Abstract
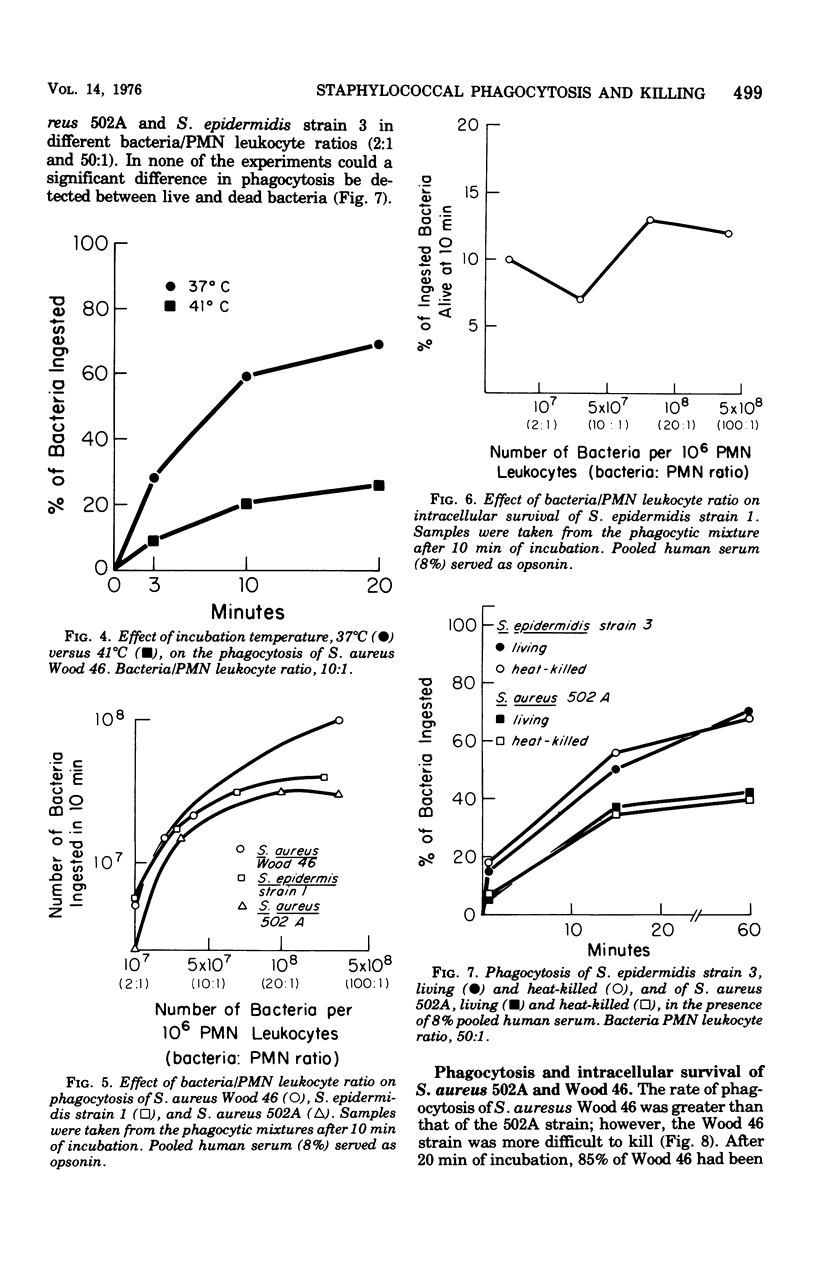
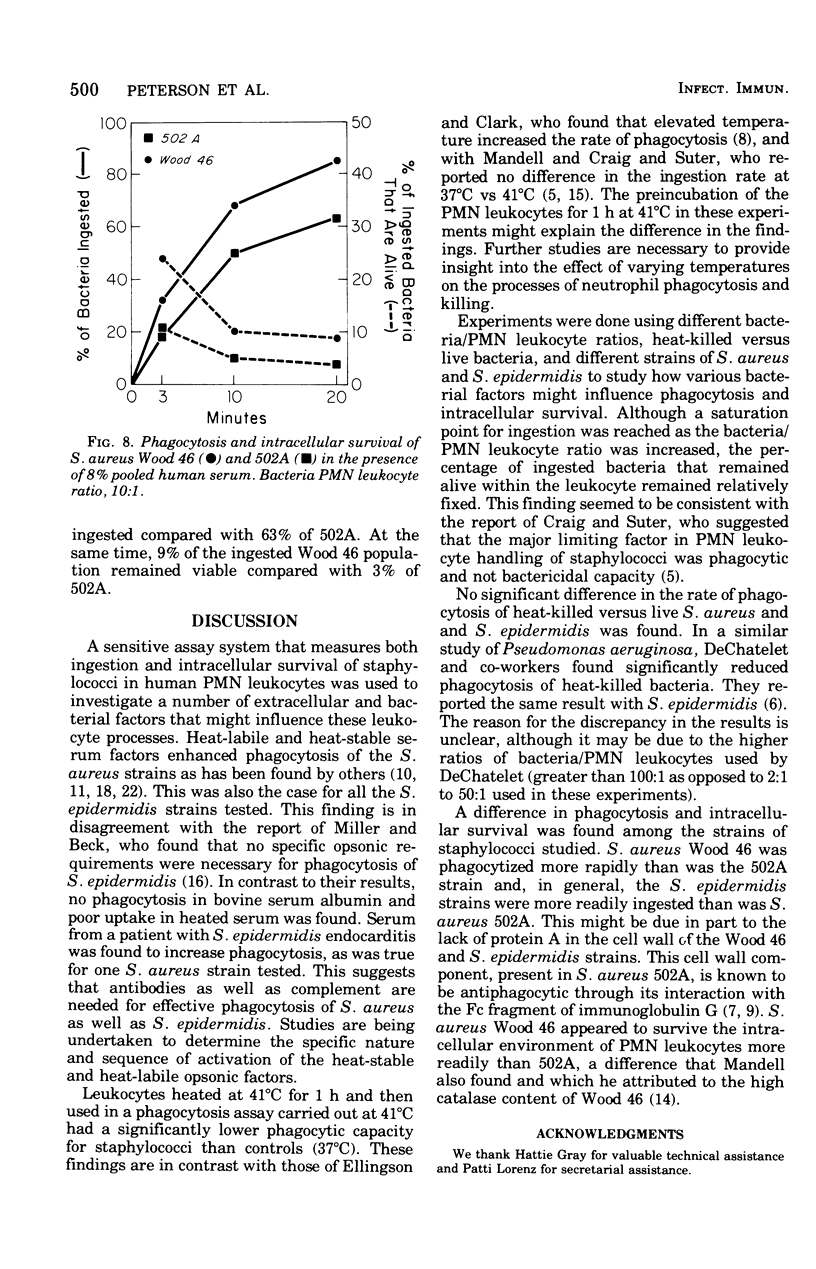
Extracellular and bacterial factors that influence the phagocytosis and killing of staphylococci by human polymorphonuclear leukocytes have been studied. Staphylococcus epidermidis strains were, in general, more rapidly phagocytized than were S. aureus strains. However, two strains of S. epidermidis had a very slow rate of ingestion. Although the rate of phagocytosis of S. aureus Wood 46 was greater than that of S. aureus 502A, the Wood 46 strain was more difficult to kill. Serum was essential for phagocytosis of both S. aureus and S. epidermidis. The opsonic titer of pooled serum was similar for S. aureus and S. epidermidis. In normal pooled serum, heat-labile factors were more important for effective phagocytosis than they were in immune serum. Although a saturation point for ingestion was reached, the percentage of ingested bacteria that remained alive within the leukocyte remained relatively fixed. Heat-killed and live staphylococci were igested in a similar fashion. The rate of phagocytosis was greatly reduced at 41 degrees C.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehner R. L., Nathan D. G., Karnovsky M. L. Correction of metabolic deficiencies in the leukocytes of patients with chronic granulomatous disease. J Clin Invest. 1970 May;49(5):865–870. doi: 10.1172/JCI106305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird-Parker A. C. The basis for the present classification of staphylococci and micrococci. Ann N Y Acad Sci. 1974 Jul 31;236(0):7–14. doi: 10.1111/j.1749-6632.1974.tb41478.x. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Functional and metabolic properties of polymorphonuclear leucocytes. I. Observations on the requirements and consequences of particle ingestion. J Exp Med. 1960 May 1;111:667–687. doi: 10.1084/jem.111.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. J. Editorial: Drugs and phagocytes. N Engl J Med. 1974 Nov 28;291(22):1187–1188. doi: 10.1056/NEJM197411282912211. [DOI] [PubMed] [Google Scholar]
- Craig C. P., Suter E. Extracellular factors influencing staphylocidal capacity of human polymorphonuclear leukocytes. J Immunol. 1966 Aug;97(2):287–296. [PubMed] [Google Scholar]
- DeChatelet L. R., Mullikin D., Shirley P. S., McCall C. E. Phagocytosis of live versus heat-killed bacteria by human polymorphonuclear leukocytes. Infect Immun. 1974 Jul;10(1):25–29. doi: 10.1128/iai.10.1.25-29.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossett J. H., Kronvall G., Williams R. C., Jr, Quie P. G. Antiphagocytic effects of staphylococcal protein A. J Immunol. 1969 Dec;103(6):1405–1410. [PubMed] [Google Scholar]
- Forsgren A., Quie P. G. Effects of staphylococcal protein A on heat labile opsonins. J Immunol. 1974 Mar;112(3):1177–1180. [PubMed] [Google Scholar]
- Forsgren A., Quie P. G. Influence of the alternate complement pathway in opsonization of several bacterial species. Infect Immun. 1974 Aug;10(2):402–404. doi: 10.1128/iai.10.2.402-404.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys D. W., Wheat L. J., White A. Staphylococcal heat-stable opsonins. J Lab Clin Med. 1974 Jul;84(1):122–128. [PubMed] [Google Scholar]
