Abstract
Seminal plasma HIV-1 RNA level is an important determinant of the risk of HIV-1 sexual transmission. We investigated potential associations between seminal plasma cytokine levels and viral concentration in the seminal plasma of HIV-1-infected men. This was a prospective, observational study of paired blood and semen samples from 18 HIV-1 chronically infected men off antiretroviral therapy. HIV-1 RNA levels and cytokine levels in seminal plasma and blood plasma were measured and analyzed using simple linear regressions to screen for associations between cytokines and seminal plasma HIV-1 levels. Forward stepwise regression was performed to construct the final multivariate model. The median HIV-1 RNA concentrations were 4.42 log10 copies/ml (IQR 2.98, 4.70) and 2.96 log10 copies/ml (IQR 2, 4.18) in blood and seminal plasma, respectively. In stepwise multivariate linear regression analysis, blood HIV-1 RNA level (p<0.0001) was most strongly associated with seminal plasma HIV-1 RNA level. After controlling for blood HIV-1 RNA level, seminal plasma HIV-1 RNA level was positively associated with interferon (IFN)-γ (p=0.03) and interleukin (IL)-17 (p=0.03) and negatively associated with IL-5 (p=0.0007) in seminal plasma. In addition to blood HIV-1 RNA level, cytokine profiles in the male genital tract are associated with HIV-1 RNA levels in semen. The Th1 and Th17 cytokines IFN-γ and IL-17 are associated with increased seminal plasma HIV-1 RNA, while the Th2 cytokine IL-5 is associated with decreased seminal plasma HIV-1 RNA. These results support the importance of genital tract immunomodulation in HIV-1 transmission.
Introduction
As approximately 85% of HIV-1 infections worldwide are acquired through sexual transmission1 it is of paramount importance to understand factors associated with that risk. It is known that the HIV-1 RNA level in semen is an important determinant of sexual transmission.2,3 Although antiretroviral therapy (ART) reduces blood HIV-1 RNA levels and concurrently seminal plasma levels in infected men, 6–48% of treated men with undetectable blood levels continue to exhibit intermittently detectable virus in seminal plasma.4–6 Furthermore, while blood HIV-1 RNA level has been demonstrated in multiple studies to be strongly correlated with semen HIV-1 RNA level,2,7 other factors also influence the level of HIV-1 RNA in semen. Sexually transmitted infections such as genital herpes,3,8 gonorrhea,9,10 and nongonococcal urethritis,10–12 as well as genital tract cytomegalovirus (CMV) reactivation,13,14 are associated with increased genital tract inflammation and increased seminal plasma HIV-1 RNA levels.
Consistent with a role for local inflammation influencing HIV-1 RNA levels in seminal plasma, several recent studies have shown correlations between increased levels of various proinflammatory cytokines and virus levels in seminal plasma. Berlier et al. described a positive correlation with interleukin (IL)-1β,15 while Storey et al. described a positive correlation with RANTES,16 and Sheth et al. showed positive correlations with IL-6, IL-8, IL-12, and interferon (IFN)-γ.17 Furthermore, Lisco et al. recently suggested that HIV-1 infection causes a “reprogrammed cytokine network” in the seminal plasma with elevated levels of several proinflammatory cytokines including IL-1α, IL-1β, IL-6, and IL-8, which do not correlate with blood plasma cytokine levels.18 This suggests that cytokine expression is compartmentalized and that seminal plasma cytokines are likely produced within the male genital tract.
In this pilot study, we examine the relationship between seminal plasma HIV-1 RNA levels and a panel of 17 blood and seminal plasma cytokines in a group of 18 HIV-1-infected men with viremia >200 copies/ml. We hypothesized that higher levels of proinflammatory cytokines in seminal plasma would be independently associated with higher seminal plasma HIV-1 RNA levels.
Materials and Methods
Study subjects and specimens
All participants provided written informed consent under a UCLA Institutional Review Board-approved protocol. The study population was composed of 18 HIV-1-infected adult men from the Los Angeles area, who were not on ART, with blood plasma HIV-1 RNA levels ≥5,000 copies/ml within the past 3–6 months by self-report, and who were willing to provide blood, urine, and semen samples. These samples were collected during a single study visit. Due to our intention to study men with detectable viremia, men with <200 HIV-1 RNA copies/ml in blood plasma at the time of the study visit were excluded.
Clinical screening
Blood and urine samples were transported to Foundation Labs and processed within 24 h for routine clinical testing for blood CD4+ T cell counts and urine Neisseria gonorrhea and Chlamydia trachomatis (APTIMA Combo 2, Hologic Gen-Probe, San Diego, CA).
