Abstract
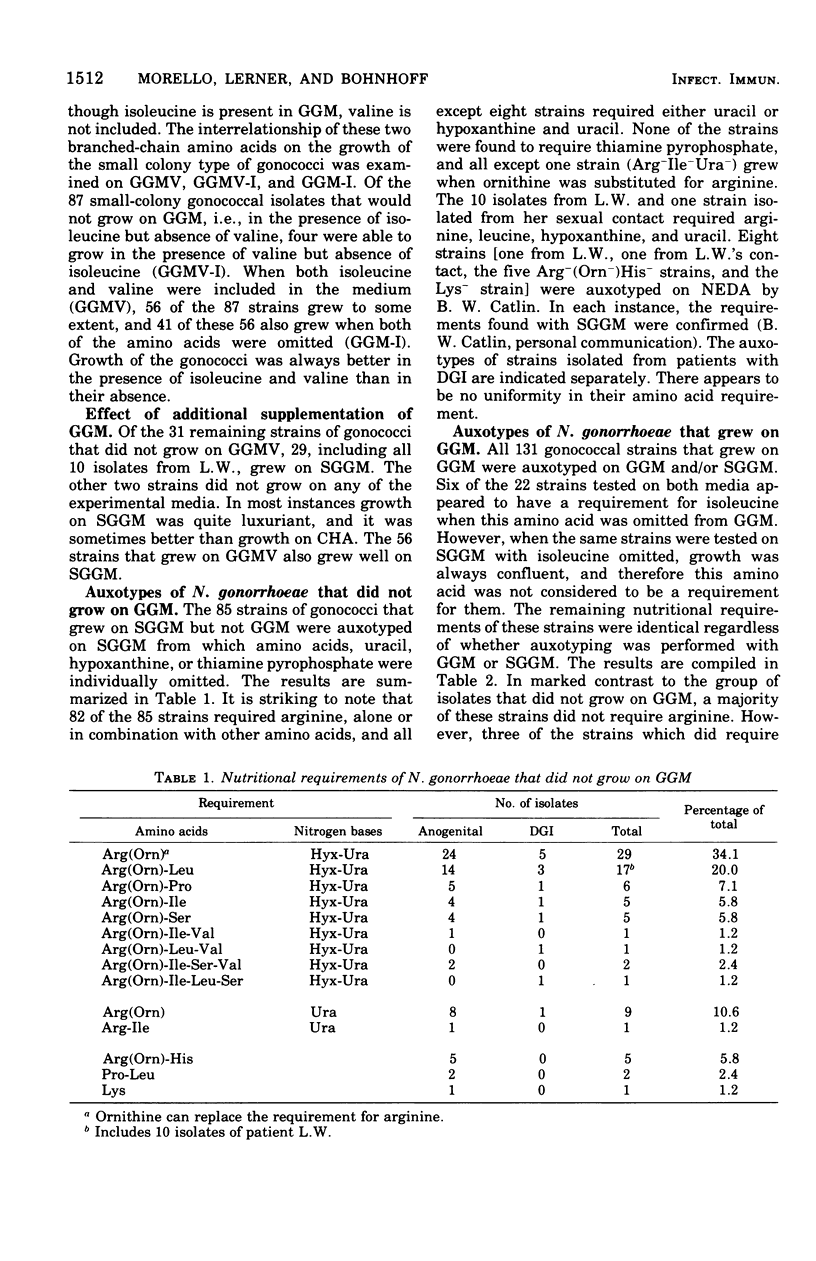
Approximately 6% of 1,200 clinical isolates of Neisseria gonorrhoeae were atypical because they produced smaller than normal colonies on conventioal chocolate agar and fermented glucose weakly. Auxotyping studies indicated that these atypical strains required for growth arginine, uracil, and, in most instances, hypoxanthine. In addition, all of them were susceptible to 0.02 U of penicillin/ml. None of the normal colony isolates, including those susceptible to the same low concentration of penicillin, had the same nutritional characteristics. Atypical strains comprised almost half of the isolates from disseminated infections, but only 5% of those from localized infections. Auxotyping was used to identify the contact of a patient who became reinfected nine times with an atypical gonoccal strain. In addition to its usefulness in such epidemiological studies, this technique has enabled us to distinguish a subgroup of gonococci with apparent increased pathogenicity.
