Abstract
Introduction:
Laparoscopic nephrectomy (LN) is likely the most common laparoscopic procedure performed by general urologists without formal laparoscopic training. The traditional technique is cumbersome because it entails making an early approach to the hilum with the risk of bleeding and need for conversion. We perform a different technique that we believe is simpler to learn and to teach. It consists of a complete dissection of the inferior and posterior aspects of the kidney, followed by en bloc stapling of the renal hilum. The present report is a detailed description of our technique including outcomes and complications.
Materials and Methods:
Perioperative data of 129 consecutive patients who underwent LN between November 2003 and September 2007 were prospectively collected and retrospectively reviewed. Complications were reported using the Clavien classification system, and follow-up was performed according to our institution's protocol and included physical examination, blood count, blood chemistry, and renal function tests at every visit, in addition to abdominal computed tomography scan six months after surgery. Additional imaging was scheduled according to disease stage and grade.
Results:
Mean patient age, tumor size, and operative time were 63 ± 15.6 years, 6.3 ± 2.4 cm, and 128 ± 41.4 minutes, respectively. Median estimated blood loss was 0 mL (0.200). Conversion to open surgery occurred in 3.1% of patients, and 8% of the patients had a blood transfusion. Complications were recorded in 26% of the patients; 91% of them had Clavien grade scores of 1 or 2.
Conclusion:
We present a standardized technique for LN. Its main advantage is that postpones any manipulation of the hilum to a later step during the procedure when it is easy to identify and control. This decreases early bleeding and main vascular complications.
Keywords: Nephrectomy, Laparoscopy
INTRODUCTION
Laparoscopic nephrectomy (LN) is likely the most common laparoscopic procedure performed by general urologists.1–3 The standard technique for LN was developed to resemble its open counterpart, thus entailing an early approach to the renal hilum with meticulous dissection of the renal vein and artery, which are subsequently divided separately with clips or staples. Dissecting the hilum early during the procedure requires advanced laparoscopic skills that may not be completely developed in the novice laparoscopic surgeon, thereby increasing the risk of bleeding and the need for open conversion.
At our institution, we perform a different technique that we believe is simpler to learn and to teach and has been our established approach for LN and laparoscopic nephroureterectomies (LNU) since 2003. It consists of a complete dissection of the inferior and posterior aspects of the kidney, followed by en bloc stapling of the renal hilum. The present report is a detailed description of our technique including outcomes and complications.
MATERIALS AND METHODS
Patients and Study Design
After institutional board review approval, the clinical data of 129 consecutive patients who underwent LN or LNU between November 2003 and September 2007 were retrospectively reviewed. Preoperative data included age, sex, operative side, American Society of Anesthesiologists score, preoperative diagnosis, and tumor size (if applicable, measured in its largest diameter by contrast-enhanced computed tomography). Intraoperative data included operative time, estimated blood loss, surgical complications, and transfusion of blood products. Postoperative data included complications, histopathology results, and outpatient follow-up. Complications were reported using the Clavien classification system,4 and follow-up was performed according to our institution's protocol and included physical examination, blood count, blood chemistry, and renal function tests at every visit, plus abdominal computed tomography six months after surgery. Additional imaging was scheduled according to disease stage and grade.
Statistical Analysis
Normally distributed quantitative data were summarized as means, and measures of variability were reported as standard deviations, whereas non-normally distributed data were summarized as median and variability reported as interquartile range (IQR). Qualitative data were reported as percentages.
Surgical Technique
A 45° to 60° flank position and a three- or four-port transperitoneal approach is preferred. On the right side, an extra port below the xiphoid process is always placed for appropriate liver retraction (Figure 1).
Figure 1.
Our preferred method of liver retraction is shown. An endoclinch atraumatic grasper is placed through a 5-mm trocar under the xiphoid process and locked to the lateral abdominal wall.