HIV-1 quantification in blood and seminal plasma and assay validation methodology
Plasma was isolated from blood and semen samples by centrifugation and aliquots were stored at −80°C prior to nucleic acid extraction. The Biomerieux NucliSENS Easy Q HIV-1 v1.1 and v2 (Durham, NC) assays were used for this study. Nucleic acid extractions were performed using the NucliSENS miniMAG extraction system, as per the manufacturer's protocol. Using the manufacturer's recommendations as a framework, the final protocol for nucleic acid isolation and subsequent real-time detection of HIV-1 RNA for both blood plasma and seminal plasma was derived following optimization of the assay.
Briefly, assay optimization was performed using matched blood and semen samples from a separate validation cohort of HIV-infected and uninfected donors (n=11). Samples were provided on two separate visits and evaluated for the degree of reproducibility for serial HIV-1 RNA measurements in both blood and seminal plasma as well as for the correlation between blood and seminal plasma HIV-1 RNA levels. Blood and seminal plasma HIV-1 RNA were found to be relatively stable between two samplings ranging from 42 to 303 days apart (median 62 days). Seminal plasma (Fig. 1A) and blood plasma (Fig. 1B) levels were highly correlated between samplings (R2=0.79 and 0.98, respectively). Comparison of the means of both samplings for semen and blood plasma demonstrated a correlation between these compartments (R2=0.43, Fig. 1C).
FIG. 1.
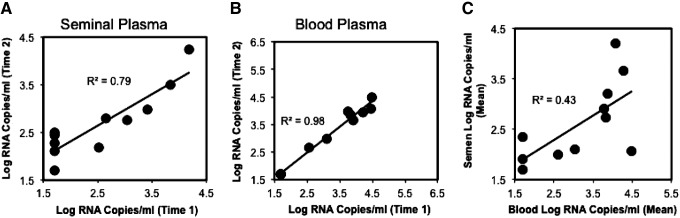
Reproducibility of serial HIV-1 RNA measurements in blood and seminal plasma and relationship of blood and seminal plasma HIV-1 RNA concentrations in a validation cohort of 11 HIV-1 infected men off antiretroviral therapy (ART). Concentrations of HIV-1 RNA from serial measurements utilizing the assay optimization cohort are plotted. Undetectable values (<1.7 log10 RNA copies/ml) were assigned a value of 1.7 log10 RNA copies/ml. (A) The mean values for seminal plasma HIV-1 RNA concentrations (log10 RNA copies/ml) across two visits for each person in the assay optimization cohort are plotted. (B) The mean values for blood plasma HIV-1 RNA concentrations (log10 RNA copies/ml) across two visits for each person in the assay optimization cohort are plotted. (C) The correlation between blood and seminal plasma HIV-1 RNA levels was significant (p=0.03).
Additionally, to verify that the optimized assay protocol accurately measured seminal plasma HIV-1 RNA levels, we conducted a series of “virus spiking” experiments using seronegative seminal plasma spiked with a serial dilution HIVNL4-3 (10,000 pg/ml–1 pg/ml). We found that the measured seminal plasma HIV-1 RNA level directly correlated with input virus (R2=0.94), indicating no measurable interference from seminal plasma protein (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/aid). During the process of assay optimization we also noted that in contrast to the manufacturer's protocol, in order to achieve reproducible and valid results from seminal plasma, the silica/nucleic acid mixtures needed to be resuspended at each extraction washing step to prevent clumping.
Cytokine quantification
Blood and seminal plasma were analyzed for concentrations of IL-1α, IL-1Rα, IL1-β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12 P70, IL-17, IFN-γ, macrophage inflammatory protein (MIP)-1β, RANTES, and tumor growth factor (TGF)-β using Luminex assays as per the manufacturer's protocol (Millipore). As done in previous studies,19,20 raw fluorescence intensities (FIs) were used as the final readout because many of the observed values were outside the range of the standard curve.
Statistical analyses
To achieve symmetric distributions, blood and seminal plasma HIV-1 RNA and cytokine values were log10 transformed. Initial screening of all cytokines for correlations with seminal plasma HIV-1 RNA levels was performed using linear regression models. The Pearson correlation was used to compare each cytokine level with seminal plasma HIV-RNA level; additionally, the partial correlation of each cytokine, when the blood plasma HIV-1 RNA level was included, was also calculated. If the partial p-value for a given cytokine was <0.20, the cytokine was included as a candidate in the multivariate model. Forward stepwise regression was used to construct the final model with seminal plasma cytokines and blood plasma HIV-1 RNA level included as candidate covariates. JMP10.0 software (SAS Institute, Cary, NC) was used with minimum AICc (Akaike Information Criterion, corrected) used as a stopping rule. Significance for all statistical tests was defined as p≤0.05.
To assess for model overfitting due to the relatively large number of variables for the small number of observations, we performed a modeling simulation to examine the likelihood that the high R2 observed for our model was due to chance. Each iteration of the simulation randomly permuted the seminal plasma cytokines while maintaining the linked blood and seminal plasma HIV-1 RNA level for each subject, permitting an assessment of the probability of selection bias in this model. Once the seminal plasma cytokines were randomly permuted, the same procedure as above was followed for model construction.
