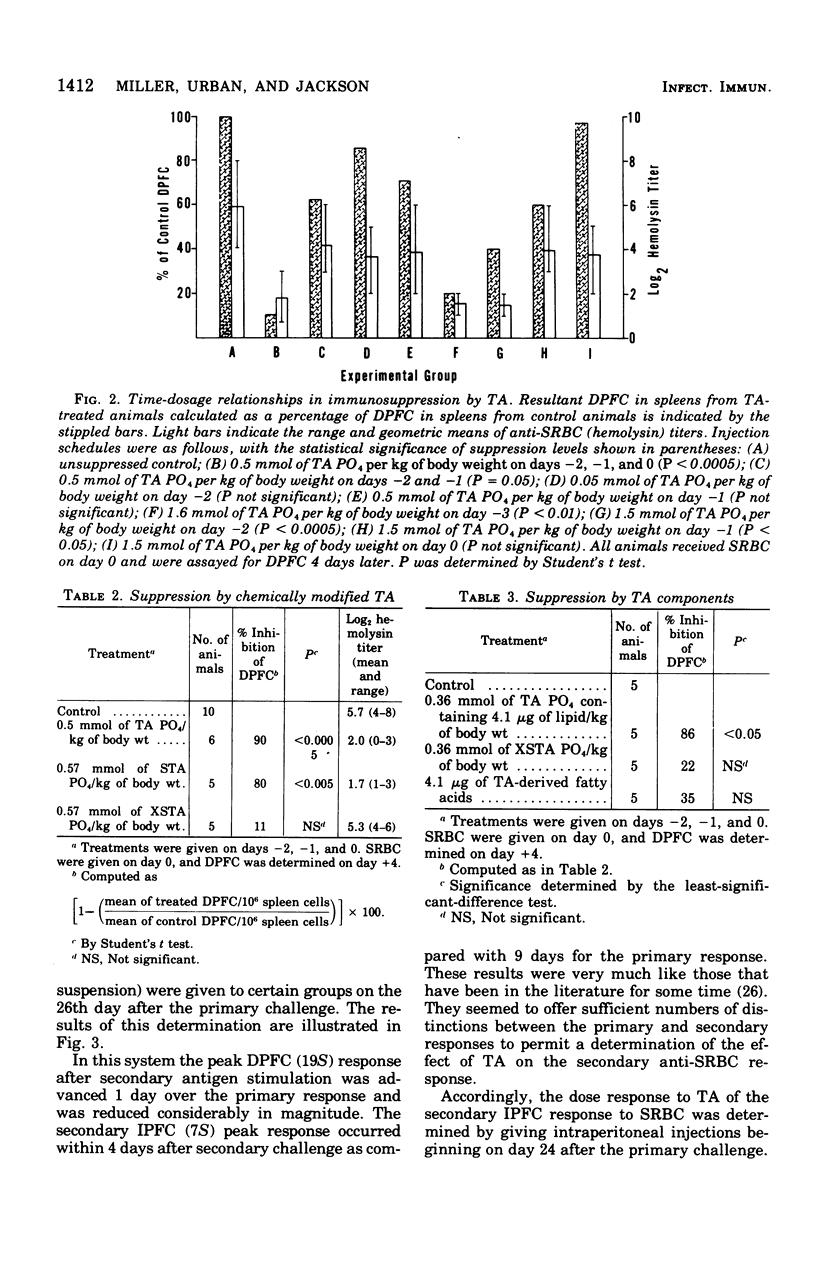
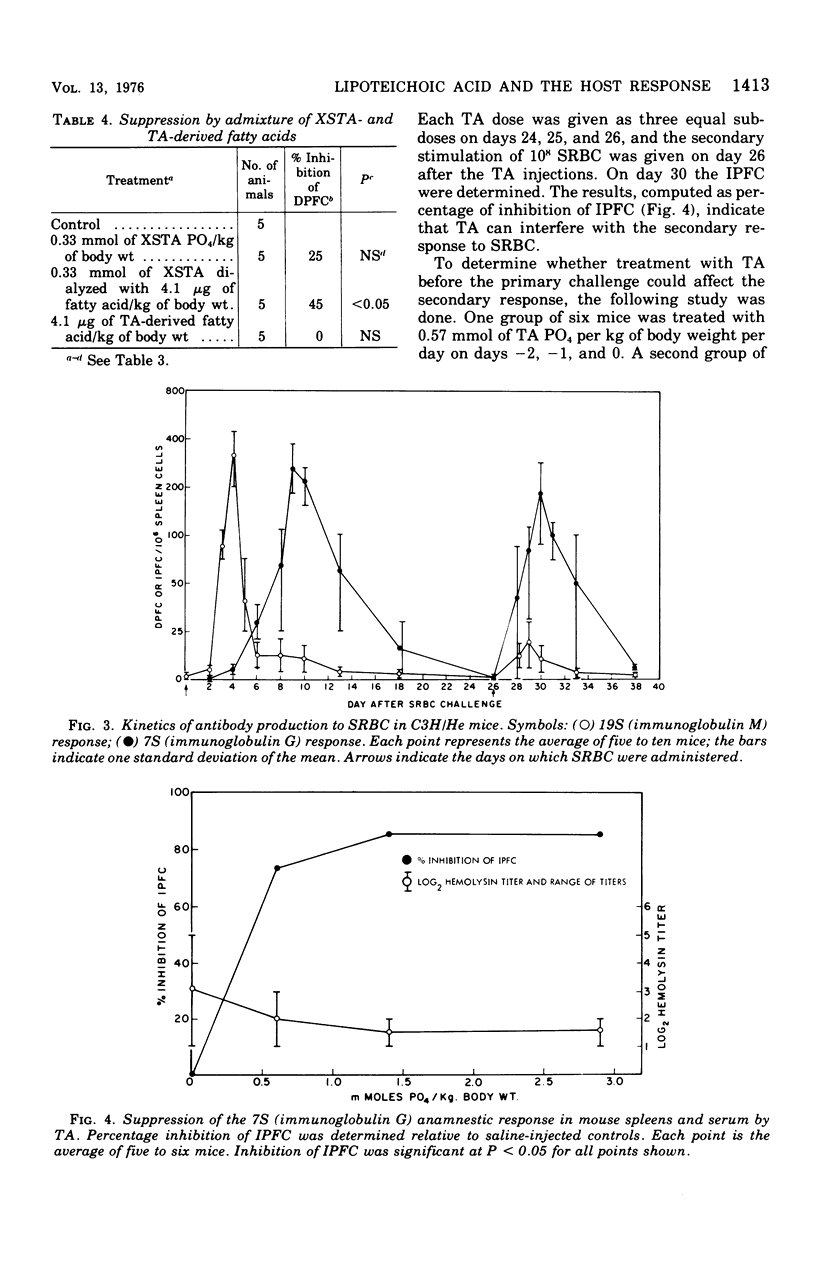
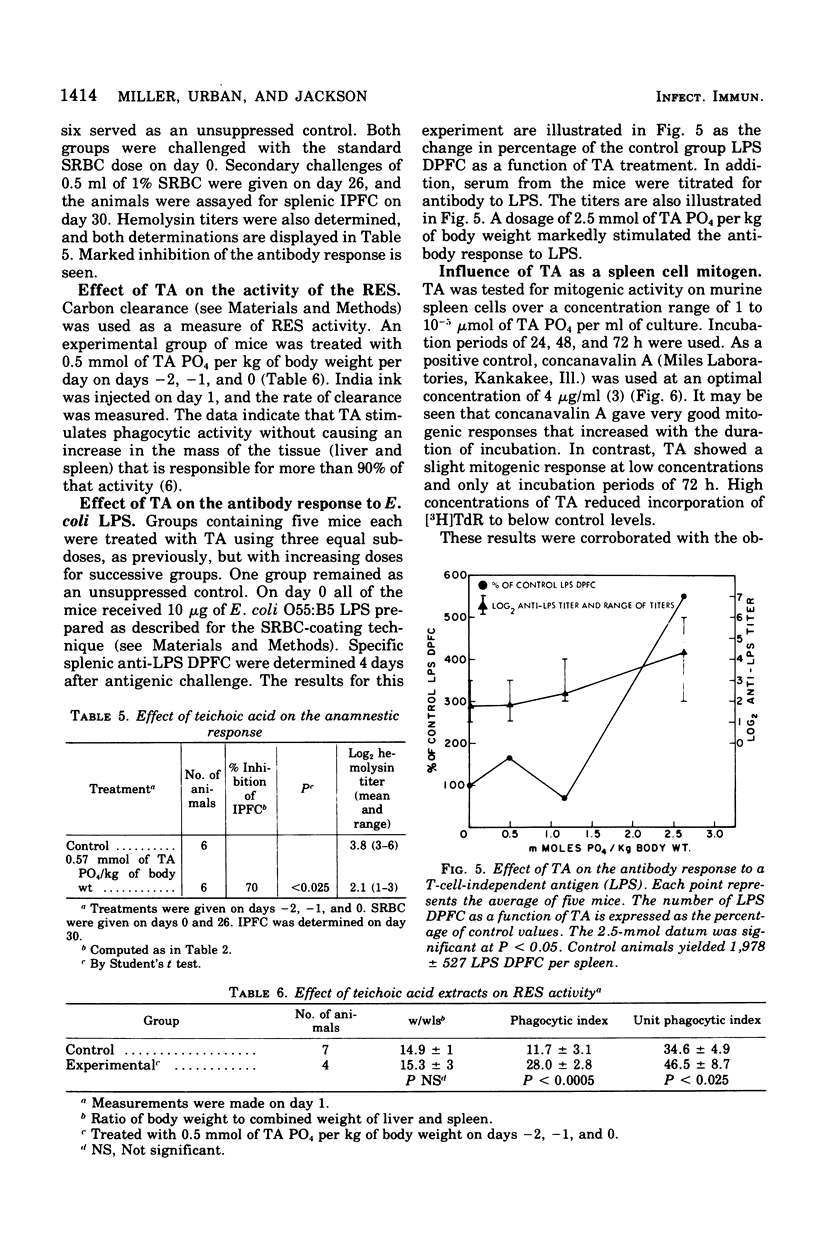
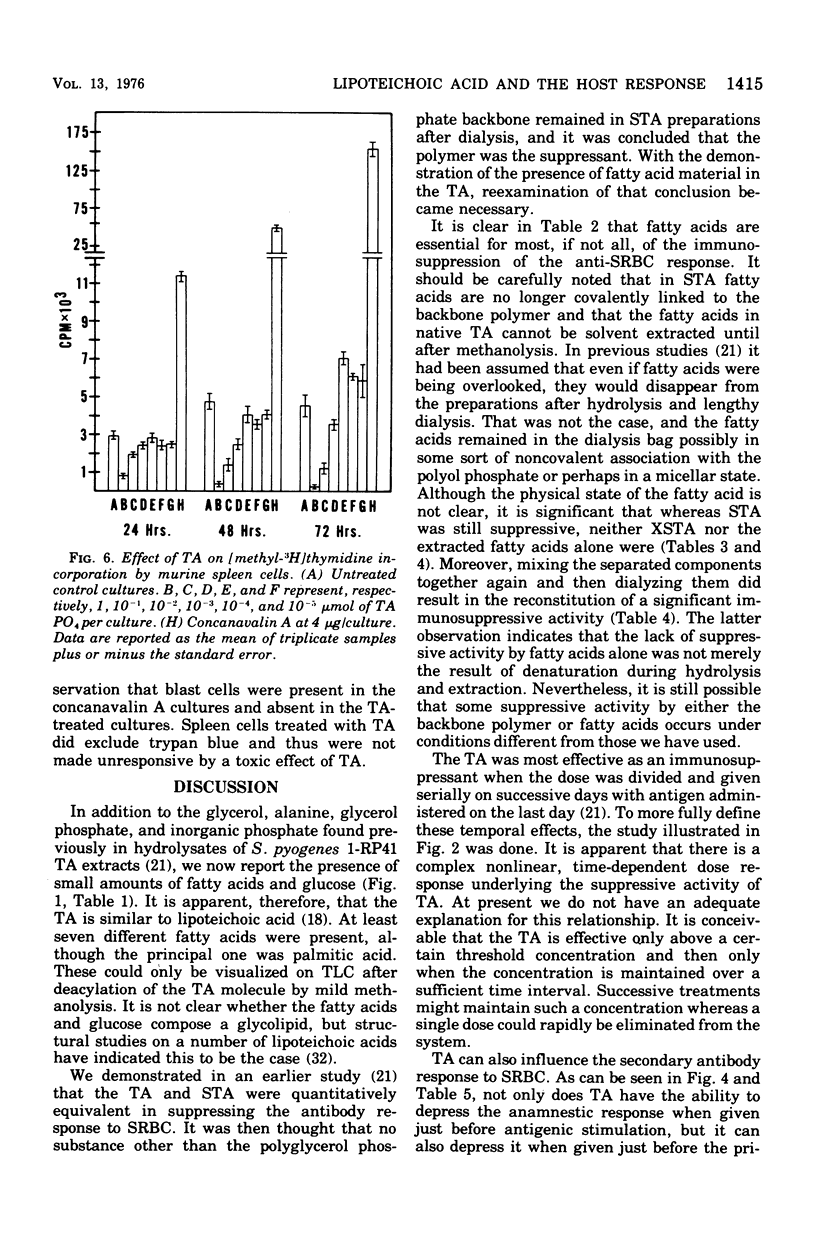
Abstract
A teichoic acid (TA) extracted from Streptococcus pyogenes 1-RP41 was previously shown to be an immunosuppressant under certain conditions (Miller and Jackson, 1973). The TA has now been shown to be a lipoteichoic acid composed of 40% glycerol, 20% alanine, 13% phosphorus, and 8% glucose, with a variable content of fatty acids. Teh presence of the polyglycerol phosphate backbone and fatty acid was required for maximum immunosuppression of the primary immunoglobulin M response to sheep cells. A complex, nonlinear, time-dependent dosage relationship in suppression of the anti-sheep erythrocyte response in mice was observed. TA depressed the anamnestic response to sheep cells in the mouse and could affect this response whether administered before the primary antigen challenge or immediately before the secondary challenge. In distinct contrast, TA enhanced antibody production to Escherichia coli O55:B5 lipopolysaccharide