Abstract
Background and Objectives:
This study evaluates our technique for robot-assisted sleeve gastrectomy for morbidly obese and super obese patients and our outcomes.
Methods:
A retrospective analysis of patients who underwent robot-assisted sleeve gastrectomy at a single center was performed. The procedure was performed with the da Vinci Si HD Surgical System (Intuitive Surgical, Sunnyvale, California). The staple line was imbricated with No. 2-0 polydioxanone in all cases. The super obese (body mass index ≥50 kg/m2) subset of patients was compared with the morbidly obese group in terms of demographic characteristics, comorbidities, operative times, perioperative complications, and excess body weight loss.
Results:
A total of 35 patients (15 female and 20 male patients) with a mean body mass index of 48.17 ± 11.7 kg/m2 underwent robot-assisted sleeve gastrectomy. Of these patients, 11 were super obese and 24 were morbidly obese. The mean operative time was 116.3 ± 24.7 minutes, and the mean docking time was 8.9 ± 5.4 minutes. Mean blood loss was 19.36 ± 4.62 mL, and there were no complications, conversions, or perioperative deaths. When compared with the morbidly obese patients, the super obese patients showed no significant difference in operative time, blood loss, and length of hospital stay. There was a steep decline in operating room times after 10 cases of robot-assisted sleeve gastrectomy.
Conclusion:
This study shows the feasibility and safety of robot-assisted sleeve gastrectomy. Robotic assistance might help overcome the operative difficulties encountered in super obese patients. It shows a rapid reduction in operative times with the growing experience of the entire operative team. Robot-assisted sleeve gastrectomy can be a good procedure by which to introduce robotics in a bariatric surgery center before going on to perform Roux-en-Y gastric bypass and revision procedures.
Keywords: Sleeve gastrectomy, Robot-assisted bariatric surgery, Super obese, Robotic surgery, Laparoscopic bariatric, Robotic sleeve
INTRODUCTION
Laparoscopic sleeve gastrectomy (SG) was introduced by Gagner and colleagues1,2 as a first-step procedure to minimize surgical risk for super-super obese (SSO) or high-risk patients, followed by either laparoscopic biliopancreatic diversion with duodenal switch or laparoscopic Roux-en-Y gastric bypass (RYGB). SG is now established as a primary bariatric procedure apart from being a first-stage procedure in a planned bariatric approach for high-risk patients.3,4
Super obese (SO) patients with a body mass index (BMI) ≥50 kg/m2 are a difficult-to-manage population because of the huge liver, leading to limited working space; excessive torque on the instruments because of the thick abdominal wall; comorbidities; and high-risk anesthesia.5 The management of patients with super-super obesity (BMI ≥60 kg/m2) also remains a challenge.6
Robotic surgery may be a suitable alternative to the laparoscopic approach in SO and SSO patients. Robotic surgery allows for more precise manipulations and increased dexterity by downscaling the surgeon's movements and filtering out physiological tremor. It liberates the surgeon from torque on the instruments because of the thick abdominal wall and decreases the port-site trauma because of remote-center technology, thereby conferring improved ergonomic positioning.7 Furthermore, a true 3-dimensional view of the intra-abdominal anatomy with robotic surgery facilitates fine tissue dissection.8 The main disadvantages associated with robotic surgery remain its high cost and longer setup time compared with the laparoscopic equivalent for many procedures. With increased experience, it may be assumed that this setup time will be reduced, and in the future, operative costs may decrease as material prices are reduced.9
This study evaluates our initial experience with and technique for robot-assisted sleeve gastrectomy (RASG) in morbidly obese (MO) and SO patients.
MATERIALS AND METHODS
This study is a retrospective analysis of our patients who underwent RASG. A robotic platform was used for bariatric surgery starting in March 2012 at our well-established center of laparoscopic bariatric surgery. The patients met the National Institutes of Health consensus criteria,10 as well as the institutional policies, for undergoing a bariatric procedure. An informed consent form was obtained from all patients.