A subanalysis was performed on the group of men with atypically high ratios of seminal plasma versus blood plasma HIV-1 RNA levels, defined as seminal/blood plasma HIV-1 RNA ≥0.5, given that seminal plasma HIV-1 RNA levels are typically ∼10% of those in blood plasma.17,21 Median levels of blood and seminal plasma cytokines were compared between men with and without atypically high ratios of seminal versus blood plasma HIV-1 RNA levels using the Wilcoxon test. Finally, a separate subanalysis was performed to compare seminal:blood plasma cytokine ratios in men with detectable seminal plasma HIV-1 RNA levels (defined as >100 copies/ml) and those with undetectable levels (≤100 copies/ml), also using the Wilcoxon test.
Results
Characteristics of study subjects
Of 31 men screened, 18 who reported not taking ART were enrolled. Thirteen were excluded for the following reasons: 11 with viremia <200 HIV-1 RNA copies/ml, one with active chlamydia infection, and one who was unable to provide semen. The median age was 45 years (IQR 35.5, 50.5), with racial distribution of 14 African American, 3 white, and 1 undeclared. The median blood CD4+ T cell count was 478 cells/mm3 (IQR 285, 603) and the median blood plasma viremia was 4.4 log10 HIV-1 RNA copies/ml (IQR 2.9, 4.7). The median seminal plasma HIV-1 RNA level was 2.97 log10 HIV-1 RNA copies/ml (IQR 2, 4.2) with 12/18 subjects (66.7%) having detectable seminal plasma HIV-1 RNA (>100 copies/ml).
Relationship between blood and seminal plasma HIV-1 levels
In our cohort of 18 men, comparison of blood plasma to seminal plasma HIV-1 RNA levels showed a significant correlation (R2=0.44), almost identical to that found in our validation cohort of 11 men (R2=0.43). While there was a linear relationship between log10 HIV-1 RNA levels in these two compartments, the concentration in blood was approximately 30-fold higher than in seminal plasma, at 4.42 log10 RNA copies/ml (IQR 2.98, 4.70) versus 2.96 log10 RNA copies/ml (IQR 2, 4.18), respectively.
Seminal and blood plasma cytokine levels
Compared to previously published data on seminal plasma cytokine concentrations in healthy men,22 our HIV-1-infected participants had higher levels of IL-1α, IL-6, and RANTES, but much lower levels of IL-17 and TGF-β (Table 1). It should be noted that our methodology for measuring seminal cytokines is different from that utilized by Politch et al.,22 so direct numerical comparisons should be interpreted with caution. Blood plasma cytokine levels are detailed in Table 2.
Table 1.
Median Concentrations and Interquartile Ranges of Seminal Plasma Cytokines
| Semen cytokine | Median concentration (pg/ml) | IQR | Median fluorescence intensity (FI) | Number out of rangea | Politch et al. valuesb |
|---|---|---|---|---|---|
| IL-1RA | 29.1 | 14.1–53.8 | 106 | 0 | Not done |
| IL-1α | 18.3 | 6.5–62.6 | 720 | 0 | 6.0 |
| IL-1β | 3.5 | 1.4–12.0 | 103 | 0 | 2.0 |
| IL-2 | 0.9 | 0.5–1.8 | 54 | 0 | <7.0 |
| IL-4 | 5.7 | 3.1–10.3 | 161 | 2 | Not done |
| IL-5 | 33.6 | 8.3–128.5 | 1,256 | 0 | 31.3 |
| IL-6 | 11.3 | 7.0–26.2 | 328 | 0 | 6.0 |
| IL-8 | 1,120 | 971–1,644 | 25,159 | 1 | 1,305 |
| IL-10 | 2.2 | 0.4–9.2 | 75 | 0 | <3.9 |
| IL-12 P70 | 1.7 | 0.7–2.5 | 59 | 2 | <5.0 |
| IL-17 | 0.9 | 0.0–1.8 | 203 | 7 | 11.6 |
| IFN-γ | 3.3 | 2.5–7.2 | 89 | 1 | <3.0 |
| MIP-1β | 37.8 | 15.5–138.2 | 690 | 0 | 66.0 |
| TNF-α | 1.6 | 0.9–4.5 | 137 | 0 | <2.0 |
| RANTES | 759 | 300–2,386 | 4,964 | 0 | 126 |
| TGF-β | 7,578 | 5,312–20,920 | 25,279 | 7 | 85,120 |
| TNF-RII | 1,075 | 374–4,710 | 5,616 | 0 | Not done |
Out of range specimens had FIs below the lowest values on the standard curves for all cytokines except TGF-β, where 6/18 semen specimens had FIs above the highest value on the standard curve and 1/18 had FIs below the lowest value on the standard curve.