- Laxdal T., Messner R. P., Williams R. C., Jr, Quie P. G. Opsonic, agglutinating, and complement-fixing antibodies in patients with subacute bacterial endocarditis. J Lab Clin Med. 1968 Apr;71(4):638–653. [PubMed] [Google Scholar]
- Mandell G. L. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal--leukocyte interaction. J Clin Invest. 1975 Mar;55(3):561–566. doi: 10.1172/JCI107963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L. Effect of temperature on phagocytosis by human polymorphonuclear neutrophils. Infect Immun. 1975 Jul;12(1):221–223. doi: 10.1128/iai.12.1.221-223.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. S., Beck S. Polymorphonuclear leukocyte phagocytosis: quantitation by a rapid radioactive method. J Lab Clin Med. 1975 Aug;86(2):344–348. [PubMed] [Google Scholar]
- Nickerson D. S., Kazmierowski J. A., Dossett J. H., Williams R. C., Jr, Quie P. G. Studies of immune and normal opsonins during experimental staphylococcal infection in rabbits. J Immunol. 1969 May;102(5):1235–1241. [PubMed] [Google Scholar]
- Quie P. G., White J. G., Holmes B., Good R. A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967 Apr;46(4):668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. Phagocytosis (first of three parts). N Engl J Med. 1974 Mar 28;290(13):717–723. doi: 10.1056/NEJM197403282901306. [DOI] [PubMed] [Google Scholar]
- Wheat L. J., Humphreys D. W., White A. Opsonization of staphylococci by normal human sera: the role of antibody and heat-labile factors. J Lab Clin Med. 1974 Jan;83(1):73–78. [PubMed] [Google Scholar]