A total of 35 patients underwent RASG from May 2012 to October 2013. The procedures were performed by 2 surgeons in the same hospital. After the required training in robotic surgery, the surgeons initially performed a few simple cases such as cholecystectomy, hernia repair, and hiatal surgery before proceeding with bariatric procedures.
Preoperative evaluation included BMI, anthropometry, routine blood parameters, nutritional markers (vitamin B12, folic acid, iron, ferritin, vitamin D3), diabetes profile (fasting blood glucose, hemoglobin A1c, fasting insulin, and C-peptide levels), lipid and thyroid profile, sleep study, upper gastrointestinal endoscopy, pulmonary function test, dobutamine stress echocardiogram, chest radiography, and ultrasonography of abdomen.
The following parameters were evaluated: patient demographic characteristics, body mass index (BMI), comorbidities, total operative time (further split into docking time and imbrication time), mean blood loss, conversions, perioperative complications and deaths, length of hospital stay, and excess body weight loss at 6 months after the procedure. The operative time was defined as the time from skin incision to skin closure, including any associated procedures. All patients were followed up at an outpatient clinic. The SO (BMI ≥50 kg/m2) subset of patients was compared with the MO patient group based on the previously mentioned parameters. Statistical analysis was performed using SPSS software, version 19.0 (IBM, Armonk, New York).
Operative Technique: RASG
The patient is placed in the supine position with his or her legs by the side. A 38F gastric calibration tube is used after induction to empty out the gastric contents and is kept in place to guide the sleeve formation. Pneumoperitoneum is created with a Veress needle at Palmer's point up to a pressure of 15 mm Hg. All distances for port placement are measured after insufflation because measurements obtained before pneumoperitoneum is achieved may be misleading. Pre-emptive infiltration of 0.5% bupivacaine is performed before incision at all port sites.
The camera port (12-mm-diameter, 150-mm-long Endopath XCEL trocar; Ethicon Endo-Surgery, Cincinnati, Ohio) is placed 20 cm below the xiphisternum slightly to the left of the midline under vision using a 0° 10-mm laparoscope. The ports for the robotic arms (R1, R2, and R3) are established next. R1 (8-mm da Vinci cannula; Intuitive Surgical, Sunnyvale, California) is placed at the left midclavicular line approximately 20 cm from the xiphisternum. R2 (5-mm da Vinci cannula) is placed in the right hypochondrium at the midclavicular line, with care taken to ensure that the entry of the port is below the margin of the liver. R3 (5-mm da Vinci cannula) is placed in the left flank at the level of the camera port. The assistant port (12-mm-diameter, 100-mm-long Endopath XCEL trocar) is placed between the camera port and R2 with a distance of at least 10 cm from both of them. A 5-mm epigastric port is made and used to place a Nathanson liver retractor for retraction of the left lobe of the liver (Cook Inc., Bloomington, IN, USA) (Figure 1).
Figure 1.
