Abstract
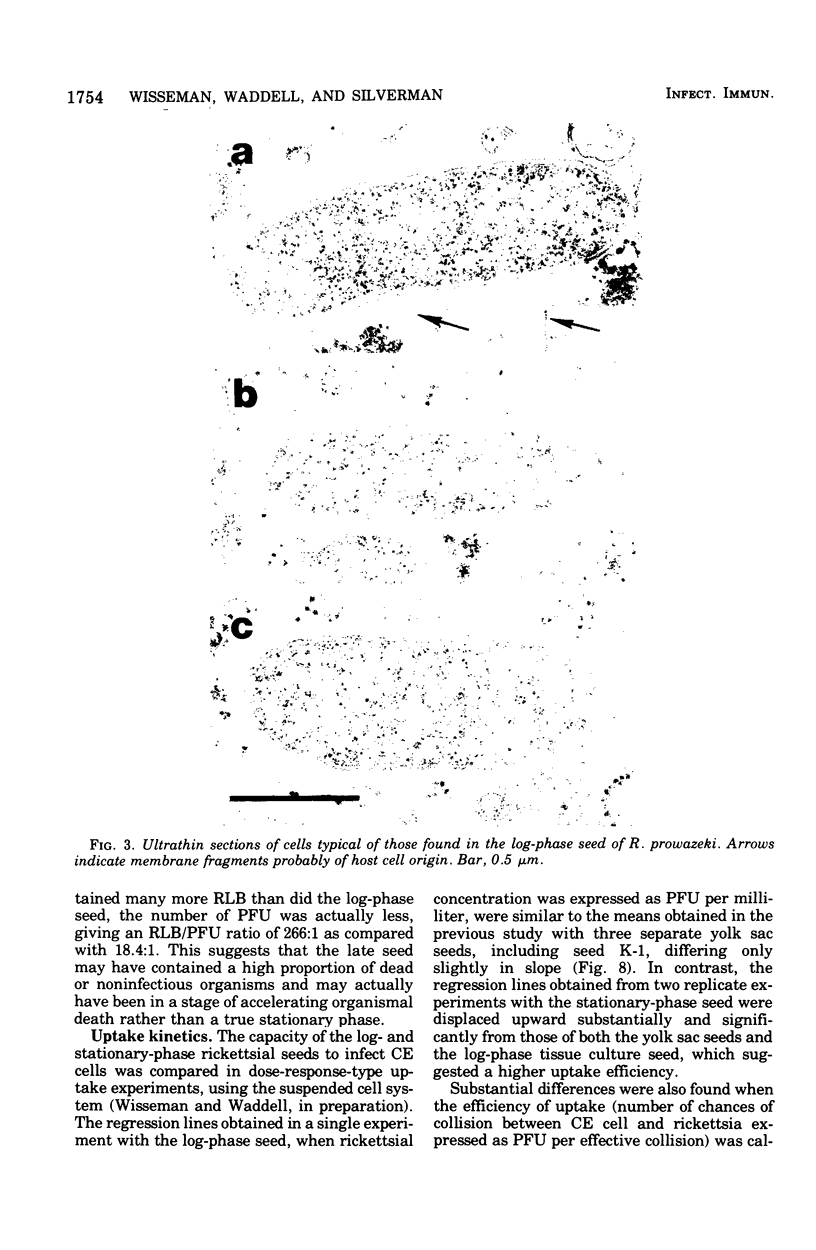
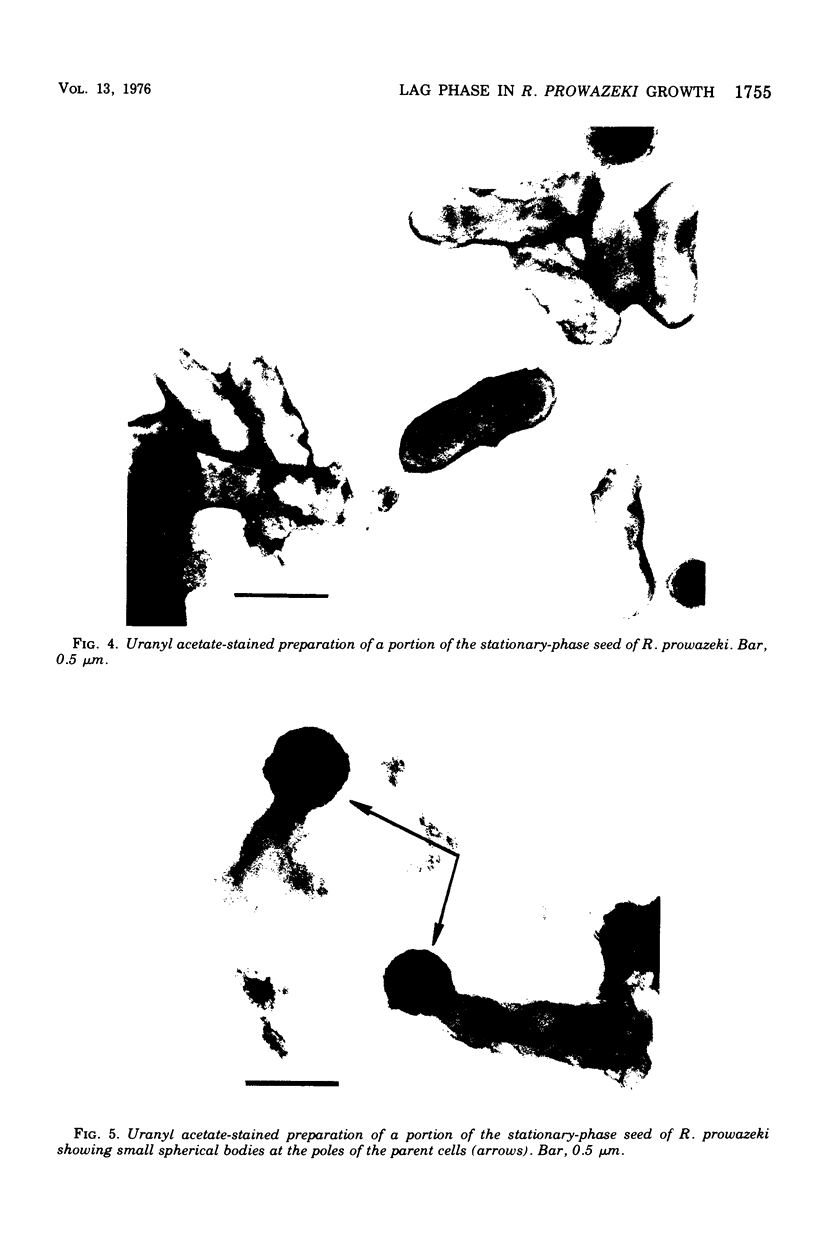
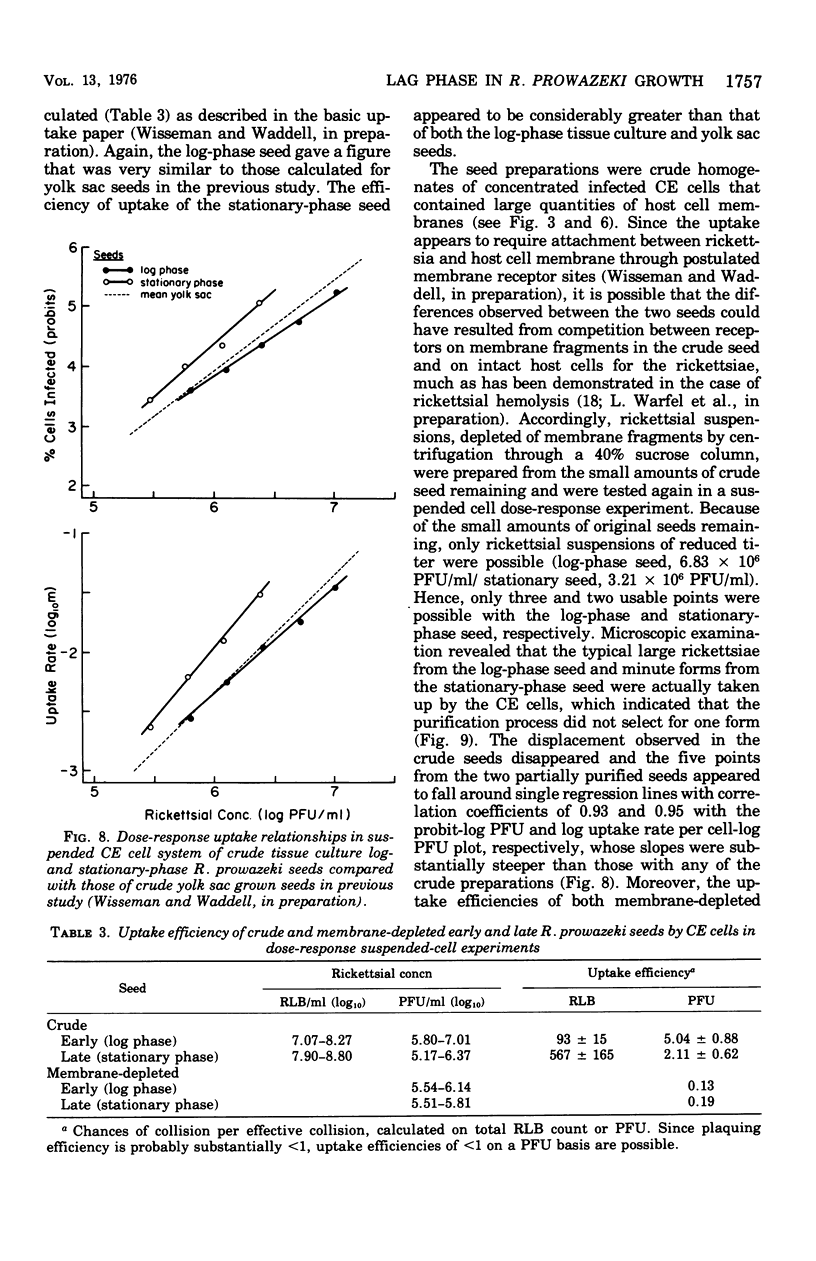
Two Rickettsia prowazeki seeds, an "early" seed in the logarithmic or exponential growth phase and a "late" seed in the stationary or possibly early decline phase, were prepared in chicken embryo (CE) cell cultures and compared with respect to morphology and infection cycle in CE cells in culture. Differences in size and ultrastructure of the organisms in the two seeds were similar to those seen in other gram-negative bacteria at comparable stages to growth. Vacuolar structures, rare in log-phase organisms, were common in stationary-phase organisms. Minute spherical forms reminiscent of minicells were seen in the stationary-phase preparations. In quantitative uptake experiments, organisms, typical in size and morphology of each preparation, had comparable capacity per plaque-forming unit to penetrate into CE cells in suspension when the seeds had been depleted of host cell membrane fragments and other debris. This suggests that host cell fragments, presumably of membrane origin, competitively inhibit rickettsial uptake by intact CE cells. Organisms of the log-phase organisms displayed a lag phase of about 7.5 h, during which they enlarged and increased in intensity of staining, before entering the log phase of growth.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. R., Hopps H. E., Barile M. F., Bernheim B. C. Comparison of the ultrastructure of several rickettsiae, ornithosis virus, and Mycoplasma in tissue culture. J Bacteriol. 