Step 1: Detachment from Intraperitoneal Structures
The posterior aspect of the peritoneum along the Told fascia is incised. The colon is displaced medially and the anterior aspect of Gerota fascia is identified. At this point, it is important to omit incising the lateral attachments of the kidney because it will fall medially and obstruct proper identification of the main structures. In addition, the dissection should continue outside of the Gerota fascia because this will allow complete medial displacement of the colon and duodenum and easy identification of the vena cava and renal vein. The incision is continued surrounding the kidney. On the right side, the hepatocolic, triangular, and hepatorenal ligaments are incised. On the left side, the splenocolic, splenorenal, and splenodiaphragmatic ligaments are incised so the spleen will freely fall medially (Figure 2).
Figure 2.
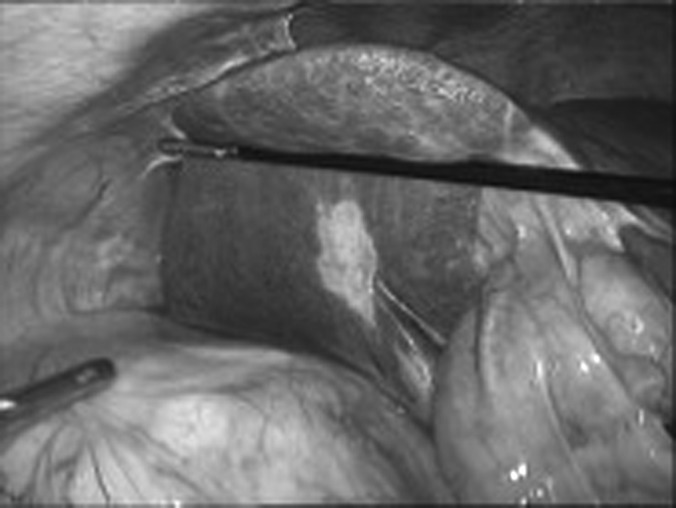
Detachment of the spleen during a LT nephrectomy. The spleen is gently lifted cranially with the shaft of an atraumatic grasper, and cutting dissection of all splenorenal and splenocolic attachments is performed. It is important not to displace the spleen with the tip of the grasper because this can cause inadvertent splenic injury.
Step 2: Inferoposterior Dissection
A window of exposure is created at the lower pole of the kidney on the anterior surface of the psoas muscle. The nondominant hand is used to lift the ureter and perinephric tissue, placing the hilum in tension and aiding in its identification. Dissection then continues cranially over the surface of the psoas muscle until the hilum is identified (Figure 3[A&B]). On the right side, the anterior wall of the inferior vena cava should be clearly identified as well (Figure 3[A]). All tissue located posterior to the renal hilum is then cleaned out. No attempt was made to identify the hilar structures.
Figure 3.
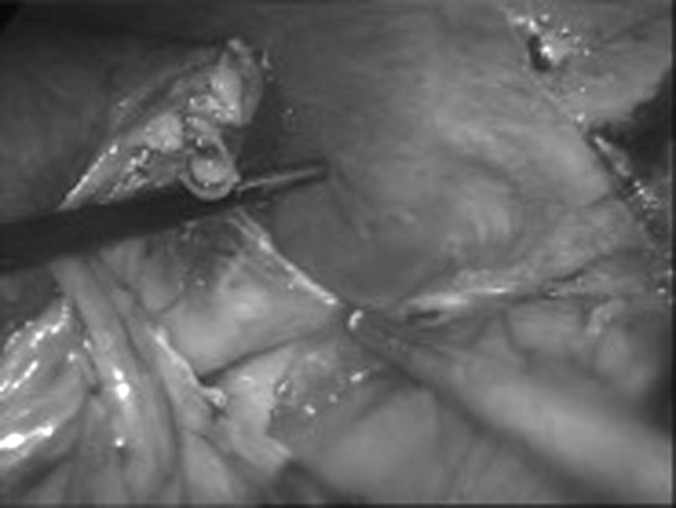
The psoas muscle is clearly identified along its anterior aspect. The kidney is lifted up from under the ureter with the goal of progressing with the dissection cranially over the surface of the psoas muscle. A, On the right side, the psoas muscle, ureter, and vena cava are clearly seen. B, On the left side, the psoas muscle and gonadal vein are also seen.