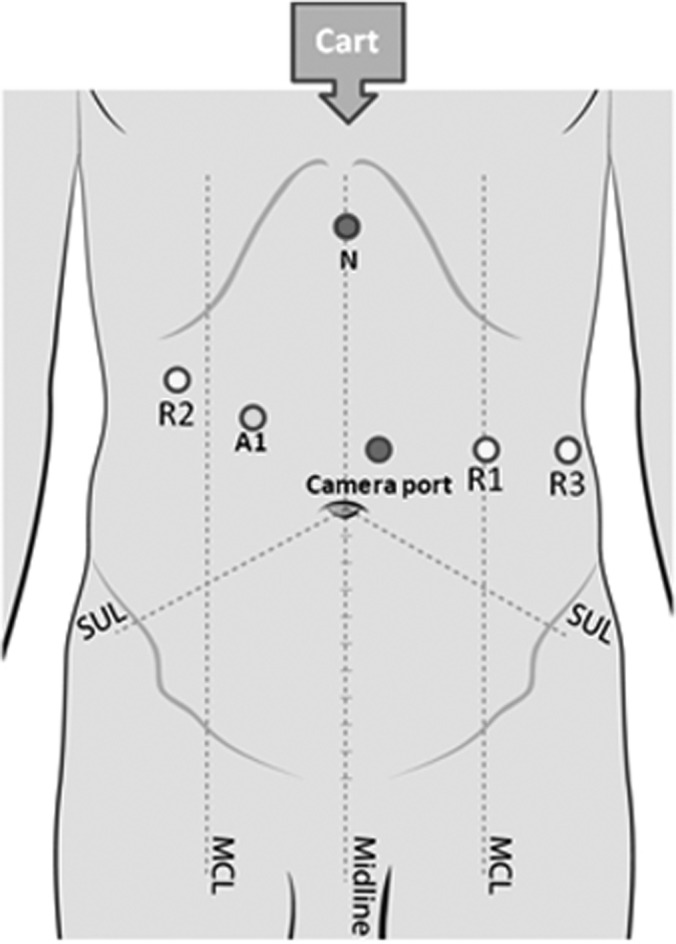
Port positions. The patient cart is brought from the head end of the patient. A1 = assistant port; N = Nathanson retractor; R1/R2/R3 = ports for robotic arms; MCL = mid clavicular line; SUL = Spino-umbilical line.
The patient is placed in the steep reverse Trendelenburg position. All 3 arms of the da Vinci Surgical System (Intuitive Surgical) are used. The patient cart is brought from the head end of the patient, and docking is performed. The instruments used for RASG are as follows: 8-mm da Vinci Harmonic ACE curved shears (Ethicon Endo-Surgery, Intuitive Surgical, Sunnyvale, CA, USA) (R1, for gastrolysis), 5-mm bowel graspers (R2 and R3), and 5-mm needle driver (R1, for over-sewing staple line). A 5-cm umbilical tape is used to measure the distance from the pylorus, and gastrolysis is performed along the greater curvature of the stomach using a Harmonic scalpel (Ethicon Endo-Surgery, Intuitive Surgical, Sunnyvale, CA, USA). The short gastric vessels from the gastrosplenic ligament and posterior gastric adhesions with the pancreas are divided. One can appreciate a bilaminar parchment–like membrane if one remains close to the stomach on the greater curvature. This layer is relatively avascular and is the right plane for gastrolysis. The left crus is completely defined so that the fundus is adequately mobilized. Care is taken to ensure that the bougie is withdrawn in the esophagus during this step; otherwise, it can lead to difficulty in dissection around the hiatus.
The gastric calibration tube is pushed forward by the anesthetist and guided by the surgeon into the first part of the duodenum using bowel graspers. Rotation of the bougie by the anesthetist and correct amount of traction by the surgeon help in negotiating the pylorus. This leads to the bougie nicely resting along the lesser curve of the stomach and helps in formation of an even sleeve. A sleeve of stomach is created using an articulating stapler from the assistant port while the console surgeon applies traction on the stomach so that it lies in the right orientation. It is important to avoid any spiraling or an uneven staple line. Sequential stapling first with a green load and subsequently with blue loads is performed while not being very snug to the bougie. A compression time of 30 seconds is allowed before and after every firing.
After the sleeve is formed, the staple line is imbricated using No. 2-0 polydioxanone running suture with a sliding loop at one end. We find that 17 inches is the appropriate length of suture for most cases. Gastroscopy with saline solution immersion is performed in all cases to rule out any leak, bleed, or obstruction.
RESULTS
A total of 35 patients (15 female and 20 male patients) with a mean BMI of 48.17 ± 11.7 kg/m2 underwent RASG. The BMI ranged from 33 to 82.6 kg/m2, with 3 patients having a BMI >80 kg/m2. The mean age of the patients was 41.8 ± 10.4 years. There were 11 SO patients. The mean number of comorbidities per patient was 1.88. The mean operative time was 116.3 ± 24.7 minutes, and the mean docking time was 8.9 ± 5.4 minutes. The mean time taken to imbricate the staple line was 21.4 ± 6.4 minutes. Mean blood loss was 19.36 ± 4.62 mL, and there were no complications, conversions, or perioperative deaths in the series. The mean length of hospital stay was 3.4 ± 0.8 days.