when assessed by counting plaque-forming cells or measuring antilipopolysaccharide serum titers. The TA failed to stimulate a large uptake of [3H]TdR by murine spleen cells; however, it significantly enhanced the clearance of carbon by the reticuloendothelial system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Andersson B., Blomgren H. Evidence for thymus-independent humoral antibody production in mice against polyvinylpyrrolidone and E. coli lipopolysaccharide. Cell Immunol. 1971 Oct;2(5):411–424. doi: 10.1016/0008-8749(71)90052-9. [DOI] [PubMed] [Google Scholar]
- Andersson J., Möller G., Sjöberg O. Selective induction of DNA synthesis in T and B lymphocytes. Cell Immunol. 1972 Aug;4(4):381–393. doi: 10.1016/0008-8749(72)90040-8. [DOI] [PubMed] [Google Scholar]
- BIOZZI G., BENACERRAF B., STIFFEL C., HALPERN B. N. Etude quantitative de l'activité granulopexique du système réticuloendothéliai chez la souris. C R Seances Soc Biol Fil. 1954 Mar;148(5-6):431–435. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Barth R. F., Stashak P. W., Amsbaugh D. F. Enhancement of the antibody response to type 3 pneumococcal polysaccharide in mice treated with antilymphocyte serum. J Immunol. 