Step 3: Medial Dissection and Hilar Identification
At this point, the kidney has been completely freed from all inferior and posterior attachments. As the dissection continues cranially, a not well-delimitated renal hilum can be identified. The goal now is to identify the medial aspect of the renal vein (Figure 4[A&B]). A small window is created outside the cranial aspect of the renal vein to allow the placement of one of the jaws of the stapler (Figure 5[A&B]). Care should be taken to not injure the main adrenal vein on the left and an accessory adrenal vein, if present on the right side. No attempt is done to dissect the plane between the renal vein and artery.
Figure 4.
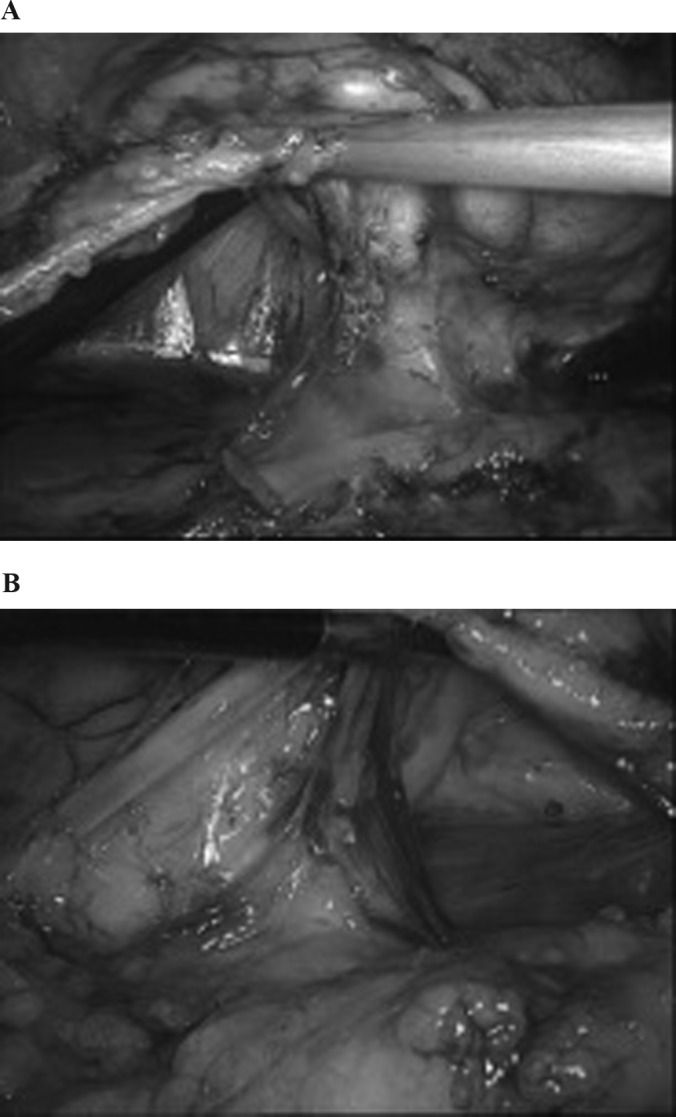
A, Right side; B, left side. The renal hilum is put in tension and all tissue located posteriorly over the surface of the psoas muscle is cleaned up. Notice that no attempt has been made to dissect in between hilar structures.
Figure 5.