A steep decline in operative time and docking time was observed after the initial 10 cases. We believe that the operating room team surpassed the learning curve for robotic bariatric surgery by the 10th case. The team was already well-versed in laparoscopic bariatric procedures and could adjust to the new system fairly quickly.
The SO group was compared with the MO group regarding patient demographic characteristics, BMI, and comorbidities (Table 1), as well as perioperative outcomes, complications, and weight loss (Table 2). The comparison showed no significant difference in operative time (P = .145), blood loss (P = .523), and length of hospital stay (P = .213). There were no perioperative complications or deaths in either group. The excess body weight loss at 6 months' follow-up was also not significantly different between the 2 groups.
Table 1.
Patient Demographic Data, BMI, and Comorbidities
| Morbidly Obese (n = 24) | Super Obese (n = 11) | P Value | |
|---|---|---|---|
| Male patients | 13 (54.2%) | 7 (63.6%) | .61 |
| Female patients | 11 (45.8%) | 4 (36.4%) | |
| Age (mean ± SD) (yr) | 42 ± 10.8 | 41.2 ± 8.7 | .81 |
| Mean No. of comorbidities | 2 | 1.64 | .77 |
| Initial BMIa (mean ± SD) (kg/m2) | 43.2 ± 4.3 | 61 ± 12.9 | .001 |
| Initial weight (mean ± SD) (kg) | 120.7 ± 18.7 | 166.6 ± 36.9 | .003 |
| Diabetes | 9 (37.5%) | 4 (36.3%) | .951 |
| Hypertension | 14 (58.3%) | 6 (54.5%) | .843 |
| Obstructive sleep apnea | 11 (45.8%) | 7 (63.6%) | .012 |
| Hypothyroidism | 14 (58.3%) | 1 (9.1%) | .776 |
BMI = body mass index.
Table 2.
Perioperative Outcomes, Complications, and EBW Loss in Morbidly Obese Versus Super Obese Patients
| Morbidly Obese (n = 24) | Super Obese (n = 11) | P Value | |
|---|---|---|---|
| Operative time (mean ± SD) (min) | 119.9 ± 26.7 | 108.6 ± 15.9 | .145 |
| Docking time (mean ± SD) (min) | 9.7 ± 5.9 | 7.3 ± 3.2 | .133 |
| Imbrication time (mean ± SD) (min) | 22.1 ± 6.8 | 19.8 ± 4.4 | .267 |
| Blood loss (mean ± SD) (mL) | 12.1 ± 10.2 | 15 ± 12.4 | .523 |
| Hospital stay (mean ± SD) (d) | 3.2 ± 0.4 | 3.7 ± 1.2 | .213 |
| Perioperative complications | 0 | 0 | >.99 |
| Perioperative mortality | 0 | 0 | >.99 |
| Conversion rate | 0 | 0 | >.99 |
| % EBWa loss at 6 mo (mean ± SD) | 43.4 ± 8.6 | 41.5 ± 10.5 | .143 |
EBW = excess body weight.
