Abstract
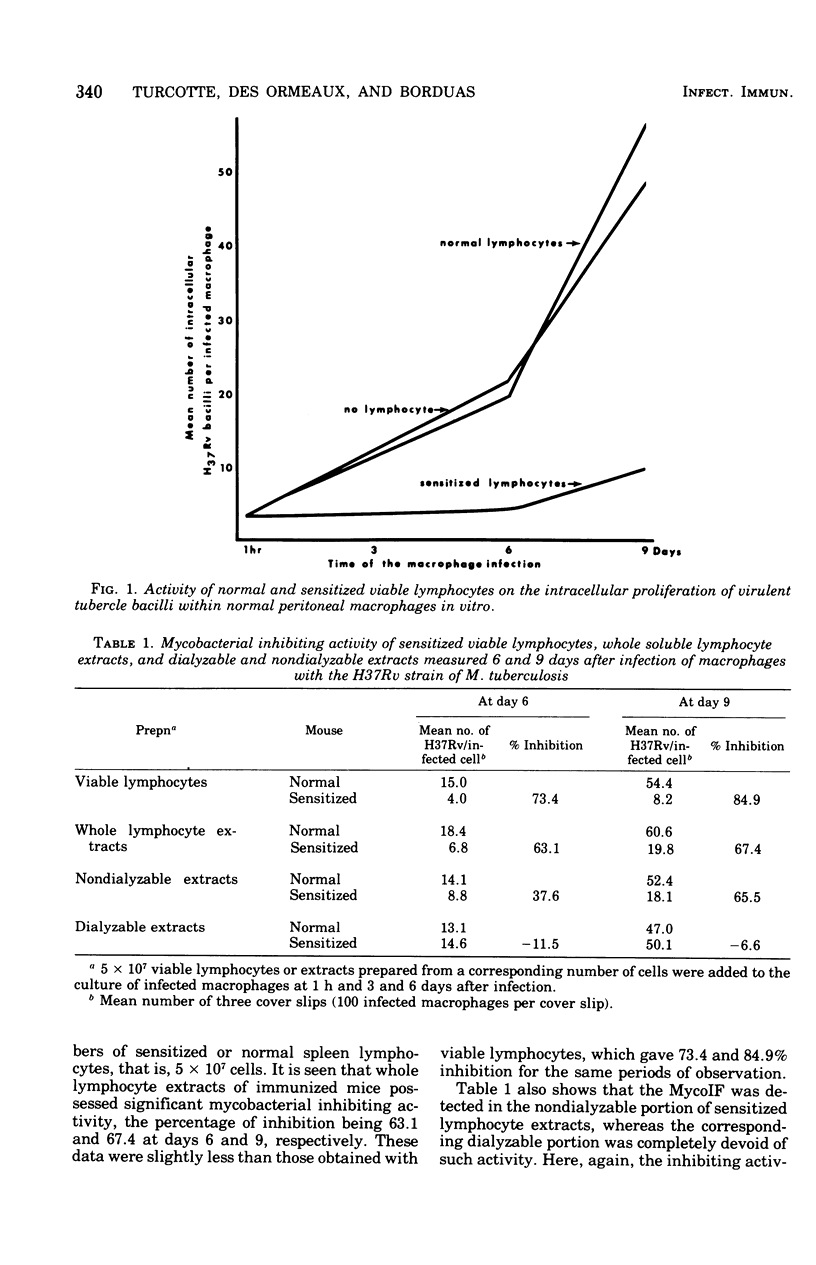
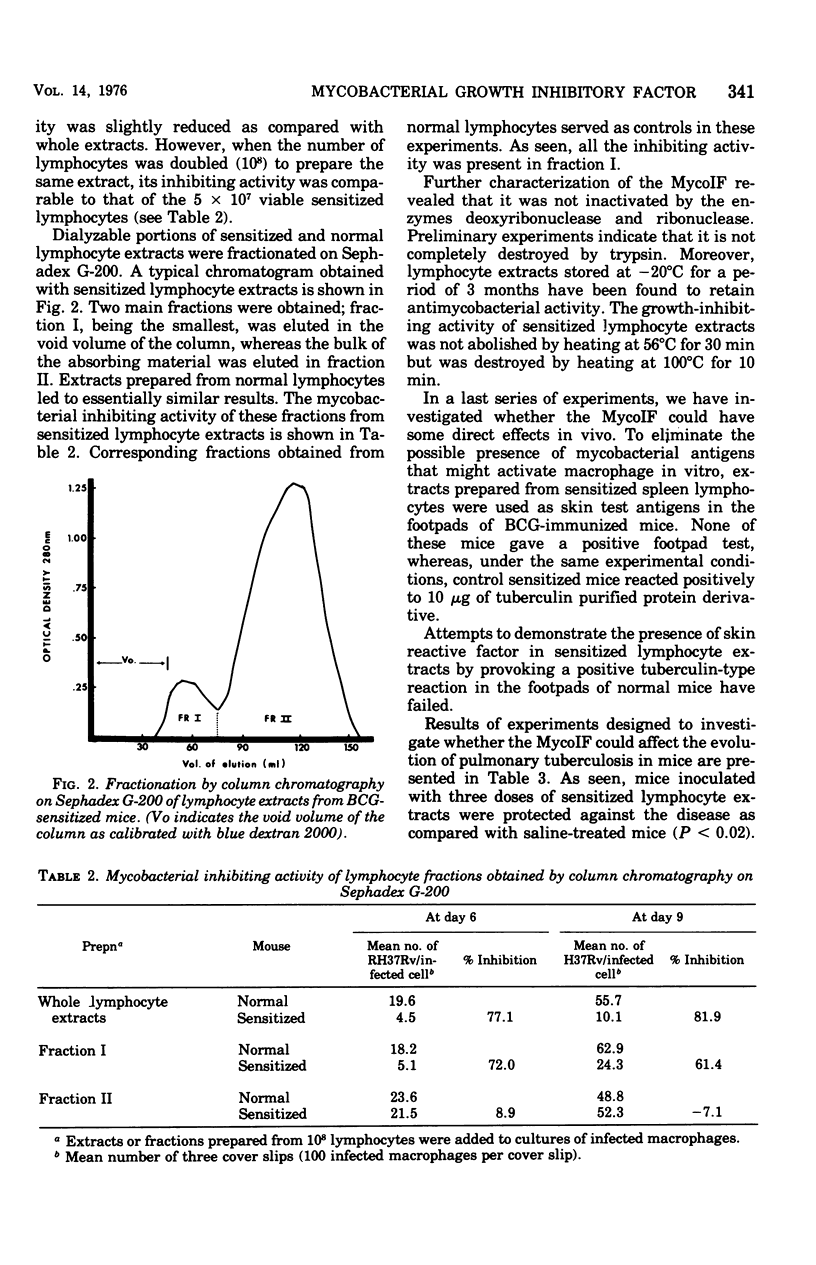
Spleen lymphocytes of BCG-immunized mice contain a soluble factor that inhibits in vitro the growth of the H37Rv strain of Mycobacterium tuberculosis within normal peritoneal macrophages. The water-soluble extracts of sensitized lymphocytes, disrupted by freezing and thawing, although less active than the corresponding viable cells retained a significant growth-inhibiting activity. Dialysis against distilled water, lyophilization, exposure to ribonuclease and deoxyribonuclease, and storage at -20 degrees C of the water-soluble extracts did not affect their antimycobacterial activity, whereas extracts heated at 100 degrees C were completely devoid of such an activity. All the inhibiting activity was recovered in the void volume of the column after chromatography on Sephadex G-200. Water-soluble constitutents of sensitized lymphocytes did not affect BCG grown in vitro, and on repeated treatments of tuberculous mice they led to a negligible protection against pulmonary tuberculosis. Preliminary observations seem to indicate that other soluble factors in lymphocytes of BCG-sensitized mice have the capacity to potentiate in vitro the phagocytic activity of normal macrophages.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CROWLE A. J. Lung density as a measure of tuberculous involvement in mice. Am Rev Tuberc. 1958 Apr;77(4):681–693. doi: 10.1164/artpd.1958.77.4.681. [DOI] [PubMed] [Google Scholar]
- Cahall D. L., Youmans G. P. Conditions for production, and some characteristics, of mycobacterial growth inhibitory factor produced by spleen cells from mice immunized with viable cells of the attenuated H37Ra strain of Mycobacterium tuberculosis. Infect Immun. 1975 Oct;12(4):833–840. doi: 10.1128/iai.12.4.833-840.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahall D. L., Youmans G. P. Macrophage migration inhibitory activity of mycobacterial growth inhibitory factor and the effect of a number of factors on mycobacterial growth inhibitory factor activity. Infect Immun. 