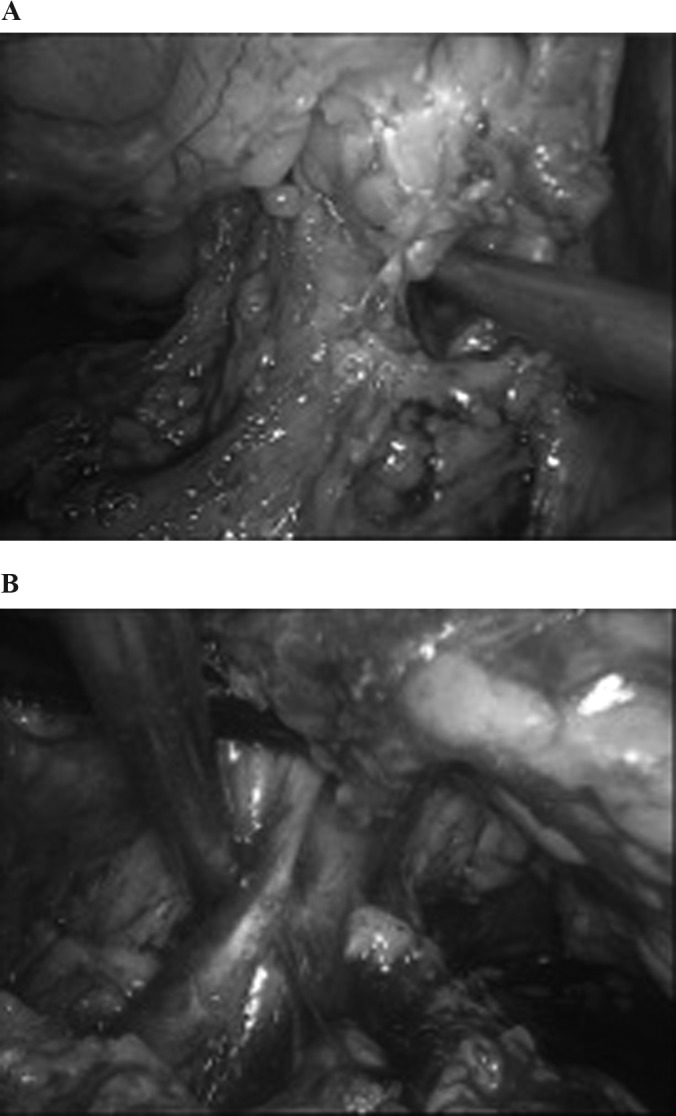
A, Right side; B, left side. A window is created with the suction cannula cranial to the renal vein to allow one of the jaws of the staple. Notice that the wide dissection allows a clear window posterolateral to the renal hilum.
Step 4: Stapling of the Hilum
Placing the kidney into tension allows the identification of the anterior wall of the renal vein, a variable bulky posterior fatty tissue containing the renal artery and a generous posterolateral window delineated by the psoas muscle and the lateral abdominal wall. A 45-mm vascular endostapler loaded with 2.5-mm titanium clips is inserted and closed, including stapling the vein and the remaining posterior hilar tissue. Correct positioning of the stapler is of maximal importance. On the right side, it is placed through a subcostal 12-mm port and runs cephalic to caudal and medial to lateral (Figure 6[A]). It is important to ensure that the stapler runs over the anterior wall of the inferior vena cava and does not catch in the duodenum. In this way, the tip reaches the already dissected posterolateral aspect of the kidney, where no elementary structures are found. On the left side, it is placed through a 12-mm port in the left lower quadrant and runs from caudal to cephalic. The direction of the jaws should be as horizontal as possible to prevent stapling the aorta and to reliably catch the renal artery that will run perpendicular to the stapler (Figure 6[B]). The tip of the staple should always be checked before firing; of special concern are the tail of the pancreas, superior mesenteric artery, splenic vessels, and part of the stomach that might descend through the lateral aspect of the spleen. After firing, a nice staple line that includes the vein and the artery is usually observed. Our preferred technique for hilar control is en bloc stapling5; however, if the thickness of the hilum does not allow safe en bloc stapling, a plane is created between the renal vein and all posterior elements to allow the entrance of one of the jaws of the stapler. One load is used to staple all posterior hilar tissue and another to staple the remaining renal vein. The remaining lateral and upper pole attachments are then released and the kidney is extracted inside a laparoscopic bag.
Figure 6.
