Abstract
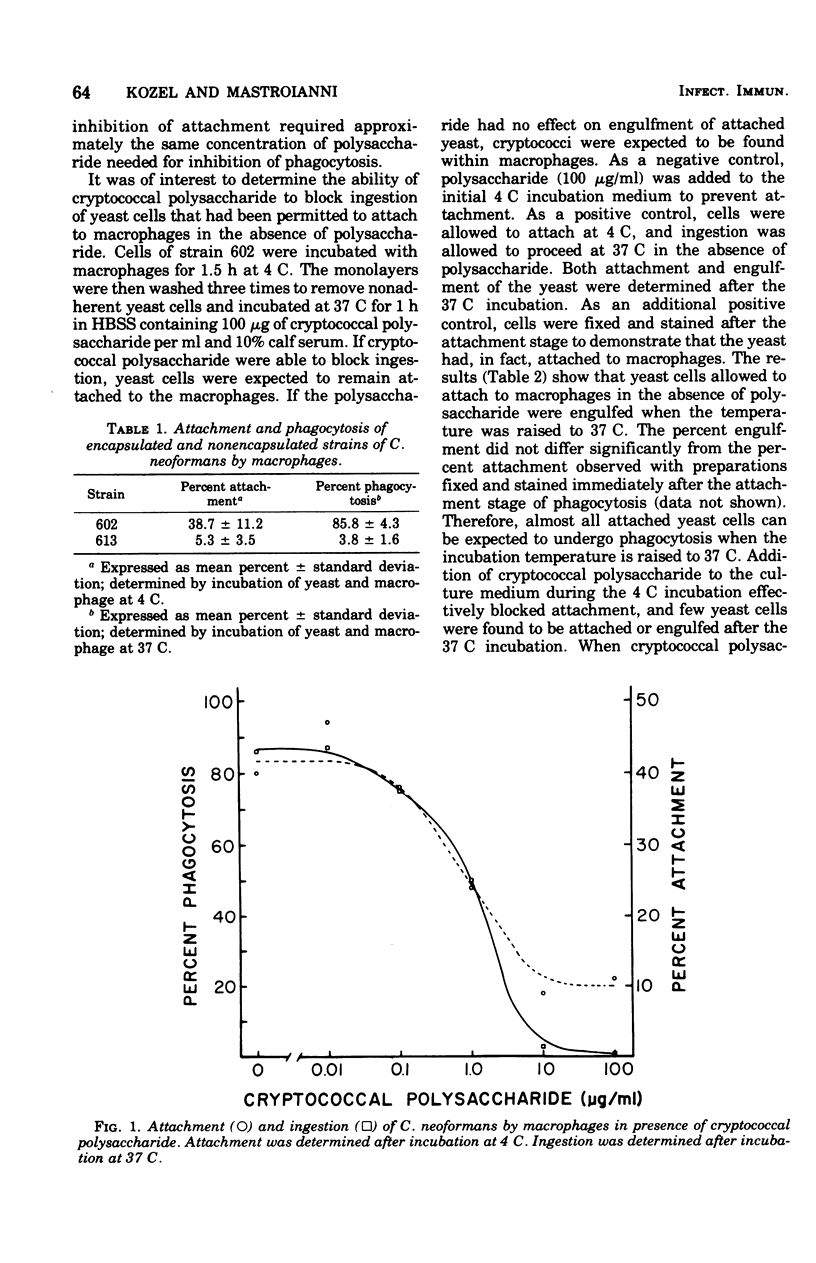
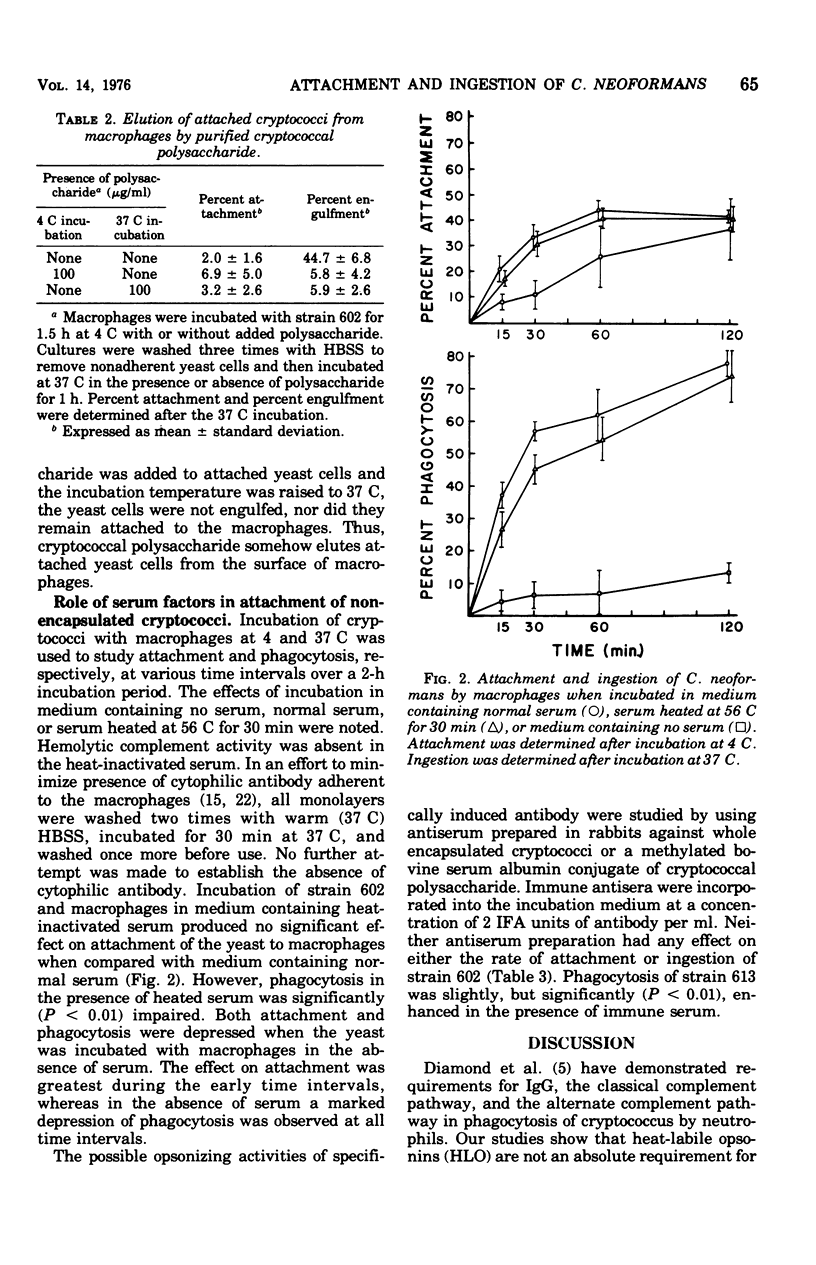
The effects of cryptococcal polysaccharide and selected serum factors on (i) the attachment of Cryptococcus neoformans to macrophages and (ii) the subsequent ingestion of yeast cells by the macrophages were investigated. Percent attachment was measured after incubation of yeast cells with macrophages at 4 C. Percent engulfment was determined after incubation of yeast cells and macrophages at 37 C. Nonencapsulated yeast cells readily attached to macrophages at the low temperature and were engulfed at a high rate at 37 C, whereas encapsulated yeast cells attached to macrophages at low rates and were engulfed at low rates. Addition of varying doses of purified cryptococcal polysaccharide to nonencapsulated yeast cells inhibited attachment at approximately the same concentration of polysaccharide required for inhibition of engulfment. Nonencapsulated yeast cells that attached to macrophages at 4 C were eluted from the macrophages by addition of purified cryptococcal polysaccharide to the incubation medium. Heat-labile opsonins were not required for attachment of yeast cells to macrophages, but they were necessary for maximal initial rates of phagocytosis. Heat-stable components of serum facilitated attachment of cryptococci, but their most important function appeared to be triggering the ingestion of attached yeast. Specific antiserum had no effect on the attachment and engulfment of nonencapsulated cryptococci, and the antiserum produced only a small enhancement of the engulfment of encapsulated cryptococci.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahams I. Further studies on acquired resistance to murine cryptococcosis: enhancing effect of Bordetella pertussis. J Immunol. 1966 Mar;96(3):525–529. [PubMed] [Google Scholar]