1965 Nov;90(5):1387–1404. doi: 10.1128/jb.90.5.1387-1404.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. A., Park R. W. Changes in morphology and cell wall structure that occur during growth of Vibrio sp. NCTC4716 in batch culture. J Gen Microbiol. 1975 Jan;86(1):12–28. doi: 10.1099/00221287-86-1-12. [DOI] [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Septum formation in Escherichia coli: characterization of septal structure and the effects of antibiotics on cell division. J Bacteriol. 1974 Jul;119(1):303–324. doi: 10.1128/jb.119.1.303-324.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. C., Curtiss R., 3rd Production, properties and utility of bacterial minicells. Curr Top Microbiol Immunol. 1975;69:1–84. doi: 10.1007/978-3-642-50112-8_1. [DOI] [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- Harvey R. J. Regulation of ribosomal protein synthesis in Escherichia coli. J Bacteriol. 1970 Feb;101(2):574–583. doi: 10.1128/jb.101.2.574-583.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman R., Fiset P. Method for counting Rickettsiae and Chlamydiae in purified suspensions. J Bacteriol. 1968 Jan;95(1):259–261. doi: 10.1128/jb.95.1.259-261.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Boese J. L., Wisseman C. L., Jr Ultrastructural studies of Rickettsia prowazeki from louse midgut cells to feces: search for "dormant" forms. Infect Immun. 1974 Jul;10(1):257–263. doi: 10.1128/iai.10.1.257-263.