Previously reported values in HIV-1-seronegative men from Politch et al.22
IL, interleukin; IFN, interferon; MIP, macrophage inflammatory protein; TNF, tumor necrosis factor; TGF, tumor growth factor; IQR, interquartile range.
Table 2.
Median Concentrations and Interquartile Ranges of Blood Plasma Cytokines
| Blood plasma cytokine | Median concentration (pg/ml) | IQR |
|---|---|---|
| IL-1RA | 56.0 | 51.0–67.8 |
| IL-1α | 158.3 | 128.8–190.5 |
| IL-1β | 54.0 | 42.8–76.4 |
| IL-2 | 27.3 | 22.9–35.5 |
| IL-4 | 124.5 | 101.9–137.0 |
| IL-5 | 44.0 | 36.8–56.2 |
| IL-6 | 87.3 | 75.0–102.0 |
| IL-8 | 300.5 | 211.8–409.3 |
| IL-10 | 97.8 | 75.4–117.0 |
| IL-12 P70 | 45.5 | 39.4–57.5 |
| IL-17 | 121.5 | 104.0–154.0 |
| IFN-γ | 56.8 | 40.0–67.3 |
| MIP-1β | 326.3 | 282.6–363.1 |
| TNF-α | 386.5 | 285.3–543.5 |
| RANTES | 16,952.5 | 6,735.1–24,317.4 |
| TGF-β | 387.8 | 134.0–861.9 |
| TNF-RII | 18,243.3 | 15,326.0–22,962.6 |
Multiple regression modeling to evaluate associations between seminal plasma and blood plasma cytokines and HIV-1 RNA levels
Initial comparisons between seminal plasma levels of individual cytokines with seminal plasma HIV-1 RNA levels demonstrated positive correlations for IL-1α (R=0.56, p=0.016) and IL-1RA (R=0.65, p=0.004). There were no statistically significant correlations between levels of specific cytokines in seminal plasma and the corresponding cytokine in blood plasma. However, linear regression analysis including blood plasma HIV-1 RNA level as a covariate revealed that seminal plasma IFN-γ, IL-1α, IL-1RA, and IL-17 showed positive correlative trends (p<0.20) with seminal plasma HIV-1 RNA levels, while IL-6, IL-8, MIP-1β, and IL-5 showed negative correlative trends (p<0.20).
Blood plasma HIV-1 RNA level as well as seminal plasma IFN- γ, IL-5, and IL-17 were included in a multiple regression model of associations between seminal plasma cytokines and HIV-1 RNA levels. Blood plasma HIV-1 RNA level (p<0.0001) as well as higher seminal plasma IFN-γ (p=0.03) and IL-17 (p=0.03) levels were positively associated with seminal plasma HIV-1 RNA levels, while seminal plasma IL-5 was negatively associated (p=0.0007). This model demonstrated a strong fit between the covariates and seminal plasma HIV-1 RNA levels (R2=0.83).
Testing of the model for variable selection bias
To differentiate the true predictive value of the above associations versus selection bias (“overfitting”), simulation modeling with 10,000 iterations was performed. Of the 10,000 iterations, only 340 (3.4%) had a higher R2 than the one observed for our final model, suggesting a less than 4% likelihood that fitting of our model is due to chance or selection bias. Note that because the relationship between blood and seminal plasma HIV-1 RNA levels was maintained in this analysis, the lowest observed R2 in this simulation was 0.44, which is the correlation between blood and seminal plasma HIV-1 RNA level in our data set.
Analysis of men with atypically high seminal plasma HIV-1 RNA levels compared to blood plasma HIV-1 RNA levels
Five of the 18 men (28%) had an atypically high level of HIV-1 RNA in seminal plasma compared to the level of HIV-1 RNA in their blood plasma (ratio of seminal plasma log10 HIV-1 RNA versus blood plasma log10 HIV-1 RNA ≥0.5). In the seminal plasma compartment, men with atypically high ratios of semen versus blood HIV-1 RNA levels demonstrated lower levels of seminal IL-5, IL-8, and IL-10 (p=0.02 for all) compared to men without atypically high ratios. Additionally, in the blood compartment, men with atypically high seminal plasma versus blood plasma HIV-1 ratios demonstrated higher levels of blood plasma IL-12 (p=0.02) and IFN-γ (p=0.0005) than men without atypically high ratios (Tables 3 and 4).
Table 3.