DISCUSSION
Laparoscopic bariatric surgery, especially in SO patients, presents a complex situation in which the surgeon has to deliver the best results despite compromised technology. With the advent of robotic technology, its application to bariatric procedures may eliminate certain limitations such as torque on rigid instruments because of the thickness of the abdominal wall and difficulty in dissection or suturing in a limited working space because of a heavy liver and excess omental fat. It also provides for a stable and ergonomically comfortable platform with 3-dimensional vision and precise movements without any tremor.11
Use of robotics in bariatric surgery has been studied more extensively in RYGB.12–15 SG, on the other hand, is considered a relatively simple procedure compared with RYGB. However, there are certain peculiarities in SG; for example, it uses a long staple line with a potential to leak, and precise and safe dissection is required in the area of the left crus and hiatus to entirely mobilize the fundus. Compared with laparoscopic surgery, robotic surgery offers the possibility of EndoWrist application (Intuitive Surgical), and this action facilitates the over-sewing of the staple line.16 Over-sewing of the staple line was accepted by most of the surgeons (95%) participating in the International Sleeve Gastrectomy Expert Panel.17 Any leak with SG is a high-pressure leak and is difficult to manage compared with an RYGB leak. These difficulties become more pronounced when operating in an SO patient because of limited working space.18 SO and SSO patients are high-risk candidates, and SG is considered the first step in a 2-stage procedure19 or, more and more often, is performed as a single-stage procedure.20 From a surgical point of view, SO patients remain a difficult population to manage, usually with outcomes slightly less favorable than those for MO patients.21,22
There have been comparisons of robot assistance in RYGB in SO patients versus MO patients,23 and there are studies that state that RASG is a safe and feasible operation.24,25 However, there are no or very few studies comparing the perioperative outcomes of using a robot for SG in SO patients versus MO patients.
The results of RASG for the SO group versus the MO group were compared to look for any advantages provided by the robotic system in the SO group. In our experience, the outcomes in both the SO and MO groups were the same, with no complications in either group. This occurred despite the fact that there were a few very high-risk SSO patients in this series, including 3 patients with a BMI >80 kg/m2. In addition, a robotic system helps in precisely over-sewing the staple line (as is the routine practice in our center) even in SSO patients with a severely limited working space. Thus, using a robotic system may help overcome many difficulties in SO patients and enables the surgeon to perform a similar procedure to that in MO patients with equal precision, similar time requirement, and little or no extra effort.
We believed that during introduction of robotic surgery to a bariatric program, SG was a relatively simple and safer procedure to start with. It enables the whole team to become accustomed to the setup of the robotic system, docking, and troubleshooting.26 Once the team is comfortable with RASG, it can proceed to robotic RYGB and revision surgical procedures. In this series the learning curve for RASG was 10 procedures for a team well-versed in laparoscopic bariatric surgery and well-trained in handling the da Vinci Surgical System. After 10 cases, our docking time, setup time, and console time plateaued with a <5% change in the operative time. The port placement and docking techniques could be standardized by the 10th case.
This study has some limitations that deserve comment. First, the study had a small sample size, and patients were not randomized. Yet, it described our initial experience with RASG and tried to address issues such as safety, outcomes, and the learning curve. Second, the study included the learning curve of the team, which may have increased the mean operating room times. Third, the study did not analyze the additional cost of using a robot, but we believe that clinical outcomes are much more important and that costs are bound to decrease with time. In addition, the study did not compare RASG with laparoscopic SG, and such a comparison is required to ascertain the real benefit of using a robotic system. Further studies on this topic are required to validate these results.
CONCLUSIONS
Our study shows the feasibility and safety of RASG. Robotic assistance might help overcome the difficulties encountered when operating on SO patients. In addition, the ease and precision with which the staple line is imbricated are definite advantages. RASG can act as a good procedure to introduce robotics in a bariatric surgery center before going on to perform RYGB and revision procedures. The learning curve for RASG is about 10 cases for an established bariatric surgery center. The real advantages in comparison with the laparoscopic approach are still being debated and require further studies for the subpopulation of SO patients.
Contributor Information
Parveen Bhatia, Institute of Minimal Access, Metabolic & Bariatric Surgery, Sir Ganga Ram Hospital, New Delhi, India..
Vivek Bindal, Division of General, Minimally Invasive & Robotic Surgery, University of Illinois at Chicago, Chicago, IL, USA..
Rahul Singh, Institute of Minimal Access, Metabolic & Bariatric Surgery, Sir Ganga Ram Hospital, New Delhi, India..
