Abstract
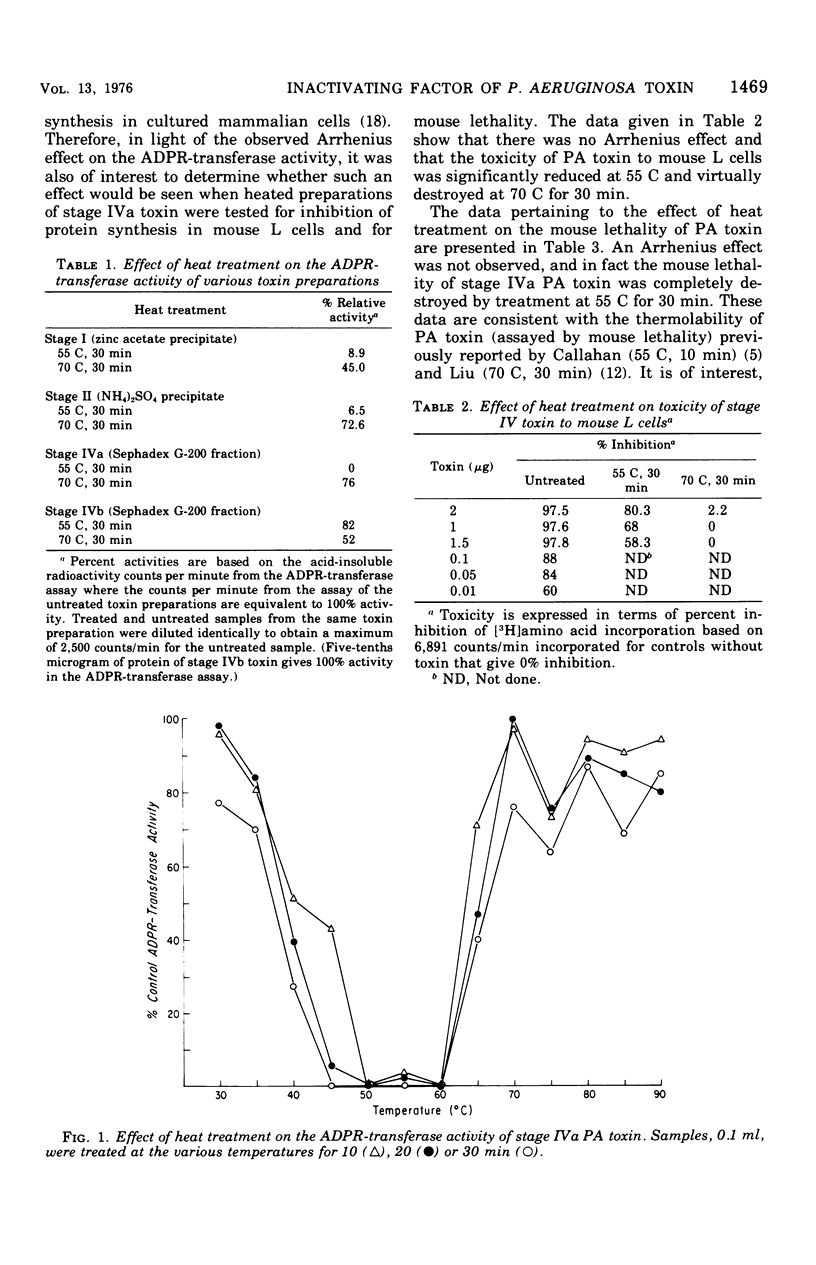
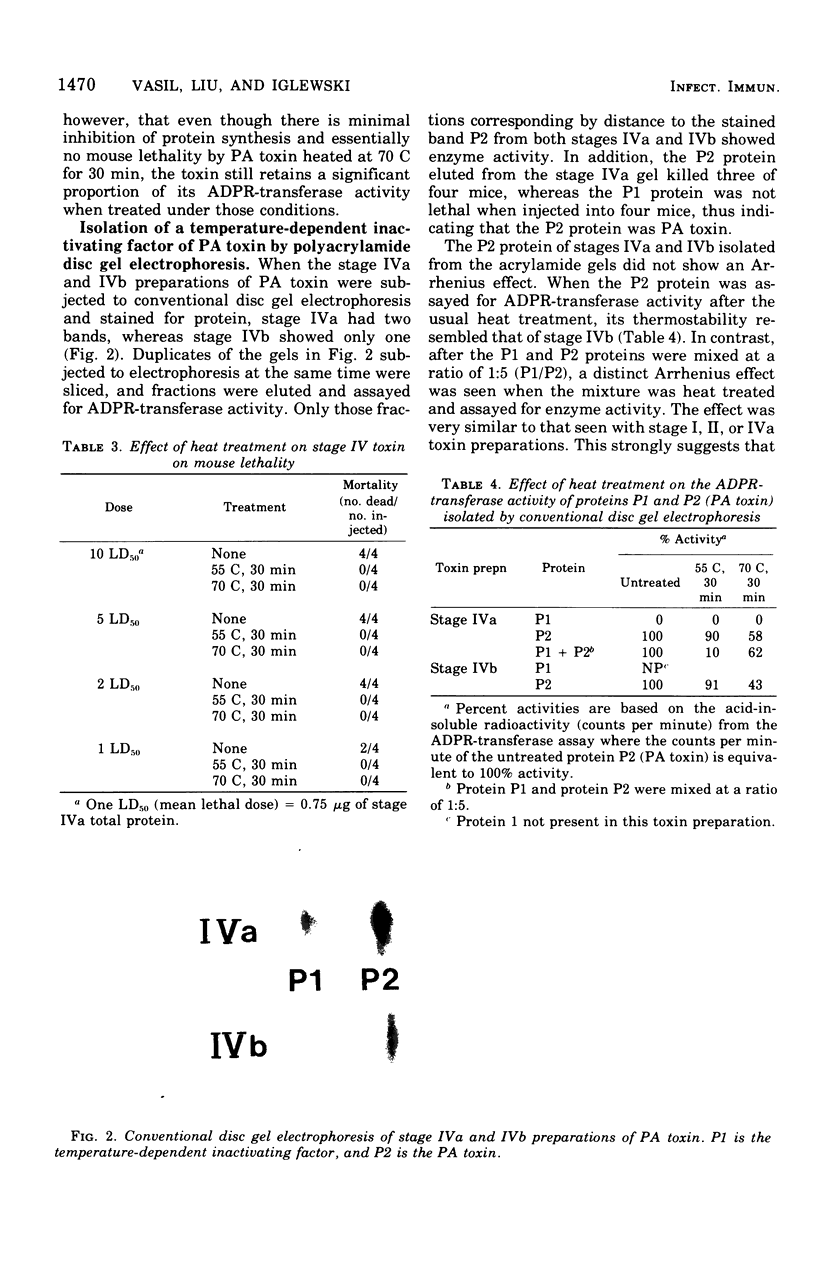
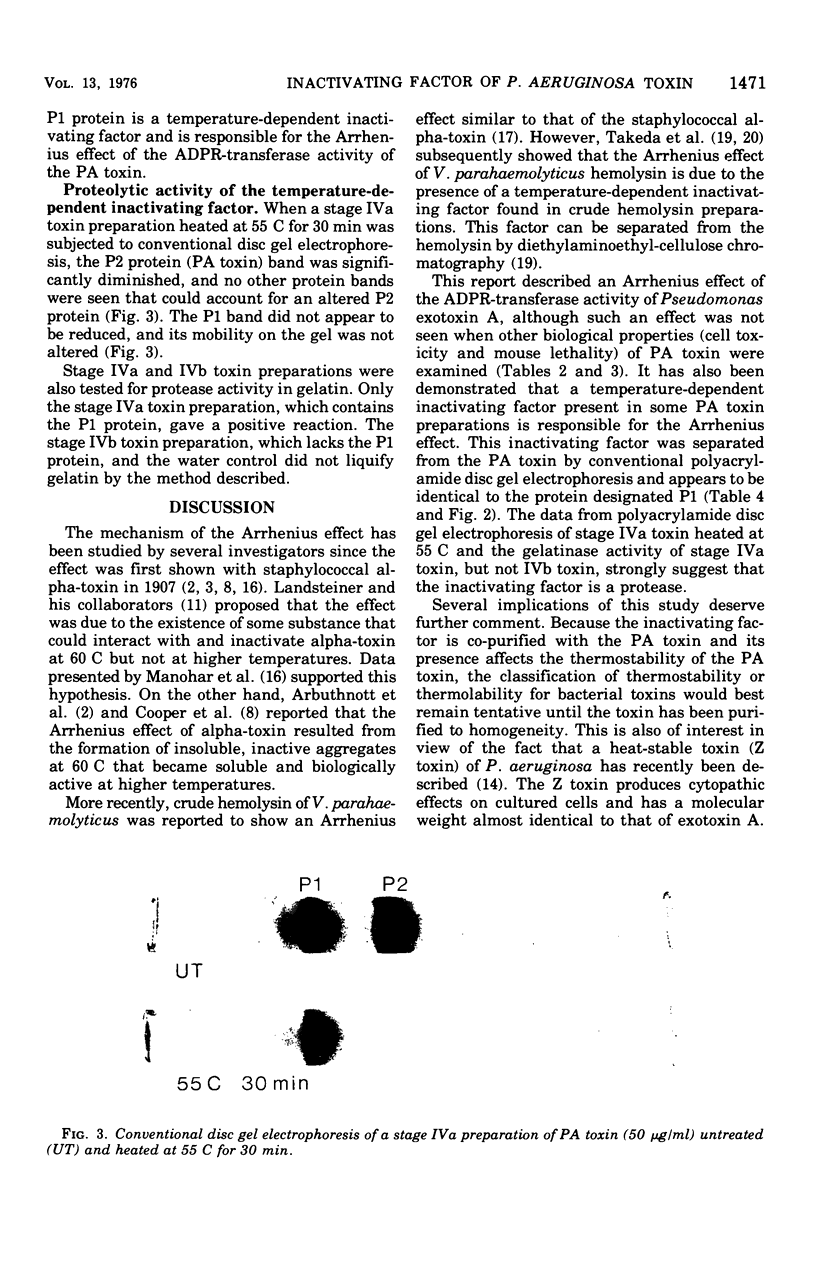
The adenosine diphosphate ribosyl transferase activity of Pseudomonas aeruginosa exotoxin A(PA toxin) was found to be rapidly destroyed by heating at 45 to 60C but not by heating at 70 to 90C (for at least 30 min). This phenomenon has been previously described for other bacterial toxins (staphylococcal alpha-toxin and Vibrio parahaemolyticus hemolysin) and is termed an Arrhenius effect. In contrast, the Arrhenius effect was not seen when the PA toxin was heat-treated as above and tested for cell toxicity or mouse lethality. Although the PA toxin treated at 70C for 30 min retained a significant proportion (is greater than 70%) of its adenosine diphosphate ribosyl transferase activity, the cell toxicity and mouse lethality of the toxin were virtually abolished. A temperature-dependent inactivating factor that has proteolytic activity and is co-purified with the PA toxin was shown to be responsible for the Arrhenius effect. PA toxin separated from the factor by conventional disc gel electrophoresis or PA toxin preparations lacking the factor did not show the Arrhenius effect.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN E. H., SCHWEET R. S. Synthesis of hemoglobin in a cell-free system. I. Properties of the complete system. J Biol Chem. 1962 Mar;237:760–767. [PubMed] [Google Scholar]