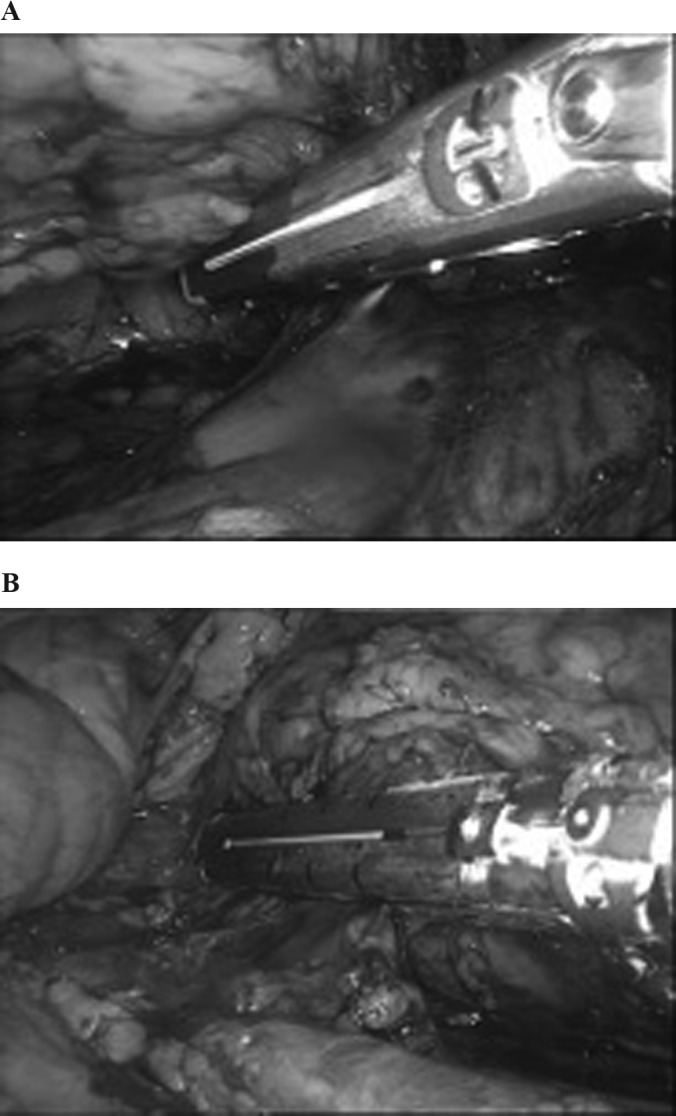
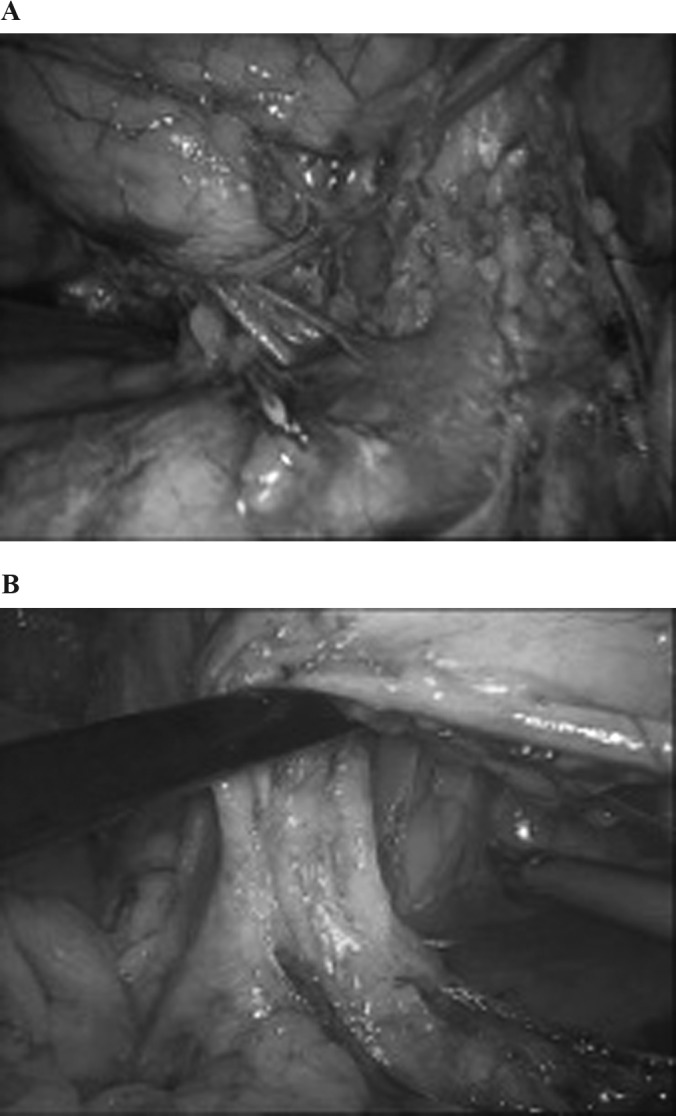
Safe en bloc stapling of the hilum. The hilum is kept in tension. A, On the right side, the staple is deployed over the inferior vena cava and away from the duodenum. B, On the left side, care should be taken to stay away from the colon, splenic hilum, and tail of the pancreas.