Raquel Gonzalez-Heredia, Division of General, Minimally Invasive & Robotic Surgery, University of Illinois at Chicago, Chicago, IL, USA..
Sudhir Kalhan, Institute of Minimal Access, Metabolic & Bariatric Surgery, Sir Ganga Ram Hospital, New Delhi, India..
Mukund Khetan, Institute of Minimal Access, Metabolic & Bariatric Surgery, Sir Ganga Ram Hospital, New Delhi, India..
Suviraj John, Institute of Minimal Access, Metabolic & Bariatric Surgery, Sir Ganga Ram Hospital, New Delhi, India..
References:
- 1. Ren CJ, Patterson E, Gagner M. Early results of laparoscopic biliopancreatic diversion with duodenal switch: a case series of 40 consecutive patients. Obes Surg. 2000;10:514–523 [DOI] [PubMed] [Google Scholar]
- 2. Regan JP, Inabnet WB, Gagner M, Pomp A. Early experience with two-stage laparoscopic Roux-en-Y gastric bypass as an alternative in the super-super-obese patient. Obes Surg. 2003;13:861–864 [DOI] [PubMed] [Google Scholar]
- 3. Himpens J, Dapri D, Cadière GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg. 2006;16:1450–1456 [DOI] [PubMed] [Google Scholar]
- 4. D'Hondt M, Vanneste S, Pottel H, Devriendt D, Rooy FV. Laparoscopic sleeve gastrectomy as a single-stage procedure for the treatment of morbid obesity and the resulting quality of life, resolution of comorbidities, food tolerance, and 6-year weight loss. Surg Endosc. 2011;25:2498–2504 [DOI] [PubMed] [Google Scholar]
- 5. Parikh MS, Shen R, Weiner M, Siegel N, Ren CJ. Laparoscopic bariatric surgery in super-obese patients (BMI>50) is safe and effective: a review of 332 patients. Obes Surg. 2005;15(6):858–863 [DOI] [PubMed] [Google Scholar]
- 6. Gagner M, Gumbs AA, Milone L, Yung E, Goldenberg L, Pomp A. Laparoscopic sleeve gastrectomy for the super-super-obese (body mass index >60 kg/m(2)). Surg Today. 2008;38(5):399–403 [DOI] [PubMed] [Google Scholar]
- 7. Cadiere GB, Himpens J, Vertruyen M, et al. Evaluation of telesurgical (robotic) Nissen fundoplication. Surg Endosc. 2001;15(9):918–923 [DOI] [PubMed] [Google Scholar]
- 8. Talamini MA, Chapman S, Horgan S, Melvin WS, Academic Robotics Group. A prospective analysis of 211 robotic-assisted procedures. Surg Endosc. 2003;17:1521–1524 [DOI] [PubMed] [Google Scholar]
- 9. Nakadi IE, Melot C, Closset J, et al. Evaluation of da Vinci Nissen fundoplication clinical results and cost minimization. World J Surg. 2006;30(6):1050–1054 [DOI] [PubMed] [Google Scholar]
- 10. Hubbard VS, Hall WH. Gastrointestinal surgery for severe obesity. Obes Surg. 1991;1:257–265 [DOI] [PubMed] [Google Scholar]
- 11. Markar SR, Karthikesalingam AP, Venkat-Ramen V, Kinross J, Ziprin P. Robotic vs. laparoscopic Roux-en-Y gastric bypass in morbidly obese patients: systematic review and pooled analysis. Int J Med Robotics Comput Assist Surg. 2011;7:393–400 [DOI] [PubMed] [Google Scholar]
- 12. Hubens G, Balliu L, Ruppert M, Gypen B, Van Tu T, Vaneerdeweg W. Roux-en-Y gastric bypass procedure performed with the Da Vinci robot system: is it worth it? Surg Endosc. 2008;22:1690–1696 [DOI] [PubMed] [Google Scholar]
- 13. Mohr CJ, Nadzam GS, Curet MJ. Totally robotic Roux-en-Y gastric bypass. Arch Surg. 2005;140:779–786 [DOI] [PubMed] [Google Scholar]
