Abstract
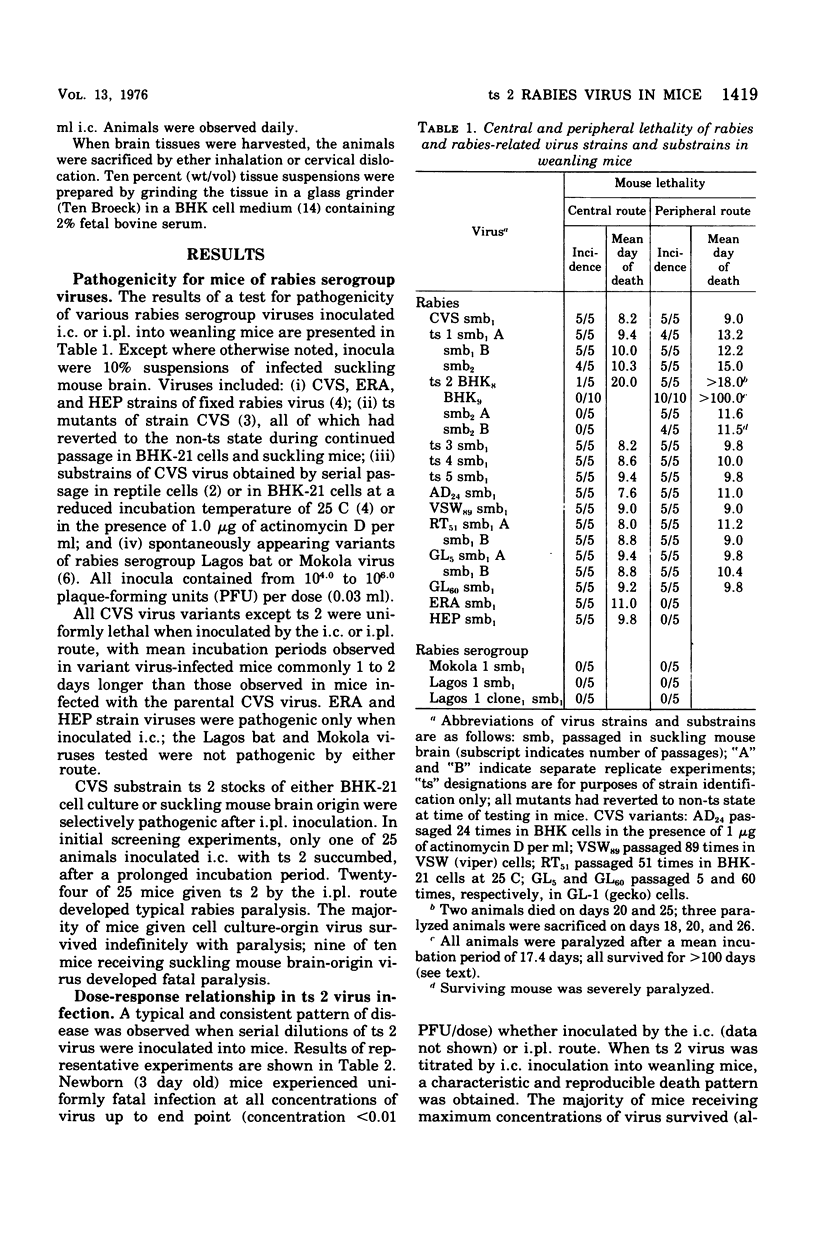
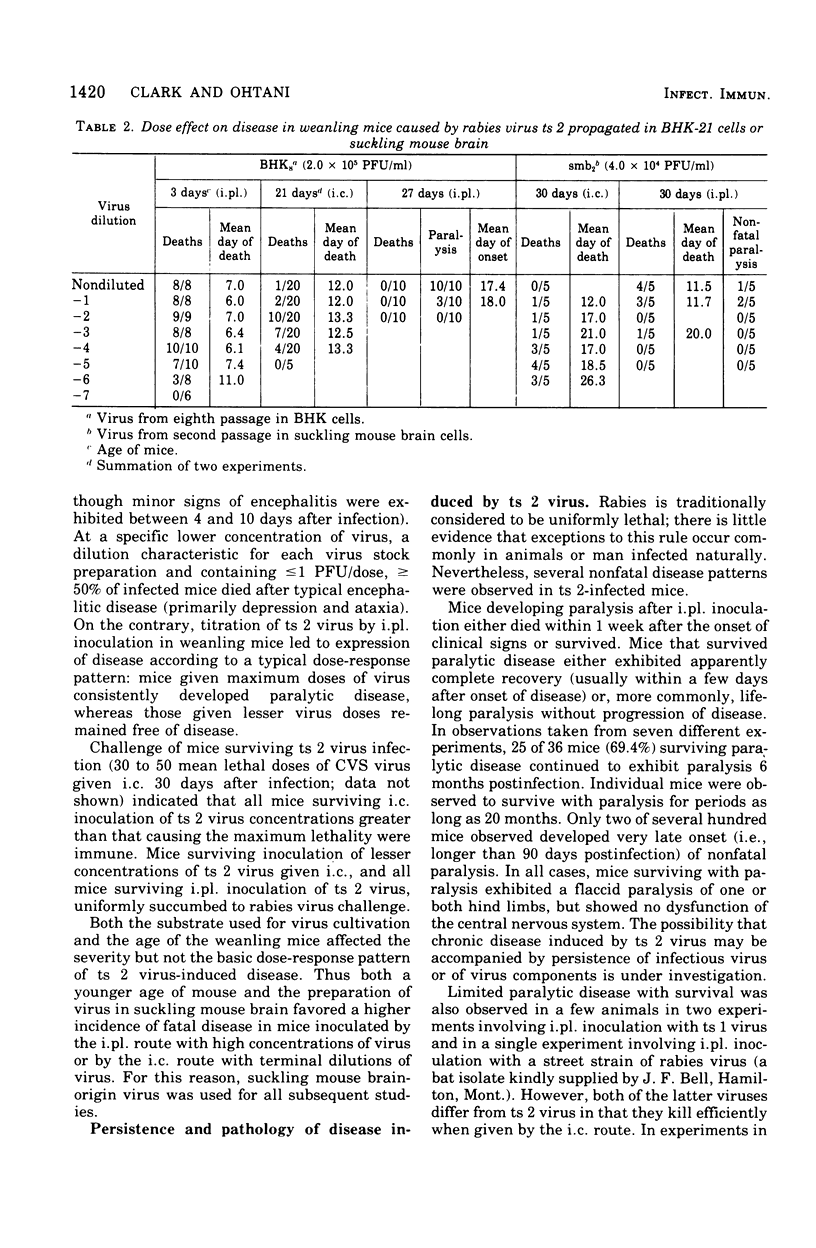
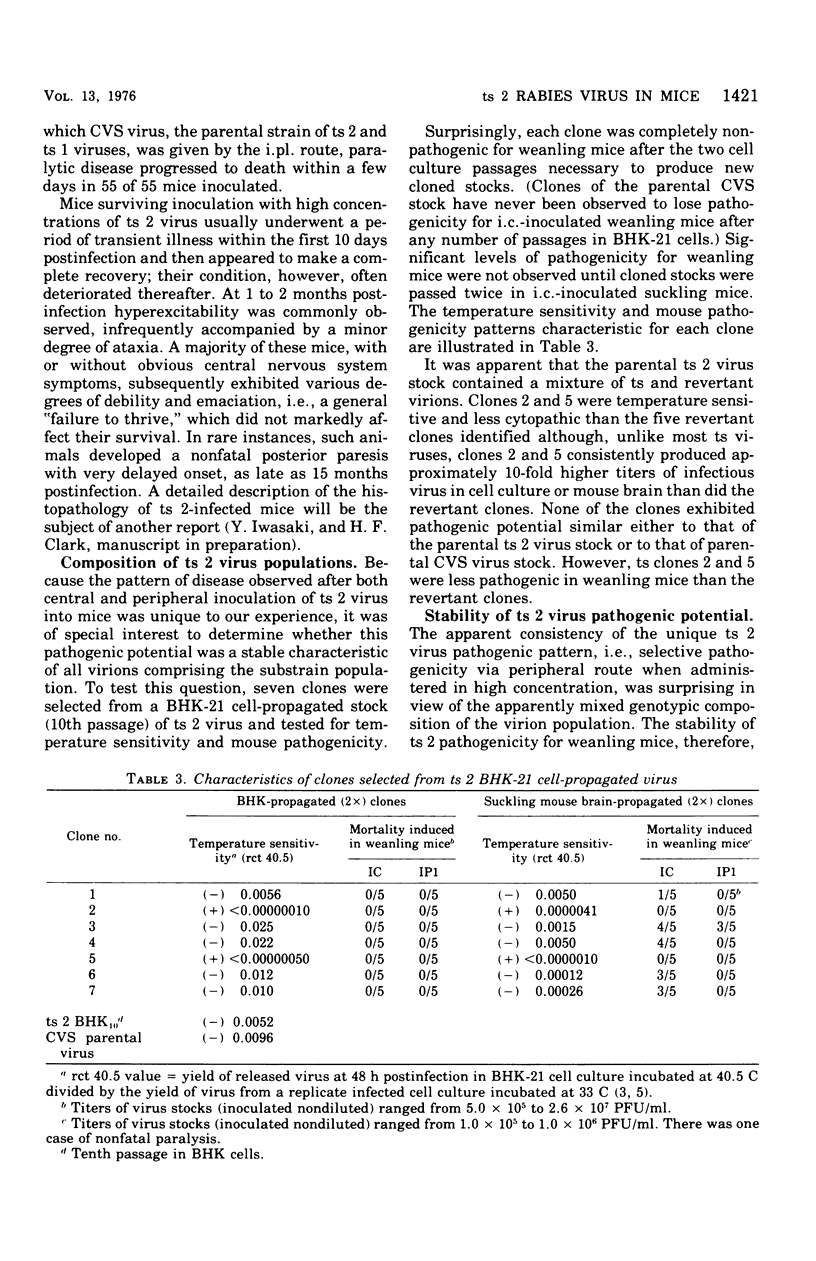
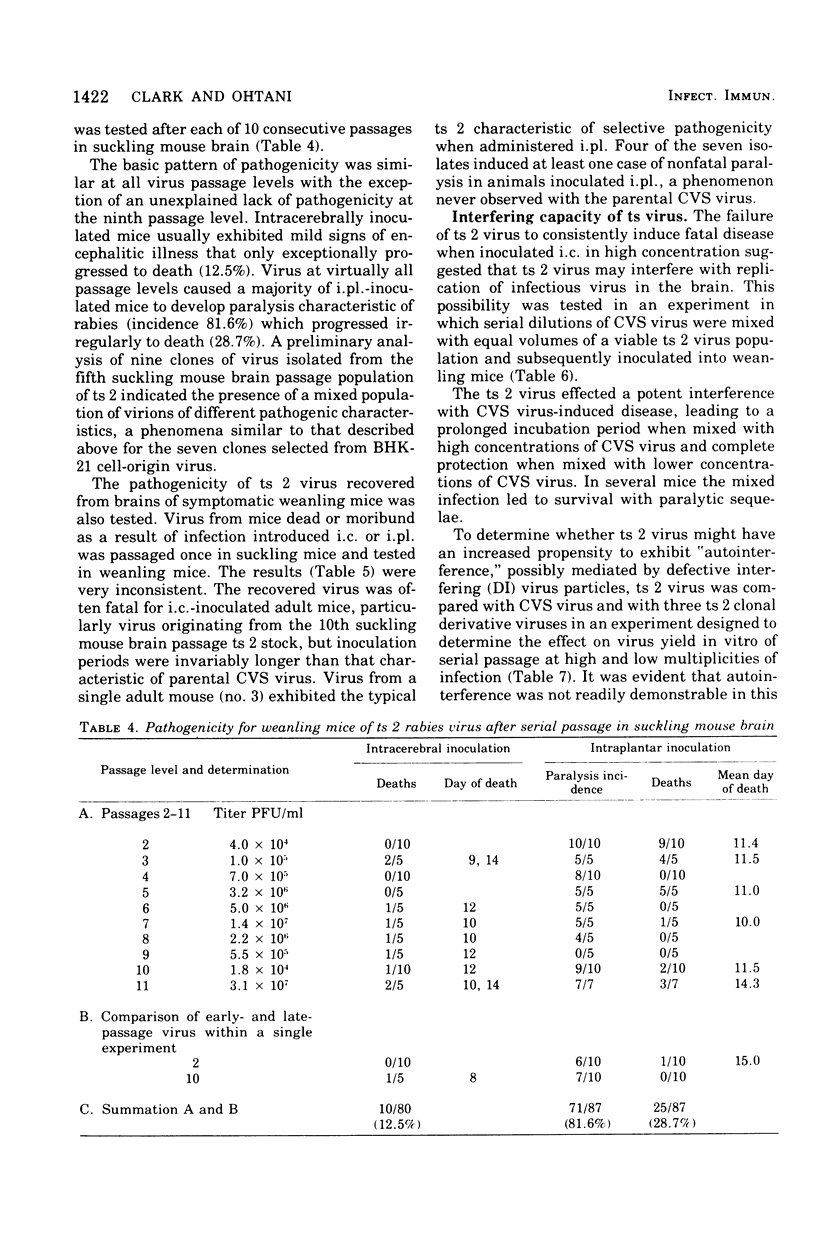
Analysis of the pathogenic potential in mice of a variety of rabies and rabies serogroup viruses revealed that an apparently revertant population of virus derived from CVS mutant ts 2 had a unique capacity to selectively induce paralytic disease when given by a peripheral [intraplantar (i.pl.)] route of inoculation. Little paralytic disease was induced by high concentrations of virus administered by the intracerebral (i.c.) route, whereas paralytic disease and death were characteristically induced in mice given only a few infectious doses of virus i.c. Disease induced by i.pl. inoculation was dose dependent. Mice frequently survived paralytic disease induced by i. pl. inoculation, with clinical signs often persisting indefinitely; mice surviving i.c. inoculation of high concentrations of virus frequently exhibited chronic nonspecific signs of minor debility. Analysis of the ts 2 virus population indicated that it was composed of a mixture of ts and revertant virions, each with characteristic pathogenic (or