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulmer G. S., Sans M. D. Cryptococcus neoformans. 3. Inhibition of phagocytosis. J Bacteriol. 1968 Jan;95(1):5–8. doi: 10.1128/jb.95.1.5-8.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., May J. E., Kane M. A., Frank M. M., Bennett J. E. The role of the classical and alternate complement pathways in host defenses against Cryptococcus neoformans infection. J Immunol. 1974 Jun;112(6):2260–2270. [PubMed] [Google Scholar]
- Diamond R. D., Root R. K., Bennett J. E. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J Infect Dis. 1972 Apr;125(4):367–376. doi: 10.1093/infdis/125.4.367. [DOI] [PubMed] [Google Scholar]
- Goren M. B. Experimental murine cryptococcosis: effect of hyperimmunization to capsular polysaccharide. J Immunol. 1967 May;98(5):914–922. [PubMed] [Google Scholar]
- Goren M. B., Warren J. Immunofluorescence studies of reactions at the Cryptococcal capsule. J Infect Dis. 1968 Apr;118(2):215–229. doi: 10.1093/infdis/118.2.215. [DOI] [PubMed] [Google Scholar]
- Graybill J. R., Alford R. H. Cell-mediated immunity in Cryptococcosis. Cell Immunol. 1974 Oct;14(1):12–21. doi: 10.1016/0008-8749(74)90164-6. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Cazin J., Jr Immune response to Cryptococcus neoformans soluble polysaccharide. I. Serological assay for antigen and antibody. Infect Immun. 1972 Jan;5(1):35–41. doi: 10.1128/iai.5.1.35-41.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Cazin J., Jr Induction of humoral antibody response by soluble polysaccharide of Cryptococcus neoformans. Mycopathol Mycol Appl. 1974 Oct 15;54(1):21–30. doi: 10.1007/BF02055969. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Cazin J. Nonencapsulated Variant of Cryptococcus neoformans I. Virulence Studies and Characterization of Soluble Polysaccharide. Infect Immun. 