1975 Oct;12(4):851–857. doi: 10.1128/iai.12.4.851-857.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahall D. L., Youmans G. P. Molecular weight and other characteristics of mycobacterial growth inhibitory factor produced by spleen cells obtained from mice immunized with viable attenuated mycobacterial cells. Infect Immun. 1975 Oct;12(4):841–850. doi: 10.1128/iai.12.4.841-850.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Montalbine V. Relative immunogenicity of streptomycin-susceptible and -resistant strains of BCG. II. Effect of the route of inoculation on growth and immunogenicity. Am Rev Respir Dis. 1975 Jan;111(1):43–51. doi: 10.1164/arrd.1975.111.1.43. [DOI] [PubMed] [Google Scholar]
- Costello R. T., Izumi T., Sakurami T. Behavior of attenuated mycobacteria in organs of neonatal and adult mice. J Exp Med. 1971 Aug 1;134(2):366–380. doi: 10.1084/jem.134.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr, Meyer O. T., Esterly J. R., Kambara T. The local nature of immunity in tuberculosis, illustrated histochemically in dermal BCG lesions. J Immunol. 1968 May;100(5):931–941. [PubMed] [Google Scholar]
- FREEDMAN S. O., TURCOTTE R., FISH A. J., SEHON A. H. The in vitro detection of "cell-fixed" hemagglutinating antibodies to tuberculin purified protein derivative (PPD) in humans. J Immunol. 1963 Jan;90:52–59. [PubMed] [Google Scholar]
- Godal T., Rees R. J., Lamvik J. O. Lymphocyte-mediated modification of blood-derived macrophage function in vitro; inhibition of growth of intracellular mycobacteria with lymphokines. Clin Exp Immunol. 1971 Apr;8(4):625–637. [PMC free article] [PubMed] [Google Scholar]
- Klun C. L., Youmans G. P. The effect of lymphocyte supernatant fluids on the intracellular growth of virulent tubercle bacilli. J Reticuloendothel Soc. 1973 Mar;13(3):263–274. [PubMed] [Google Scholar]