RESULTS
An attending urologist performed the surgeries with a resident or fellow, with the trainee performing variable steps of the procedure including kidney dissection and hilar control.
Patients' characteristics and outcomes are described in Table 1. Conversion to open surgery was recorded in four cases (3.1%): two caused by failure to progress secondary to thick perirenal inflammatory tissue, one caused by bleeding from a missed aberrant artery, and the remaining a result of venous bleeding that did not allow proper visualization. No patients had a vascular injury to the renal hilum. Thirty-nine complications were registered in 33 patients (Table 2). Three patients underwent reinterventions: one as a result of eventration at the specimen retrieval incision; one because of postoperative bleeding and hypotension, the source of which was found to be the renal vein stump with inadequate staple lining; and the remaining as a result of peritonitis secondary to inadvertent bowel injury. In addition, one patient had an intraabdominal abscess after a simple nephrectomy for a nonfunctioning kidney and was treated successfully with percutaneous drainage. Most of the patients had minor intraoperative bleeding, with 82 (64%) of them having <50 mL of quantifiable blood loss. After a median follow-up of 28 months, no cases of arteriovenous fistula were detected.
Table 1.
Patients' Characteristics and Outcomes
| N = 129 | SD | |
|---|---|---|
| Mean age (y) | 63 y | 15.6 |
| Male (%) | 58 | |
| Mean BMI | 27 | 5.1 |
| Right side (%) | 38 | |
| Type of surgery (%) | ||
| RN | 62 | |
| NU | 20 | |
| SN | 18 | |
| Mean tumor size in RN | 6.3 cm | 2.4 |
| Mean operative time | 128 min | 41.4 |
| Median estimated blood loss | 0 mL | (0.200)* |
| Intraoperative transfusion rates (%) | 8 | |
| Conversion to open surgery (%) | 3.1 | |
| Overall complications (%) | 26 | |
| Histopathology report (%) | ||
| RCC | 61 | |
| UC | 12 | |
| Benign | 27 |
25th and 75th percentiles.
BMI, body mass index; NU, nephroureterectomy; RCC, renal cell carcinoma; RN radical nephrectomy; SN, simple nephrectomy; UC, urothelial carcinoma.
Table 2.
Complications According to the Clavien Classification System
| Overall number of patients | 129 | |
| No patients with registered complications | 33 | |
| Total number of complications | 39 | |
| N | % | |
| Grade of complications | ||
| Grade 1 (not requiring pharmacological treatment) | ||
| Skin hematoma | 2 | 2 |
| Lung atelectasia | 1 | 1 |
| Wound infection | 1 | 1 |
| Grade 2 (requiring pharmacological treatment) | ||
| In-hospital blood transfusion | 23 | 18 |
| Urinary infection | 3 | 2 |
| Grade 3 (requiring surgical or percutaneous treatment) | ||
| Bowel injury | 1 | 1 |
| Bowel eventration | 1 | 1 |
| Abdominal abscess | 1 | 1 |
| Bleeding | 1 | 1 |
| Grade 4 (requiring treatment in intensive care unit) | ||
| Pulmonary edema | 2 | 2 |
| Acute coronary syndrome | 2 | 2 |
| Seizures | 1 | 1 |
| Grade 5 (death of the patient) | 0 | 0 |
A brief description of corresponding grade is given in the parentheses.
