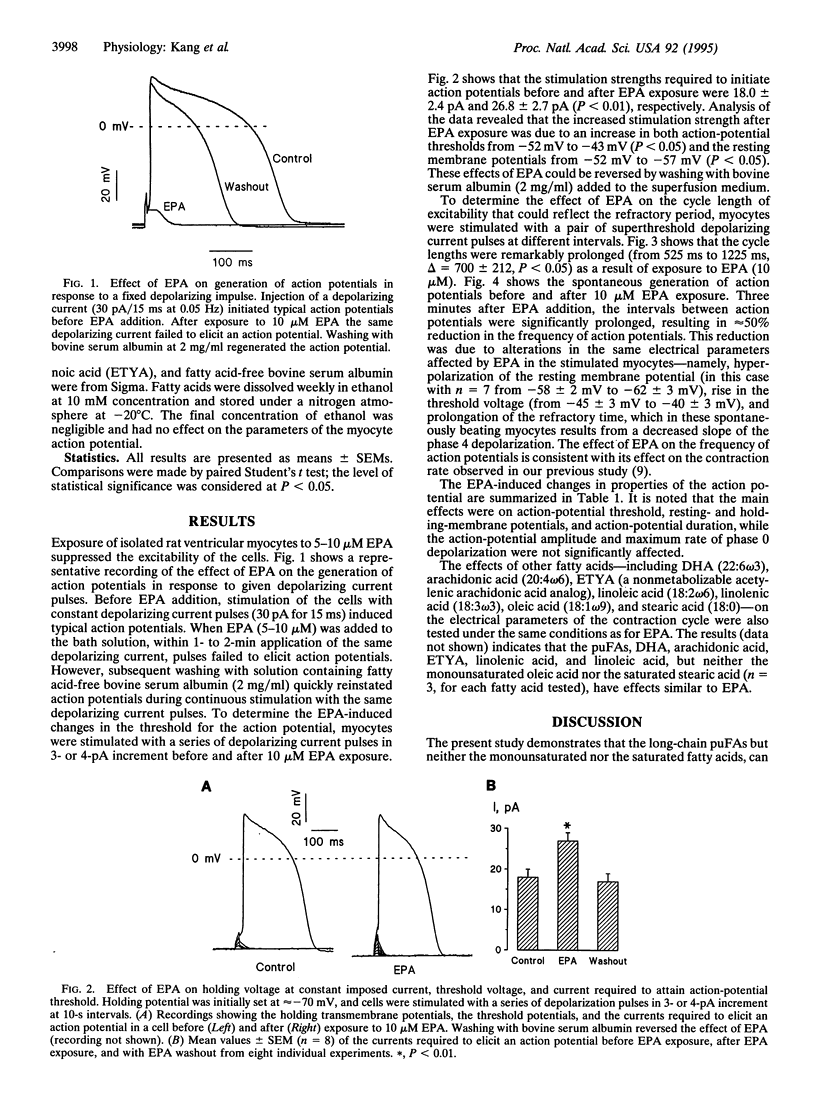
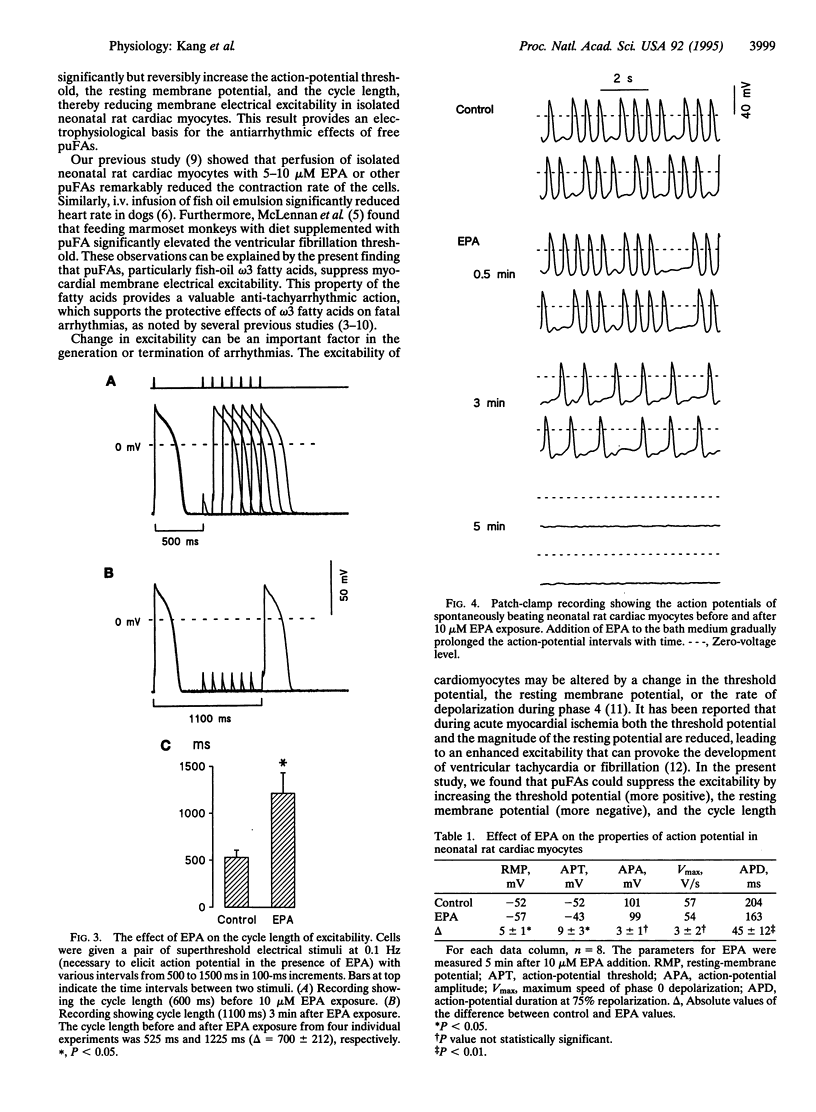
Abstract
Because previous studies showed that polyunsaturated fatty acids can reduce the contraction rate of spontaneously beating heart cells and have antiarrhythmic effects, we examined the effects of the fatty acids on the electrophysiology of the cardiac cycle in isolated neonatal rat cardiac myocytes. Exposure of cardiomyocytes to 10 microM eicosapentaenoic acid for 2-5 min markedly increased the strength of the depolarizing current required to elicit an action potential (from 18.0 +/- 2.4 pA to 26.8 +/- 2.7 pA, P < 0.01) and the cycle length of excitability (from 525 ms to 1225 ms, delta = 700 +/- 212, P < 0.05). These changes were due to an increase in the threshold for action potential (from -52 mV to -43 mV, delta = 9 +/- 3, P < 0.05) and a more negative resting membrane potential (from -52 mV to -57 mV, delta = 5 +/- 1, P < 0.05). There was a progressive prolongation of intervals between spontaneous action potentials and a slowed rate of phase 4 depolarization. Other polyunsaturated fatty acids--including docosahexaenoic acid, linolenic acid, linoleic acid, arachidonic acid, and its nonmetabolizable analog eicosatetraynoic acid, but neither the monounsaturated oleic acid nor the saturated stearic acid--had similar effects. The effects of the fatty acids could be reversed by washing with fatty acid-free bovine serum albumin. These results show that free polyunsaturated fatty acids can reduce membrane electrical excitability of heart cells and provide an electrophysiological basis for the antiarrhythmic effects of these fatty acids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billman G. E., Hallaq H., Leaf A. Prevention of ischemia-induced ventricular fibrillation by omega 3 fatty acids. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4427–4430. doi: 10.1073/pnas.91.10.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr M. L., Fehily A. M., Gilbert J. F., Rogers S., Holliday R. M., Sweetnam P. M., Elwood P. C., Deadman N. M. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet. 1989 Sep 30;2(8666):757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- Charnock J. S., Sundram K., Abeywardena M. Y., McLennan P. L., Tan D. T. Dietary fats and oils in cardiac arrhythmia in rats. Am J Clin Nutr. 1991 Apr;53(4 Suppl):1047S–1049S. doi: 10.1093/ajcn/53.4.1047S. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Gross R. W., Sobel B. E. Amphipathic metabolites and membrane dysfunction in ischemic myocardium. Circ Res. 1984 Aug;55(2):135–154. doi: 10.1161/01.res.55.2.135. [DOI] [PubMed] [Google Scholar]
- Elharrar V., Zipes D. P. Cardiac electrophysiologic alterations during myocardial ischemia. Am J Physiol. 1977 Sep;233(3):H329–H345. doi: 10.1152/ajpheart.1977.233.3.H329. [DOI] [PubMed] [Google Scholar]