- Klun C. L., Youmans G. P. The induction by Listeria monocytogenes and plant mitogens of lymphocyte supernatant fluids which inhibit the growth of Mycobacterium tuberculosis within macrophages in vitro. J Reticuloendothel Soc. 1973 Mar;13(3):275–285. [PubMed] [Google Scholar]
- Krahenbuhl J. L., Remington J. S. In vitro induction of nonspecific resistance in macrophages by specifically sensitized lymphocytes. Infect Immun. 1971 Oct;4(4):337–343. doi: 10.1128/iai.4.4.337-343.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Remold H. G., David J. R. Characterization of a lymphocyte factor which alters macrophage functions. J Exp Med. 1973 Feb 1;137(2):275–290. doi: 10.1084/jem.137.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Multiplication of Mycobacterium tuberculosis Within Normal and "Immune" Mouse Macrophages Cultivated With and Without Streptomycin. Infect Immun. 1970 Jan;1(1):30–40. doi: 10.1128/iai.1.1.30-40.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portelance V., Boulanger R. P. Mice tuberculosis quantified by the lung density technique. Can J Microbiol. 1974 Sep;20(9):1247–1255. doi: 10.1139/m74-192. [DOI] [PubMed] [Google Scholar]
- SANSONNENS R. [A propos d'un nouveau vaccin BCG homogène]. Bibl Paediatr. 1954;58:551–557. [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Cellular immunity in vitro. I. Immunologically mediated enhancement of macrophage bactericidal capacity. J Exp Med. 1971 Jun 1;133(6):1377–1389. doi: 10.1084/jem.133.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Migration inhibitory factor and macrophage bactericidal function. Infect Immun. 1972 Aug;6(2):101–103. doi: 10.1128/iai.6.2.101-103.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svejcar J., Pekárek J., Johanovský J. Specific stimulation of mycobacteria phagocytosis by substances liberated during the cultivation of lymph node cells from tuberculin hypersensitive rabbits with the specific antigen. Experientia. 1972 Apr 15;28(4):467–470. doi: 10.1007/BF02008346. [DOI] [PubMed] [Google Scholar]