1971 Feb;3(2):287–294. doi: 10.1128/iai.3.2.287-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTMAN M. L., TSUBURA E. Effect of degree of encapsulation upon virulence of Cryptococcus neoformans. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:773–777. doi: 10.3181/00379727-101-25090. [DOI] [PubMed] [Google Scholar]
- Mitchell T. G., Friedman L. In vitro phagocytosis and intracellular fate of variously encapsulated strains of Cryptococcus neoformans. Infect Immun. 1972 Apr;5(4):491–498. doi: 10.1128/iai.5.4.491-498.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M., De Stefano M. J. Particle recognition by cultivated macrophages. J Immunol. 1973 Mar;110(3):695–701. [PubMed] [Google Scholar]
- Rabinovitch M. Studies on the immunoglobulins which stimulate the ingestion of glutaraldehyde-treated red cells attached to macrophages. J Immunol. 1967 Dec;99(6):1115–1120. [PubMed] [Google Scholar]
- Rabinovitch M. The dissociation of the attachment and ingestion phases of phagocytosis by macrophages. Exp Cell Res. 1967 Apr;46(1):19–28. doi: 10.1016/0014-4827(67)90405-3. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. Quantitative studies of phagocytosis. Kinetic effects of cations and heat-labile opsonin. J Cell Biol. 1973 Aug;58(2):346–356. doi: 10.1083/jcb.58.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacker J. R., Farhi F., Bulmer G. S. Intracellular fate of Cryptococcus neoformans. Infect Immun. 1972 Aug;6(2):162–167. doi: 10.1128/iai.6.2.162-167.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizard I. R. Elution of macrophage-bound immunoglobulins by temperature changes in vitro. Immunology. 1972 Jan;22(1):69–73. [PMC free article] [PubMed] [Google Scholar]