- 14. Yu SC, Clapp BL, Lee MJ, Albrecht WC, Scarborough TK, Wilson EB. Robotic assistance provides excellent outcomes during the learning curve for laparoscopic Roux-en-Y gastric bypass: results from 100 robotic-assisted gastric bypasses. Am J Surg. 2006;192:746–749 [DOI] [PubMed] [Google Scholar]
- 15. Fourman MM, Saber AA. Robotic bariatric surgery; a systematic review. Surg Obes Relat Dis. 2012;8:483–488 [DOI] [PubMed] [Google Scholar]
- 16. Vilallonga R, Fort JM, Caubet E, Gonzalez O, Armengol M. Robotic sleeve gastrectomy versus laparoscopic sleeve gastrectomy: a comparative study with 200 patients. Obes Surg. 2013;23:1501–1507 [DOI] [PubMed] [Google Scholar]
- 17. Rosenthal RJ, International Sleeve Gastrectomy Expert Panel, Diaz AA, et al. International Sleeve Gastrectomy Expert Panel consensus statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8(1):8–19 [DOI] [PubMed] [Google Scholar]
- 18. Gould JC, Garren MJ, Boll V, Starling JR. Laparoscopic gastric bypass: risks vs. benefits up to two years following surgery in super-super obese patients. Surgery. 2006;140(4):524–529; discussion 529–531 [DOI] [PubMed] [Google Scholar]
- 19. Mukherjee S, Devalia K, Rahman MG, Mannur KR. Sleeve gastrectomy as a bridge to a second bariatric procedure in superobese patients—a single institution experience. Surg Obes Relat Dis. 2012;8(2):140–144 [DOI] [PubMed] [Google Scholar]
- 20. Gentileschi P. Laparoscopic sleeve gastrectomy as a primary operation for morbid obesity: experience with 200 patients. Gastroenterol Res Pract. 2012;2012:801325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kakarla VR, Nandipati K, Lalla M, Castro A, Marola S. Are laparoscopic bariatric procedures safe in superobese (BMI >50 kg/m2) patients? An NSQIP data analysis. Surg Obes Relat Dis. 2011;7(4):452–458 [DOI] [PubMed] [Google Scholar]
- 22. Suter M, Calmes JM, Paroz A, Romi S, Giusti V. Results of Roux-en-Y gastric bypass in morbidly obese vs superobese patients: similar bodyweight loss, correction of comorbidities, and improvement of quality of life. Arch Surg. 2009;144(4):312–318; discussion 318 [DOI] [PubMed] [Google Scholar]
- 23. Buchs NC, Pugin F, Chassot G, et al. Robot-assisted Roux-en-Y gastric bypass for super obese patients: a comparative study. Obes Surg. 2013;23(3):353–357 [DOI] [PubMed] [Google Scholar]
- 24. Romero RJ, Kosanovic R, Rabaza JR, et al. Robotic sleeve gastrectomy: experience of 134 cases and comparison with a systematic review of the laparoscopic approach. Obes Surg. 2013;23:1743–1752 [DOI] [PubMed] [Google Scholar]
- 25. Ayloo S, Buchs NC, Addeo P, Bianco FM, Giulianotti PC. Robot-assisted sleeve gastrectomy for super-morbidly obese patients. J Laparoendosc Adv Surg Tech A. 2011;21(4):295–299 [DOI] [PubMed] [Google Scholar]
- 26. Vilallonga R, Fort JM, Gonzalez O, et al. The initial learning curve for robot-assisted sleeve gastrectomy: A surgeon's experience while introducing the robotic technology in a bariatric surgery department. Minim Invasive Surg. 2012;2012:347131. [DOI] [PMC free article] [PubMed] [Google Scholar]