Median Seminal and Blood Plasma Cytomine Concentrations
| Median cytokine concentration in pg/ml (IQR) | |||
|---|---|---|---|
| Cytokine | Higher ratio semen:blood HIV-1 | Lower ratio semen:blood HIV-1 | Wilcoxon p-value |
| Blood | |||
| IL-1β | 0.8 (0.0, 2.0) | 0.0 (0.0, 0.5) | 0.09 |
| TNF-α | 4.4 (2.5, 11.0) | 5.5 (4.3, 6.6) | 0.59 |
| IL-2 | 0.0 (0.0, 11.1) | 0.0 (0.0, 0.0) | 0.09 |
| IL-4 | 2.3 (1.1, 9.8) | 0.7 (0.0, 2.6) | 0.14 |
| IL-6 | 0.4 (0.2, 0.9) | 0.3 (0.1, 0.6) | 0.43 |
| IL-8 | 1.3 (0.9, 2.8) | 2.1 (0.8, 3.7) | 0.35 |
| IL-10 | 5.6 (3.7, 132.9) | 5.0 (3.1, 7.4) | 0.73 |
| IL-12 P70 | 1.0 (0.3, 100.8) | 0.0 (0.0, 0.4) | 0.02 |
| IFN-γ | 1.7 (1.2, 78.0) | 0.0 (0.0, 0.7) | 0.01 |
| MIP-1β | 2.4 (0.0, 3.6) | 0.0 (0.0, 4.6) | 0.66 |
| IL-1ra | 14.5 (7.7, 87.4) | 10.5 (9.2, 14.5) | 0.28 |
| IL-1α | 0.0 (0.0, 11.1) | 0.0 (0.0, 0.0) | 0.81 |
| IL-5 | 0.4 (0.2, 1.0) | 0.2 (0.1, 0.3) | 0.17 |
| IL-17 | 0.0 (0.0, 2.1) | 0.0 (0.0, 0.0) | 0.09 |
| RANTES | 126,031 (57,026, 178,632) | 103,284 (52,428, 193,511) | 0.07 |
| TGF-β | 3,727 (3112, 6827) | 5,916 (4,600, 5,915) | 0.18 |
| TNF-RII | 6,397 (3,300, 10,949) | 6,676 (4,834, 13,933) | 0.59 |
| Semen | |||
| IL-1β | 1.5 (0.8, 9.0) | 3.7 (1.6, 12.3) | 0.26 |
| TNF-α | 0.9 (0.7, 2.8) | 1.8 (1.0, 7.4) | 0.13 |
| IL-2 | 0.3 (0.2, 3.4) | 1.2 (0.8, 1.8) | 0.07 |
| IL-4 | 3.5 (2.9, 11.9) | 6.3 (3.0, 12.3) | 0.73 |
| IL-6 | 8.3 (6.4, 10.0) | 18.7 (7.0, 68.0) | 0.13 |
| IL-8 | 1,013 (953, 1,069) | 1,294 (1,013, 2,707) | 0.03 |
| IL-10 | 0.4 (0.2, 1.3) | 3.7 (1.4, 23.6) | 0.03 |
| IL-12 P70 | 0.9 (0.3, 3.5) | 1.8 (1.0, 3.0) | 0.46 |
| IFN-γ | 2.6 (2.1, 7.9) | 3.3 (2.6, 9.0) | 0.52 |
| MIP-1β | 20.2 (13.1, 31.0) | 58.0 (17.5, 233.4) | 0.07 |
| IL-1ra | 30.4 (12.8, 97.5) | 27.8 (16.2, 60.2) | 0.96 |
| IL-1α | 58.4 (6.4, 239.5) | 15.7 (6.0, 46.1) | 0.35 |
| IL-5 | 3.3 (12.0, 28.3) | 80.0 (13.2, 210.0) | 0.02 |
| IL-17 | 1.5 (0.0, 4.3) | 0.9 (0.0, 1.9) | 0.96 |
| RANTES | 589 (149, 2,154) | 794 (370, 2,646) | 0.59 |
| TGF-β | 7,494 (6,165, 19,406) | 7,661 (4,971, 21,914) | 0.59 |
| TNF-RII | 554 (183, 9,965) | 1,316 (592, 3,977) | 0.88 |
Concentrations for men with atypically high seminal versus blood plasma HIV-1 RNA levels (n=5, seminal/blood plasma HIV-1 RNA >0.5) versus men without atypically high seminal versus blood plasma HIV-1 RNA levels (n=13, seminal/blood plasma HIV-1 RNA <0.5).
Table 4.
Cytokine Differences in Seminal and Blood Plasma of Men with Higher (>0.5) Versus Lower (<0.5) Ratios of Seminal to Blood Plasma HIV-1 RNA
| Men with atypically high seminal plasma versus blood plasma HIV-1 RNA ratios | ||
|---|---|---|
| Higher cytokines | Lower cytokines | |
| Blood plasma | IL-12** | RANTES* |
| IFN-γ** | ||
| IL-1β* | ||
| IL-2* | ||
| IL-17* | ||
| Seminal plasma | IL-2* | IL-5** |
| IL-8** | ||
| IL-10** | ||
| MIP-1β* | ||
p≤0.10.
p≤0.05.