DISCUSSION
Laparoscopic surgery is being increasingly performed by general urologists who did not undergo formal laparoscopic training. European surveys6,7 showed that laparoscopy was performed in as much as 71% of sampled departments, with most of the remaining intending to establish it during the upcoming years. Others2,3 found strong correlation between performing laparoscopy and receiving laparoscopic training during residency programs. Surprisingly, case burden was not a deciding factor, because 73% of residents who performed fewer than 15 cases continued to perform laparoscopy afterwards, with LN being one of those primarily performed.1,2 Short “on hand” courses have become another popular source of training. A report of a five-day “mini-residency” course for postgraduate urologists3 showed that 81% of the participants were performing laparoscopy within 8 months after the course—especially LN—although none of them had formal fellowship training in laparoscopy.
The standard technique for LN entails early and meticulous dissection of the renal hilum, individual clipping of all encountered arteries, and stapling or clipping of the vein. Limitations in retraction and exposure, loss of adequate vision even with a small amount of bleeding, and a well-hidden and posteriorly located artery can render this step cumbersome and even hazardous in the hands of an inexperienced laparoscopic surgeon. Early bleeding during the procedure can severely compromise success and has been a frequent cause of conversion to open surgery. A Japanese study reported 11.5% of vascular complications in 78 laparoscopic nephrectomies done at the beginning of their experience.8 In this interesting report, a wide range of accidents secondary to dissection of the renal hilum were encountered: renal artery lesions during dissection of periarterial tissue, bleeding from the renal artery after stapling over an existing clip, clipping of the superior mesenteric artery after mistaking it for the left renal artery, stapling of the inferior vena cava instead of the right renal vein after division of the renal artery, and injuries to the vena cava during right hilar dissections. They concluded, in agreement with Vallancien et al,9 that a minimum of 50 cases is required to acquire the necessary skills for LN. Others reported that the learning curve could be overcome after 12 months of dedicated laparoscopic training.10
We believe our technique represents an evolution to the traditional approach to LN that corresponds better to current practice patterns, because we have found our residents to easily assimilate its concepts with excellent feedback. Because our technique differs from the traditional way the hilum is approached and controlled, considerations specifically concerning this approach should be undertaken. The technique of vascular control by stapling is different from that of clipping or tying. To perform a safe hilar stapling during LN, certain technical steps should be undertaken in advance. Complete dissection and release of the spleen when performing a left nephrectomy is of paramount importance. This allows the spleen to be rolled medially, where it has no risk of being inadvertently torn during upper-pole dissection. In addition, this maneuver also places the tail of the pancreas and splenic vessels out of the operative field and, more important, out of the line of the stapler. The appropriate technique to release the spleen consists of cutting dissection exclusively. The assisting grasper is placed under the spleen with its tip resting at the lateral abdominal wall (Figure 2). In this way, the spleen is gently lifted with the shaft of the instrument and previously mentioned ligaments cut. The spleen should never be lifted with the tip of the grasper because this might produce inadvertent stabbing, a frequent cause of postoperative bleeding and reintervention, even if it is discovered during the intraoperative. On the right side, the anterior wall of the vena cava must be clearly identified to place the stapler over the vena cava and avoid injuring it inadvertently.
To perform safe stapling and cutting, the renal hilum must be kept in tension at all times and there must be no tissue located posterolaterally to the hilum. This is achieved by exposing the psoas muscle inferiorly and laterally to the renal hilum by maintaining the kidney attachments to the lateral abdominal wall. These maneuvers allow the exposure of a clear window for stapling when the hilum is lifted up on tension.
A perfectly aligned staple line should be ensured before the staple is fired. Solid structures trapped between the staple jaws (e.g., clips), stapling of bulky tissue, or tissue being trapped at the tip of the stapler will all prevent appropriate alignment of the staple line, thus the cutting will be performed through nonproperly secured borders. To appropriately assess for these pitfalls, a wide window should be created before the stapler is placed and the closure of the jaws evaluated throughout its entire length.
