Abstract
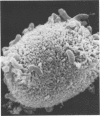
Adhesion of vibrios to the small intestine may occur (i) by association of the bacteria with secreted mucus gel or (ii) by adherence of the bacteria to the surface of epithelial cells. In the present study, vibrios readily adhered to isolated brush border membranes obtained from rabbit intestinal epithelial cells. Adhesion was temperature dependent and required the presence of divalent cations such as calcium. The agglutination of human O erythrocytes by Vibrio cholerae was observed also, and the hemagglutination test appeared to detect the same mechanism that was involved in the adhesion of vibrios to brush borders. When the bacteria were grown in broth they were adhesive and hemagglutinating, but vibrios grown on agar plates or suspended in buffer for 15 min at 37 C lacked these abilities, even though they retained undiminished motility. These two model systems differed, however, in that strontium promoted only adhesion to brush borders. The significance of this difference remains to be determined. Vibrios were observed to penetrate intestinal mucus gel and occasionally to become entrapped in it. However, there was no evidence that vibrios attached to mucus gel.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bangham A. D. Lipid bilayers and biomembranes. Annu Rev Biochem. 1972;41:753–776. doi: 10.1146/annurev.bi.41.070172.003541. [DOI] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. M protein-associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect Immun. 1972 May;5(5):826–830. doi: 10.1128/iai.5.5.826-830.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. M., Wrigglesworth J. M., Burdett K., Pover W. F. Studies on epithelial cells isolated from guinea pig small intestine. J Cell Biol. 1971 Nov;51(21):452–464. doi: 10.1083/jcb.51.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner G. G. Release of intestinal surface-membrane glycoproteins associated with enzyme activity by brief digestion with papain. Biochem J. 1971 Mar;121(5):781–789. doi: 10.1042/bj1210781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R. Parameters affecting the association of vibrios with the intestinal surface in experimental cholera. Infect Immun. 1972 Aug;6(2):134–141. doi: 10.1128/iai.6.2.134-141.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fubara E. S., Freter R. Protection against enteric bacterial infection by secretory IgA antibodies. J Immunol. 1973 Aug;111(2):395–403. [PubMed] [Google Scholar]
- Gibbons R. A., Jones G. W., Sellwood R. An attempt to identify the intestinal receptor for the K88 adhesin by means of a haemagglutination inhibition test using glycoproteins and fractions from sow colostrum. J Gen Microbiol. 1975 Feb;86(2):228–240. doi: 10.1099/00221287-86-2-228. [DOI] [PubMed] [Google Scholar]
- Guentzel M. N., Berry L. J. Motility as a virulence factor for Vibrio cholerae. Infect Immun. 1975 May;11(5):890–897. doi: 10.1128/iai.11.5.890-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S. Structure and function of the glycocalyx. Fed Proc. 1969 Jan-Feb;28(1):12–25. [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. The association of K88 antigen with haemagglutinating activity in porcine strains of Escherichia coli. J Gen Microbiol. 1974 Sep;84(1):135–144. doi: 10.1099/00221287-84-1-135. [DOI] [PubMed] [Google Scholar]
- MILLER D., CRANE R. K. The digestive function of the epithelium of the small intestine. II. Localization of disaccharide hydrolysis in the isolated brush border portion of intestinal epithelial cells. Biochim Biophys Acta. 1961 Sep 16;52:293–298. doi: 10.1016/0006-3002(61)90678-3. [DOI] [PubMed] [Google Scholar]
- Rutter J. M., Jones G. W. Protection against enteric disease caused by Escherichia coli--a model for vaccination with a virulence determinant? Nature. 1973 Apr 20;242(5399):531–532. doi: 10.1038/242531a0. [DOI] [PubMed] [Google Scholar]
- Savage D. C., Blumershine R. V. Surface-surface associations in microbial communities populating epithelial habitats in the murine gastrointestinal ecosystem: scanning electron microscopy. Infect Immun. 1974 Jul;10(1):240–250. doi: 10.1128/iai.10.1.240-250.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrank G. D., Verwey W. F. Distribution of cholera organisms in experimental Vibrio cholerae infections: proposed mechanisms of pathogenesis and antibacterial immunity. Infect Immun. 1976 Jan;13(1):195–203. doi: 10.1128/iai.13.1.195-203.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellwood R., Gibbons R. A., Jones G. W., Rutter J. M. Adhesion of enteropathogenic Escherichia coli to pig intestinal brush borders: the existence of two pig phenotypes. J Med Microbiol. 1975 Aug;8(3):405–411. doi: 10.1099/00222615-8-3-405. [DOI] [PubMed] [Google Scholar]
- Tweedy J. M., Park R. W., Hodgkiss W. Evidence for the presence of fimbriae (pili) on vibrio species. J Gen Microbiol. 1968 Apr;51(2):235–244. doi: 10.1099/00221287-51-2-235. [DOI] [PubMed] [Google Scholar]