Analysis of seminal and blood plasma cytokine ratios in men with detectable versus undetectable seminal plasma HIV-1 RNA levels
The ratio of seminal to blood plasma IL-10 was significantly lower in men with detectable HIV-1 RNA (>100 copies/ml) in seminal plasma (p=0.03) compared to men without detectable virus in seminal plasma (≤100 copies/ml), and there was a trend toward a higher ratio of seminal to blood plasma IL-1α (p=0.06) in men with detectable HIV-1 RNA in seminal plasma. Several other cytokines demonstrated markedly different seminal-to-blood plasma ratios in persons with detectable seminal plasma HIV-1 RNA, but these differences did not reach statistical significance (Table 5).
Table 5.
Median Seminal Plasma to Blood Plasma Cytokine Ratios in Men with Detectable (>100 Copies/ml) Versus Undetectable (≤100 Copies/ml) Seminal Plasma HIV-1 RNA
| Median semen/blood cytokine ratio | ||||
|---|---|---|---|---|
| Cytokine | Overall (n=18) | Detectable semen HIV-1 RNA (>100 copies/ml) (n=12) | Undetectable semen HIV-1 RNA (≤100 copies/ml) (n=6) | Wilcoxon p-value |
| IL-1β | 1.44 | 1.49 (0.97, 3.92) | 1.35 (1.14, 6.73) | 0.85 |
| TNF-α | 0.40 | 0.34 (0.22, 0.69) | 0.73 (0.26, 1.27) | 0.51 |
| IL-2 | 2.30 | 2.62 (1.11, 3.72) | 2.22 (1.43, 2.37) | 0.40 |
| IL-4 | 1.35 | 1.15 (1.01, 2.10) | 1.37 (1.21, 2.16) | 0.64 |
| IL-6 | 3.30 | 3.22 (2.17, 11.24) | 4.29 (2.96, 89.19) | 0.45 |
| IL-8 | 90.5 | 88.6 (66.8, 125.6) | 90.5 (61.4, 98.2) | 0.57 |
| IL-10 | 0.68 | 0.55 (0.40, 1.11) | 1.25 (0.71, 7.03) | 0.03 |
| IL-12 | 1.13 | 1.09 (0.82, 1.62) | 1.25 (1.09, 1.77) | 0.35 |
| IFN-γ | 1.80 | 1.72 (1.02, 2.80) | 2.61 (1.59, 4.07) | 0.26 |
| MIP-1β | 2.08 | 1.58 (1.30, 5.95) | 2.34 (1.63, 7.60) | 0.48 |
| IL-1ra | 1.82 | 2.16 (1.13, 5.02) | 1.49 (0.73, 2.15) | 0.22 |
| IL-1α | 3.73 | 6.24 (3.20, 18.54) | 2.86 (1.93, 5.28) | 0.06 |
| IL-5 | 21.5 | 20.3 (3.8, 98.2) | 50.0 (12.6, 262.9) | 0.26 |
| IL-17 | 1.56 | 1.39 (0.96, 2.31) | 1.93 (1.14, 2.29) | 0.40 |
| RANTES | 0.64 | 0.86 (0.07, 0.95) | 0.39 (0.10, 0.85) | 0.78 |
| TGF-β | 64.9 | 66.4 (40.6, 187.8) | 43.7 (1.9, 188.5) | 0.35 |
| TNFR2 | 0.37 | 0.37 (0.10, 0.96) | 0.36 (0.13, 0.84) | 0.93 |
Discussion
Our study adds to the growing evidence that untreated HIV-1 infection in men is associated with genital compartment inflammation. Compared to previously reported seminal plasma cytokine levels for healthy men,22 we noted elevated levels of the proinflammatory cytokines IL-1α, IL-6, and RANTES and decreased levels of the immunosuppressive cytokine TGF-β.
We demonstrate significant associations between seminal plasma IFN-γ, IL-17, and IL-5 and seminal plasma HIV-1 RNA levels. We replicate the previously reported association between seminal plasma IFN-γ and seminal plasma HIV-1 RNA levels.17 Our finding of a positive correlation between IL-17 and HIV-1 RNA levels in seminal plasma directs further attention to the potential role of Th17 cells and/or NK cells in driving inflammation that increases HIV-1 RNA levels.23
In our cohort, HIV-1 RNA levels in blood plasma were approximately 30-fold higher than in seminal plasma. In the context of reports of compartmentalization of HIV-1 in semen,5,24 our results suggest that local factors contribute to seminal plasma HIV-1 RNA levels. Additionally, we observed altered seminal plasma-to-blood plasma ratios of IL-1α and IL-10 in subjects with detectable HIV-1 RNA levels in seminal plasma. These findings are consistent with previous work by Lisco et al. reporting increased compartmentalization of many proinflammatory cytokines in the setting of HIV-1 infection.18 It is also interesting to note that men with atypically high seminal plasma HIV-1 RNA levels relative to blood had increased Th1 cytokines IFN-γ and IL-12 in the blood plasma and decreased levels of Th2 cytokines IL-5, IL-8, and IL-10 in the seminal plasma. This suggests that increased inflammation in the blood and decreased immunomodulatory activity in the genital compartment are associated with atypically high levels of seminal plasma HIV-1 RNA levels relative to blood.