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R. M., Frazier W. C. Physiological Characteristics of Lactic Acid Bacteria Near the Maximum Growth Temperature: I. Growth and Acid Production. J Bacteriol. 1941 Oct;42(4):479–499. doi: 10.1128/jb.42.4.479-499.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wike D. A., Tallent G., Peacock M. G., Ormsbee R. A. Studies of the rickettsial plaque assay technique. Infect Immun. 1972 May;5(5):715–722. doi: 10.1128/iai.5.5.715-722.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Ramm L. E. Adsorption of typhus rickettsiae to ghosts of sheep erythrocytes. Infect Immun. 1975 Jun;11(6):1244–1251. doi: 10.1128/iai.11.6.1244-1251.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. D. In vitro studies on rickettsia-host cell interactions: intracellular growth cycle of virulent and attenuated Rickettsia prowazeki in chicken embryo cells in slide chamber cultures. Infect Immun. 1975 Jun;11(6):1391–1404. doi: 10.1128/iai.11.6.1391-1401.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. D., Walsh W. T. In vitro studies of the action of antibiotics on Rickettsia prowazeki by two basic methods of cell culture. J Infect Dis. 1974 Dec;130(6):564–574. doi: 10.1093/infdis/130.6.564. [DOI] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. D., Walsh W. T. Mechanisms of immunity in typhus infections. IV. Failure of chicken embryo cells in culture to restrict growth of antibody-sensitized Rickettsia prowazeki. Infect Immun. 1974 Mar;9(3):571–575. doi: 10.1128/iai.9.3.571-575.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]