nonpatholgnic) propensities, none of which was identical to the original composite ts 2 virus populations. Despite the heterogeneity of the ts 2 virus population, the typical pathogenic pattern of selective pathogenic capicity after i. pl. inoculation at high doses was retained during 11 ocnsecutive passages in suckling mouse brain. ts 2 virus was demonstrated to interfere with the disease-producing capacity of CVS fixed rabies virus when ts 2-CVS mixtures were inoculated i.c. However, attempts to demonstrate a particular propensity for induction in vitro of "autointerference" by ts 2 in serial passage in BHK-21 cell culture inoculated at high multiplicity were unsuccessful.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell J. F., Moore G. J. Effects of high ambient temperature on various stages of rabies virus infection in mice. Infect Immun. 1974 Sep;10(3):510–515. doi: 10.1128/iai.10.3.510-515.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H. F., Koprowski H. Isolation of temperature-sensitive conditional lethal mutants of "fixed" rabies virus. J Virol. 1971 Mar;7(3):295–300. doi: 10.1128/jvi.7.3.295-300.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H. F., Kritchevsky D. Growth and attenuation of rabies virus in cell cultures of reptilian origin. Proc Soc Exp Biol Med. 1972 Apr;139(4):1317–1325. doi: 10.3181/00379727-139-36354. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Wiktor T. J. Plasticity of phenotypic characters of rabies-related viruses: spontaneous variation in the plaque morphology, virulence, and temperature-sensitivity characters of serially propagated Lagos bat and Mokola viruses. J Infect Dis. 1974 Dec;130(6):608–618. doi: 10.1093/infdis/130.6.608. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Wiktor T. J. Temperature-sensitivity characteristics distinguishing substrains of fixed rabies virus: lack of correlation with plague-size markers or virulence for mice. J Infect Dis. 1972 Jun;125(6):637–646. doi: 10.1093/infdis/125.6.637. [DOI] [PubMed] [Google Scholar]
- Crick J., Brown F. An interfering component of rabies virus which contains RNA. J Gen Virol. 1974 Jan;22(1):147–151. doi: 10.1099/0022-1317-22-1-147. [DOI] [PubMed] [Google Scholar]
- Doyle M., Holland J. J. Prophylaxis and immunization in mice by use of virus-free defective T particles to protect against intracerebral infection by vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2105–2108. doi: 10.1073/pnas.70.7.