Arteriovenous fistula has been a traditional concern during en bloc control of the renal hilum. Although this might be true for en bloc ligation with silk sutures as previously reported,11 it does not seem to be the case with nonreactive titanium clips. In 400 cases of en bloc stapling reported, including a recent randomized trial, no cases of AVFs have been found.12,13
We are aware of the study limitations—mainly the retrospective design and lack of comparison with other nephrectomy techniques. We believe our technique simplifies the teaching of LN and brings it closer to the general urologist. Its main advantage is that postpones any manipulation of the hilum to a later step during the procedure when it is easy to identify and control. This decreases early bleeding and main vascular complications. At the moment the hilum is boarded, the kidney has been released from its inferior and posterior adherences in a stepwise fashion and its upper pole freed from surrounding structures. At the moment the stapler is positioned, there is plenty of space for a safe and soft entrapment of the renal pedicle.
Contributor Information
Oscar Schatloff, Department of Urology, Assaf HaRofeh Medical Center, Tel Aviv, Israel..
Andrei Nadu, Department of Urology, Rabin Medical Center, Petah Tikva, Israel..
Uri Lindner, Department of Urology, Kaplan Medical Center, Rehovot, Israel..
Jacob Ramon, Department of Urology, The Chaim Sheba Medical Center, Ramat Gan, Israel..
References:
- 1. Abdelshehid CS, Eichel L, Lee D, et al. Current trends in urologic laparoscopic surgery. J Endourol. 2005;19(1):15–20 [DOI] [PubMed] [Google Scholar]
- 2. Shay BF, Thomas R, Monga M. Urology practice patterns after residency training in laparoscopy. J Endourol. 2002;16(4):251–256 [DOI] [PubMed] [Google Scholar]
- 3. Corica FA, Boker JR, Chou DS, et al. Short-term impact of a laparoscopic “mini-residency” experience on postgraduate urologists' practice patterns. J Am Coll Surg. 2006;203(5):692–698 [DOI] [PubMed] [Google Scholar]
- 4. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schatloff O, Ramon J, Lindner U, et al. Is postoperative arteriovenous fistula still a concern after en bloc stapling of the renal hilum during laparoscopic nephrectomy? J Endourol. 2009;23:639–643 [DOI] [PubMed] [Google Scholar]
- 6. Vögeli TA, Burchardt M, Fornara P, Rassweiler J, Sulser T; Laparoscopic Working Group of the German Urological Association. Current laparoscopic practice patterns in urology: results of a survey among urologists in Germany and Switzerland. Eur Urol. 2002;42(5):441–446 [DOI] [PubMed] [Google Scholar]
- 7. Laguna MP, Schreuders LC, Rassweiler JJ, et al. ; European Society of Uro-Technology. Development of laparoscopic surgery and training facilities in Europe: results of a survey of the European Society of Uro-Technology (ESUT). Eur Urol. 2005;47(3):346–351 [DOI] [PubMed] [Google Scholar]
- 8. Kanno T, Shichiri Y, Oida T, Kanamaru H, Takao N, Shimizu Y. Complications and the learning curve for a laparoscopic nephrectomy at a single institution. Int J Urol. 2006;13:101–104 [DOI] [PubMed] [Google Scholar]
- 9. Vallancien G, Cathelineau X, Baumert H, Doublet JD, Guillonneau B. Complications of transperitoneal laparoscopic surgery in urology: review of 1,311 procedures at a single center. J Urol. 2002;168:23–26 [PubMed] [Google Scholar]
- 10. Cadeddu JA, Wolfe JS, Jr, Nakada S, et al. Complications of laparoscopic procedures after concentrated training in urological laparoscopy. J Urol. 2001;166:2109–2011 [PubMed] [Google Scholar]
- 11. Lacombe M. Renal arteriovenous fistula following nephrectomy. Urology. 1985;25:13–16 [DOI] [PubMed] [Google Scholar]
- 12. Schatloff O, Lindner U, Lindner A. Current status of en bloc stapling of the renal hilum during laparoscopic nephrectomy. J Laparoendosc Adv Surg Tech A. 2010;20:631–633 [DOI] [PubMed] [Google Scholar]
- 13. Chung JH, Lee SW, Lee KS, Cho WY, Kim TH. Safety of en bloc ligation of the renal hilum during laparoscopic radical nephrectomy for renal cell carcinoma: a randomized controlled trial. J Laparoendosc Adv Surg Tech A. 2013;23:489–494 [DOI] [PubMed] [Google Scholar]