- Gudbjarnason S. Dynamics of n-3 and n-6 fatty acids in phospholipids of heart muscle. J Intern Med Suppl. 1989;731:117–128. doi: 10.1111/j.1365-2796.1989.tb01445.x. [DOI] [PubMed] [Google Scholar]
- Hallaq H., Sellmayer A., Smith T. W., Leaf A. Protective effect of eicosapentaenoic acid on ouabain toxicity in neonatal rat cardiac myocytes. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7834–7838. doi: 10.1073/pnas.87.20.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallaq H., Smith T. W., Leaf A. Modulation of dihydropyridine-sensitive calcium channels in heart cells by fish oil fatty acids. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1760–1764. doi: 10.1073/pnas.89.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E., Barhanin J., Attali B., Lesage F., Lazdunski M. External blockade of the major cardiac delayed-rectifier K+ channel (Kv1.5) by polyunsaturated fatty acids. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1937–1941. doi: 10.1073/pnas.91.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. M., Xian H., Bacaner M. Long-chain fatty acids activate calcium channels in ventricular myocytes. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6452–6456. doi: 10.1073/pnas.89.14.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J. X., Leaf A. Effects of long-chain polyunsaturated fatty acids on the contraction of neonatal rat cardiac myocytes. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9886–9890. doi: 10.1073/pnas.91.21.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A. M., Messineo F. C. Lipid-membrane interactions and the pathogenesis of ischemic damage in the myocardium. Circ Res. 1981 Jan;48(1):1–16. doi: 10.1161/01.res.48.1.1. [DOI] [PubMed] [Google Scholar]
- Kim D., Clapham D. E. Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science. 1989 Jun 9;244(4909):1174–1176. doi: 10.1126/science.2727703. [DOI] [PubMed] [Google Scholar]
- Kim D., Duff R. A. Regulation of K+ channels in cardiac myocytes by free fatty acids. Circ Res. 1990 Oct;67(4):1040–1046. doi: 10.1161/01.res.67.4.1040. [DOI] [PubMed] [Google Scholar]
- Kirber M. T., Ordway R. W., Clapp L. H., Walsh J. V., Jr, Singer J. J. Both membrane stretch and fatty acids directly activate large conductance Ca(2+)-activated K+ channels in vascular smooth muscle cells. FEBS Lett. 1992 Feb 3;297(1-2):24–28. doi: 10.1016/0014-5793(92)80319-c. [DOI] [PubMed] [Google Scholar]
- Linden D. J., Routtenberg A. cis-Fatty acids, which activate protein kinase C, attenuate Na+ and Ca2+ currents in mouse neuroblastoma cells. J Physiol. 1989 Dec;419:95–119. doi: 10.1113/jphysiol.1989.sp017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan P. L., Abeywardena M. Y., Charnock J. S. Dietary fish oil prevents ventricular fibrillation following coronary artery occlusion and reperfusion. Am Heart J. 1988 Sep;116(3):709–717. doi: 10.1016/0002-8703(88)90328-6. [DOI] [PubMed] [Google Scholar]