- Arbuthnott J. P., Freer J. H., Bernheimer A. W. Physical states of staphylococcal alpha-toxin. J Bacteriol. 1967 Oct;94(4):1170–1177. doi: 10.1128/jb.94.4.1170-1177.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atik M., Liu P. V., Hanson B. A., Amini S., Rosenberg C. F. Pseudomonas exotoxin shock. A preliminary report of studies in dogs. JAMA. 1968 Jul 15;205(3):134–140. doi: 10.1001/jama.205.3.134. [DOI] [PubMed] [Google Scholar]
- Callahan L. T., 3rd Purification and characterization of Pseudomonas aeruginosa exotoxin. Infect Immun. 1974 Jan;9(1):113–118. doi: 10.1128/iai.9.1.113-118.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J., Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J Biol Chem. 1971 Mar 10;246(5):1496–1503. [PubMed] [Google Scholar]
- Cooper L. Z., Madoff M. A., Weinstein L. Heat stability and species range of purified staphylococcal alpha-toxin. J Bacteriol. 1966 May;91(5):1686–1692. doi: 10.1128/jb.91.5.1686-1692.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Rittenberg M. B., Iglewski W. J. Preferential inhibition of growth and protein synthesis in Rous sarcoma virus transformed cells by diphtheria toxin. Virology. 1975 May;65(1):272–275. doi: 10.1016/0042-6822(75)90029-x. [DOI] [PubMed] [Google Scholar]
- Liu P. V. Exotoxins of Pseudomonas aeruginosa. I. Factors that influence the production of exotoxin A. J Infect Dis. 1973 Oct;128(4):506–513. doi: 10.1093/infdis/128.4.506. [DOI] [PubMed] [Google Scholar]
- Liu P. V. Exotoxins of Pseudomonas aeruginosa. I. Factors that influence the production of exotoxin A. J Infect Dis. 1973 Oct;128(4):506–513. doi: 10.1093/infdis/128.4.506. [DOI] [PubMed] [Google Scholar]