We have expanded upon previous studies examining the relationship between seminal plasma HIV-1 RNA level and seminal plasma cytokines by controlling for blood plasma HIV-1 RNA level in our model. Blood plasma HIV-1 RNA level is known to be the strongest single predictor of seminal plasma HIV-1 RNA level, with reported Pearson R2 correlations in the range of 0.40–0.50.2,7 This strong correlation may have obscured the effects of various cytokines on seminal plasma HIV-1 RNA levels. However, when we performed analyses for associations between seminal plasma cytokines and HIV-1 RNA levels without including blood plasma HIV-1 RNA as a model covariate, we still did not observe a statistically significant association between seminal plasma HIV-1 RNA level and specific seminal plasma cytokines that have been reported in prior studies (data not shown), including a study by Berlier et al.15 that found a correlation between seminal plasma levels of IL-1β and seminal plasma HIV-1 RNA concentration and a study by Storey et al.16 that reported a correlation between seminal plasma RANTES and seminal plasma HIV-1 RNA levels.
Differences between our findings and those of other research groups may have been due to technical differences in analysis methods or differences in laboratory technique. We analyzed the Luminex data using units of fluorescence intensity, rather than calculated concentrations from standard curves, which allowed analysis of low cytokine levels that were clearly detectable but below the lowest standard. This methodology has been used by some investigators in other fields,19,20 but most prior HIV-1 studies have used calculated concentrations. Standardizing laboratory techniques has been the focus of working groups such as the Semen Best Practices Working Group at the National Institutes of Health, and will provide much needed guidance for future studies.
Our study has several limitations. Our sample size was small due to the exclusion of 11 subjects (35% of the sample) with HIV-1- RNA levels <200 copies/ml. Also, we had limited information on other factors that can influence genital tract inflammation, such as CMV serostatus.14,18 Additionally, we measured seminal plasma HIV-1 RNA and did not quantify cell-associated virus. Our model is exploratory, and did not control for multiple testing. Finally, the performance of the model may be overly optimistic due to variable selection bias (a large number of candidate cytokines compared to the number of samples).
In conclusion, this study adds to the growing knowledge about seminal plasma cytokines and HIV-1 RNA levels in untreated men with chronic infection. Although seminal plasma HIV-1 RNA levels are greatly decreased by ART, a substantial subset of patients continue to have intermittently elevated seminal plasma HIV-1 RNA levels and remain at risk for transmitting to their sexual partners. Comprehensive studies are needed to advance our understanding of the relationship between the blood and genital compartments among those both on and off ART, as well as specific factors within the genital tract that drive seminal plasma HIV-1 RNA levels.
Supplementary Material
Acknowledgments
We would like to express our deep gratitude to the participants in our study. We would also like to acknowledge the contributions of the staff of the Center for HIV Prevention Research, without whom this study would not have been possible, particularly Charina McDonald, Terry Saunders, Elena Khanukhova, and Justin Akin. This study was funded by UCLA AIDS Institute and the UCLA Center for AIDS Research (AI28697): Administrative Core (seed grant) and Cores of Mucosal Immunology and Virology, and by the UCLA Department of Medicine Statistics Core. Dr. J. Hoffman was supported by an NRSA Fellowship Award T32 MH 80634-6.
Previously presented as a poster at the 20th Conference on Retroviruses and Opportunistic Infections, Atlanta, Georgia, March 3–6, 2013, abstract number 1081.