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Doyle M. Attempts to detect homologous autointerference in vivo with influenza virus and vesicular stomatitis virus. Infect Immun. 1973 Apr;7(4):526–531. doi: 10.1128/iai.7.4.526-531.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOPROWSKI H., BLACK J., NELSEN D. J. Studies on chick-embryo-adapted-rabies virus. VI. Further changes in pathogenic properties following prolonged cultivation in the developing chick embryo. J Immunol. 1954 Jan;72(1):94–106. [PubMed] [Google Scholar]
- Kaplan M. M., Wiktor T. J., Koprowski H. Pathogenesis of rabies in immunodeficient mice. J Immunol. 1975 Jun;114(6):1761–1765. [PubMed] [Google Scholar]
- Kawai A., Matsumoto S., Tanabe K. Characterization of rabies viruses recovered from persistently infected BHK cells. Virology. 1975 Oct;67(2):520–533. doi: 10.1016/0042-6822(75)90452-3. [DOI] [PubMed] [Google Scholar]
- Lodmell D. L., Bell J. F., Moore G. J., Raymond G. H. Comparative study of abortive and nonabortive rabies in mice. J Infect Dis. 1969 Jun;119(6):569–580. doi: 10.1093/infdis/119.6.569. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Murphy B. R., Spring S. B., Coleman M. T., Chanock R. M. Temperature sensitive mutants of influenza virus. IX. Genetic and biological characterization of TS-1[E] lesions when transferred to a 1972 (H3N2) influenza A virus. Virology. 1975 Aug;66(2):551–562. doi: 10.1016/0042-6822(75)90227-5. [DOI] [PubMed] [Google Scholar]
- Sedwick W. D., Wiktor T. J. Reproducible plaquing system for rabies, lymphocytic choriomeningitis,k and other ribonucleic acid viruses in BHK-21-13S agarose suspensions. J Virol. 1967 Dec;1(6):1224–1226. doi: 10.1128/jvi.1.6.1224-1226.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R. Pathogenicity and immunogenicity for mice of temperature-sensitive mutants of vesicular stomatitis virus. Infect Immun. 1974 Aug;10(2):309–315. doi: 10.1128/iai.10.2.309-315.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Koprowski H., Rorke L. B. Localized rabies infection in mice. Proc Soc Exp Biol Med. 1972 Jul;140(3):759–764. doi: 10.3181/00379727-140-36547. [DOI] [PubMed] [Google Scholar]
- Yoshino K., Taniguchi S., Arai K. Autointerference of rabies virus in chick embryo fibroblasts. Proc Soc Exp Biol Med. 1966 Nov;123(2):387–392. doi: 10.3181/00379727-123-31496. [DOI] [PubMed] [Google Scholar]