1970 May;104(5):1313–1315. [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B., Barth R. F. Evidence for the existence of two functionally distinct types of cells which regulate the antibody response to type 3 pneumococcal polysaccharide. J Immunol. 1970 Dec;105(6):1581–1583. [PubMed] [Google Scholar]
- Coutinho A., Möller G. B cell mitogenic properties of thymus-independent antigens. Nat New Biol. 1973 Sep 5;245(140):12–14. doi: 10.1038/newbio245012a0. [DOI] [PubMed] [Google Scholar]
- Duncombe W. G. The colorimetric micro-determination of long-chain fatty acids. Biochem J. 1963 Jul;88(1):7–10. doi: 10.1042/bj0880007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Rietschel E. T., Lüderitz O., Westphal O., Kim Y. B., Watson D. W. Biological activities of lipid A complexed with bovine-serum albumin. Eur J Biochem. 1972 Dec 4;31(2):230–233. doi: 10.1111/j.1432-1033.1972.tb02524.x. [DOI] [PubMed] [Google Scholar]
- HURLBERT R. B., SCHMITZ H., BRUMM A. F., POTTER V. R. Nucleotide metabolism. II. Chromatographic separation of acid-soluble nucleotides. J Biol Chem. 1954 Jul;209(1):23–39. [PubMed] [Google Scholar]
- Jackson R. W., Moskowitz M. Nature of a red cell sensitizing substance from streptococci. J Bacteriol. 1966 Jun;91(6):2205–2209. doi: 10.1128/jb.91.6.2205-2209.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K., Nordin A. A. Plaque Formation in Agar by Single Antibody-Producing Cells. Science. 1963 Apr 26;140(3565):405–405. doi: 10.1126/science.140.3565.405. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Immunological properties of teichoic acids. Bacteriol Rev. 1973 Jun;37(2):215–257. doi: 10.1128/br.37.2.215-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller G. A., Jackson R. W. The effect of a streptococcus pyogenes teichoic acid on the immune response of mice. J Immunol. 1973 Jan;110(1):148–156. [PubMed] [Google Scholar]
- PERKINS E. H., MAKINODAN T. RELATIVE POOL SIZE OF POTENTIALLY COMPETENT ANTIBODY-FORMING CELLS OF PRIMED AND NONPRIMED SPLEEN CELLS GROWN IN IN VIVO CULTURE. J Immunol. 1964 Feb;92:192–200. [PubMed] [Google Scholar]
- Peavy D. L., Adler W. H., Smith R. T. The mitogenic effects of endotoxin and staphylococcal enterotoxin B on mouse spleen cells and human peripheral lymphocytes. J Immunol. 1970 Dec;105(6):1453–1458. [PubMed] [Google Scholar]
- Schwab J. H. Suppression of the immune response by microorganisms. Bacteriol Rev. 1975 Jun;39(2):121–143. doi: 10.1128/br.39.2.121-143.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S., Park A. B., Nordin A. A. Immunoglobulin classes of antibody-forming cells in mice. I. Localized hemolysis-in-agar plaque-forming cells belonging to five immunoglobulin classes. J Immunol. 1970 Feb;104(2):483–494. [PubMed] [Google Scholar]
- Strong D. M., Ahmed A. A., Scher I., Knudsen R. C., Sell K. W. Specificity of in vitro murine B cell activation by protein and polysaccharide polymers. J Immunol. 1974 Nov;113(5):1429–1437. [PubMed] [Google Scholar]
- Veit B. C., Michael J. G. The lack of thymic influence in regulating the immune response to Escherichia coli 0127 endotoxin. J Immunol. 1972 Sep;109(3):547–553. [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]