J.H. performed the data and statistical analyses and wrote the primary manuscript; P.A. participated in the study design, subject recruitment/sample collection, and data analysis, and revised the manuscript; G.C.B. participated in the study design, performed laboratory experiments, and revised the manuscript; J.E., D.A.P., and K.T. participated in the study design and in the optimization/performance of laboratory experiments; D.E. performed statistical analyses and revised the manuscript; C.S. performed statistical analyses; O.Y. participated in the study design, performed data analysis, and revised the manuscript; R.H. designed the study, enrolled participants, participated in data analysis, and revised the manuscript. All authors read and approved the final manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hladik F. and McElrath MJ: Setting the stage: Host invasion by HIV. Nat Rev Immunol 2008;8(6):447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, Kahle E, Lingappa JR, et al. : Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med 2011;3(77):77ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler DM, Smith DM, Cachay ER, et al. : Herpes simplex virus 2 serostatus and viral loads of HIV-1 in blood and semen as risk factors for HIV transmission among men who have sex with men. AIDS 2008;22(13):1667–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert-Niclot S, Tubiana R, Beaudoux C, et al. : Detection of HIV-1 RNA in seminal plasma samples from treated patients with undetectable HIV-1 RNA in blood plasma on a 2002–2011 survey. AIDS 2012;26(8):971–975 [DOI] [PubMed] [Google Scholar]
- 5.Sheth PM, Yi TJ, Kovacs C, et al. : Mucosal correlates of isolated HIV semen shedding during effective antiretroviral therapy. Mucosal Immunol 2012;5(3):248–257 [DOI] [PubMed] [Google Scholar]
- 6.Politch JA, Mayer KH, Welles SL, et al. : Highly active antiretroviral therapy does not completely suppress HIV in semen of sexually active HIV-infected men who have sex with men. AIDS 2012;26(12):1535–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalichman SC, Di Berto G, and Eaton L: Human immunodeficiency virus viral load in blood plasma and semen: Review and implications of empirical findings. Sex Transm Dis 2008;35(1):55–60 [DOI] [PubMed] [Google Scholar]
- 8.Zuckerman RA, Lucchetti A, Whittington WL, et al. : HSV suppression reduces seminal HIV-1 levels in HIV-1/HSV-2 co-infected men who have sex with men. AIDS 2009;23(4):479–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen MS, Hoffman IF, Royce RA, et al. : Reduction of concentration of HIV-1 in semen after treatment of urethritis: Implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet 1997;349(9069):1868–1873 [DOI] [PubMed] [Google Scholar]
- 10.Sadiq ST, Taylor S, Copas AJ, et al. : The effects of urethritis on seminal plasma HIV-1 RNA loads in homosexual men not receiving antiretroviral therapy. Sex Transm Infect 2005;81(2):120–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson LF. and Lewis DA: The effect of genital tract infections on HIV-1 shedding in the genital tract: A systematic review and meta-analysis. Sex Transm Dis 2008;35(11):946–959 [DOI] [PubMed] [Google Scholar]
- 12.Winter AJ, Taylor S, Workman J, et al. : Asymptomatic urethritis and detection of HIV-1 RNA in seminal plasma. Sex Transm Infect 1999;75(4):261–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheth PM, Danesh A, Sheung A, et al. : Disproportionately high semen shedding of HIV is associated with compartmentalized cytomegalovirus reactivation. J Infect Dis 2006;193(1):45–48 [DOI] [PubMed] [Google Scholar]
- 14.Gianella S, Morris SR, Anderson C, et al. : Herpes viruses and HIV-1 drug resistance mutations influence the virologic and immunologic milieu of the male genital tract. AIDS 2013;27(1):39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berlier W, Bourlet T, Levy R, et al. : Amount of seminal IL-1beta positively correlates to HIV-1 load in the semen of infected patients. J Clin Virol 2006;36(3):204–207 [DOI] [PubMed] [Google Scholar]
- 16.Storey DF, Dolan MJ, Anderson SA, et al. : Seminal plasma RANTES levels positively correlate with seminal plasma HIV-1 RNA levels. AIDS 1999;13(15):2169–2171 [DOI] [PubMed] [Google Scholar]
- 17.Sheth PM, Danesh A, Shahabi K, et al. : HIV-specific CD8+ lymphocytes in semen are not associated with reduced HIV shedding. J Immunol 2005;175(7):4789–4796 [DOI] [PubMed] [Google Scholar]
- 18.Lisco A, Munawwar A, Introini A, et al. : Semen of HIV-1-infected individuals: Local shedding of herpesviruses and reprogrammed cytokine network. J Infect Dis 2012;205(1):97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Connor MJ, Lind C, Tang X, et al. : Persistence of anti-human leukocyte antibodies in congenital heart disease late after surgery using allografts and whole blood. J Heart Lung Transplant 2013;32(4):390–397 [DOI] [PubMed] [Google Scholar]
- 20.Ling M, Marfo K, Masiakos P, et al. : Pretransplant anti-HLA-Cw and anti-HLA-DP antibodies in sensitized patients. Hum Immunol 2012;73(9):879–883 [DOI] [PubMed] [Google Scholar]
- 21.Sheth PM, Shahabi K, Rebbapragada A, et al. : HIV viral shedding in semen: Lack of correlation with systemic virus-specific CD8 responses. AIDS 2004;18(16):2202–2205 [DOI] [PubMed] [Google Scholar]
- 22.Politch JA, Tucker L, Bowman FP, and Anderson DJ: Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum Reprod 2007;22(11):2928–2935 [DOI] [PubMed] [Google Scholar]
- 23.Ibarrondo FJ, Wilson SB, Hultin LE, et al. : Preferential depletion of gut CD4-expressing iNKT cells contributes to systemic immune activation in HIV-1 infection. Mucosal Immunol 2013;6(3):591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diem K, Nickle DC, Motoshige A, et al. : Male genital tract compartmentalization of human immunodeficiency virus type 1 (HIV). AIDS Res Hum Retroviruses 2008;24(4):561–571 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