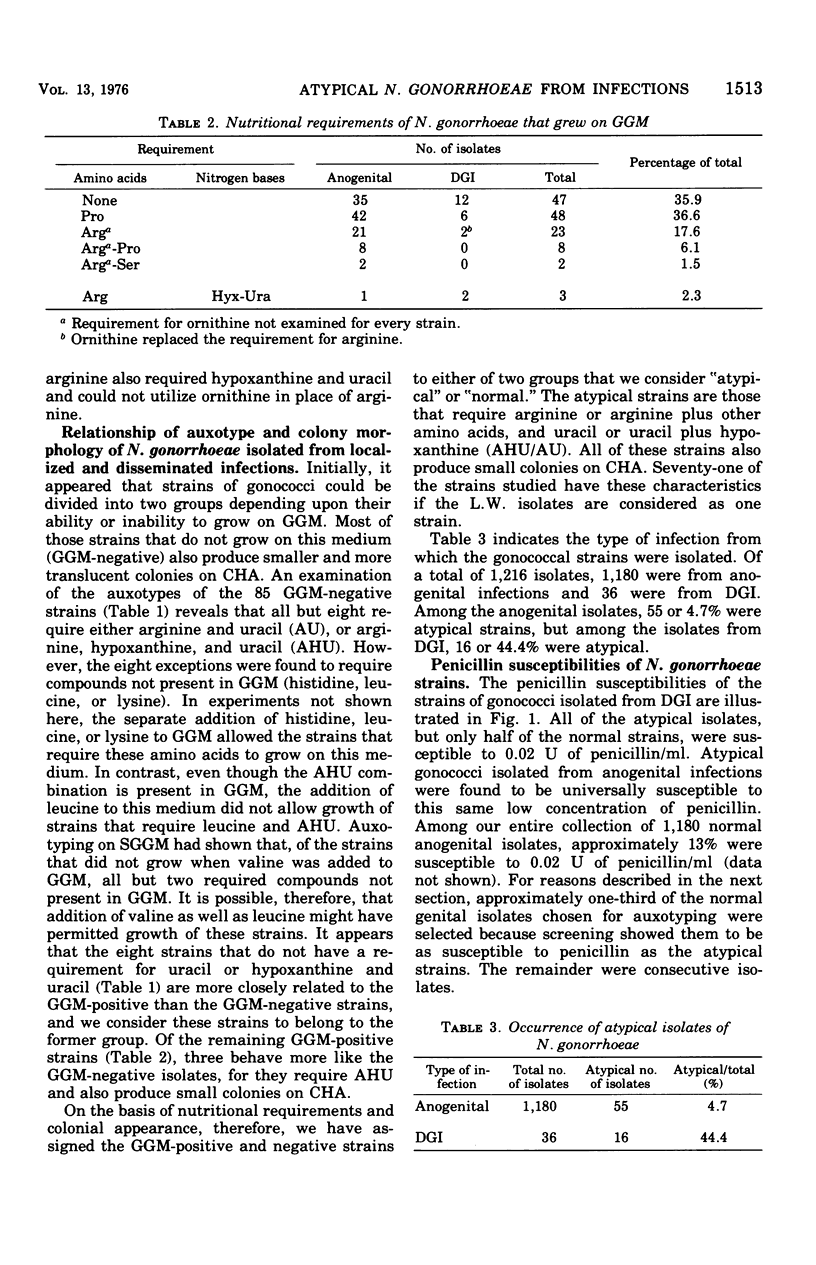
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carifo K., Catlin B. W. Neisseria gonorrhoeae auxotyping: differentiation of clinical isolates based on growth responses on chemically defined media. Appl Microbiol. 1973 Sep;26(3):223–230. doi: 10.1128/am.26.3.223-230.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Holmes K. K. Disseminated gonococcal infections caused by Neisseria gonorrhoeae with unique nutritional requirements. J Infect Dis. 1975 Aug;132(2):204–208. doi: 10.1093/infdis/132.2.204. [DOI] [PubMed] [Google Scholar]
- La Scolea L. J., Jr, Young F. E. Development of a defined minimal medium for the growth of Neisseria gonorrhoeae. Appl Microbiol. 1974 Jul;28(1):70–76. doi: 10.1128/am.28.1.70-76.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. 3. MAP ORDER OF THE STRUCTURAL GENES AND OPERATOR GENES. J Bacteriol. 1965 Mar;89:661–664. doi: 10.1128/jb.89.3.661-664.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. I. GENETIC DEREPRESSION OF ENZYME FORMATION. J Bacteriol. 1964 Mar;87:566–573. doi: 10.1128/jb.87.3.566-573.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiffler P. W., Lerner S. A., Bohnhoff M., Morello J. A. Plasmid deoxyribonucleic acid in clinical isolates of Neisseria gonorrhoeae. J Bacteriol. 1975 Jun;122(3):1293–1300. doi: 10.1128/jb.122.3.1293-1300.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walstad D. L., Reitz R. C., Sparling P. F. Growth inhibition among strains of Neisseria gonorrhoeae due to production of inhibitory free fatty acids and lysophosphatidylethanolamine: absence of bacteriocins. Infect Immun. 1974 Sep;10(3):481–488. doi: 10.1128/iai.10.3.481-488.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