- McLennan P. L., Bridle T. M., Abeywardena M. Y., Charnock J. S. Dietary lipid modulation of ventricular fibrillation threshold in the marmoset monkey. Am Heart J. 1992 Jun;123(6):1555–1561. doi: 10.1016/0002-8703(92)90809-a. [DOI] [PubMed] [Google Scholar]
- McLennan P. L. Relative effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on cardiac arrhythmias in rats. Am J Clin Nutr. 1993 Feb;57(2):207–212. doi: 10.1093/ajcn/57.2.207. [DOI] [PubMed] [Google Scholar]
- Miller B., Sarantis M., Traynelis S. F., Attwell D. Potentiation of NMDA receptor currents by arachidonic acid. Nature. 1992 Feb 20;355(6362):722–725. doi: 10.1038/355722a0. [DOI] [PubMed] [Google Scholar]
- Ordway R. W., Walsh J. V., Jr, Singer J. J. Arachidonic acid and other fatty acids directly activate potassium channels in smooth muscle cells. Science. 1989 Jun 9;244(4909):1176–1179. doi: 10.1126/science.2471269. [DOI] [PubMed] [Google Scholar]
- Pepe S., Bogdanov K., Hallaq H., Spurgeon H., Leaf A., Lakatta E. Omega 3 polyunsaturated fatty acid modulates dihydropyridine effects on L-type Ca2+ channels, cytosolic Ca2+, and contraction in adult rat cardiac myocytes. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8832–8836. doi: 10.1073/pnas.91.19.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzaire-Dubois B., Gérard V., Dubois J. M. Modification of K+ channel properties induced by fatty acids in neuroblastoma cells. Pflugers Arch. 1991 Nov;419(5):467–471. doi: 10.1007/BF00370790. [DOI] [PubMed] [Google Scholar]
- Takenaka T., Horie H., Hori H., Kawakami T. Effects of arachidonic acid and the other long-chain fatty acids on the membrane currents in the squid giant axon. J Membr Biol. 1988 Dec;106(2):141–147. doi: 10.1007/BF01871396. [DOI] [PubMed] [Google Scholar]
- Wieland S. J., Fletcher J. E., Gong Q. H., Rosenberg H. Effects of lipid-soluble agents on sodium channel function in normal and MH-susceptible skeletal muscle cultures. Adv Exp Med Biol. 1991;301:9–19. doi: 10.1007/978-1-4684-5979-1_2. [DOI] [PubMed] [Google Scholar]
- de Lorgeril M., Renaud S., Mamelle N., Salen P., Martin J. L., Monjaud I., Guidollet J., Touboul P., Delaye J. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994 Jun 11;343(8911):1454–1459. doi: 10.1016/s0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]
- van der Vusse G. J., Glatz J. F., Stam H. C., Reneman R. S. Fatty acid homeostasis in the normoxic and ischemic heart. Physiol Rev. 1992 Oct;72(4):881–940. doi: 10.1152/physrev.1992.72.4.881. [DOI] [PubMed] [Google Scholar]