- Ludovici P. P., Roinestad F. A., Wong F. S. Column chromatography and cell culture assay of Pseudomonas aeruginosa toxin Z preparations. Appl Microbiol. 1975 Aug;30(2):293–297. doi: 10.1128/am.30.2.293-297.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar M., Kumar S., Lindorfer R. K. Heat reactivation of the alpha-hemolytic, dermonecrotic, lethal activities of crude and purified staphylococcal alpha-toxin. J Bacteriol. 1966 May;91(5):1681–1685. doi: 10.1128/jb.91.5.1681-1685.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwatani T., Takeda Y., Sakurai J., Yoshihara A., Taga S. Effect of heat (Arrhenius effect) on crude hemolysin of Vibrio parahaemolyticus. Infect Immun. 1972 Dec;6(6):1031–1033. doi: 10.1128/iai.6.6.1031-1033.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovskis O. R., Gordon F. B. Pseudomonas aeruginosa exotoxin: effect on cell cultures. J Infect Dis. 1972 Jun;125(6):631–636. doi: 10.1093/infdis/125.6.631. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Hori Y., Miwatani T. Demonstration of a temperature-dependent inactivating factor of the thermostable direct hemolysin in Vibrio parahaemolyticus. Infect Immun. 1974 Jul;10(1):6–10. doi: 10.1128/iai.10.1.6-10.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Hori Y., Taga S., Sakurai J., Miwatani T. Characterization of the temperature-dependent inactivating factor of the thermostable direct hemolysin in Vibrio parahaemolyticus. Infect Immun. 1975 Sep;12(3):449–454. doi: 10.1128/iai.12.3.449-454.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]




