Abstract
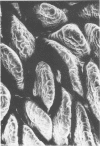
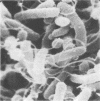
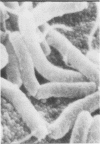
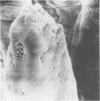
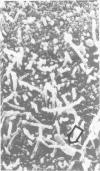
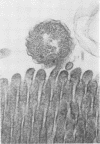
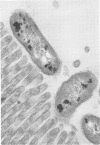
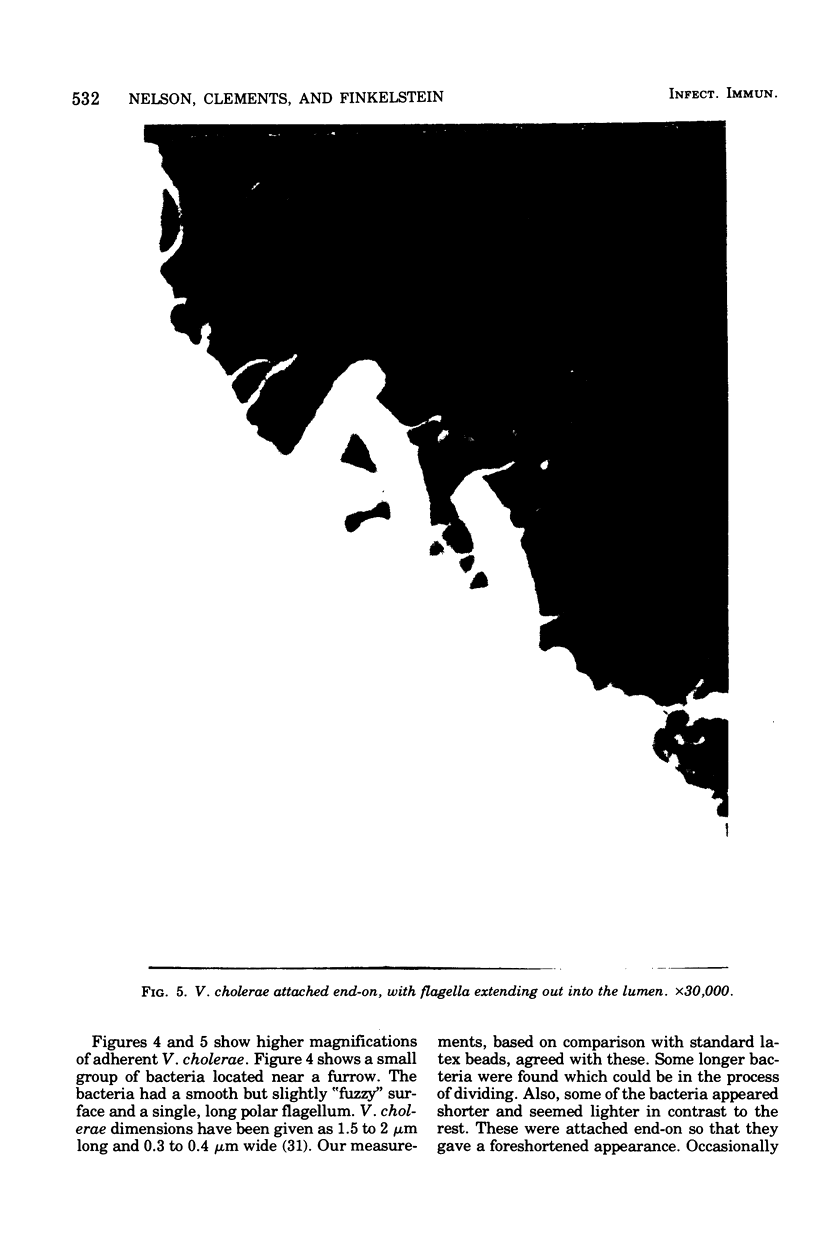
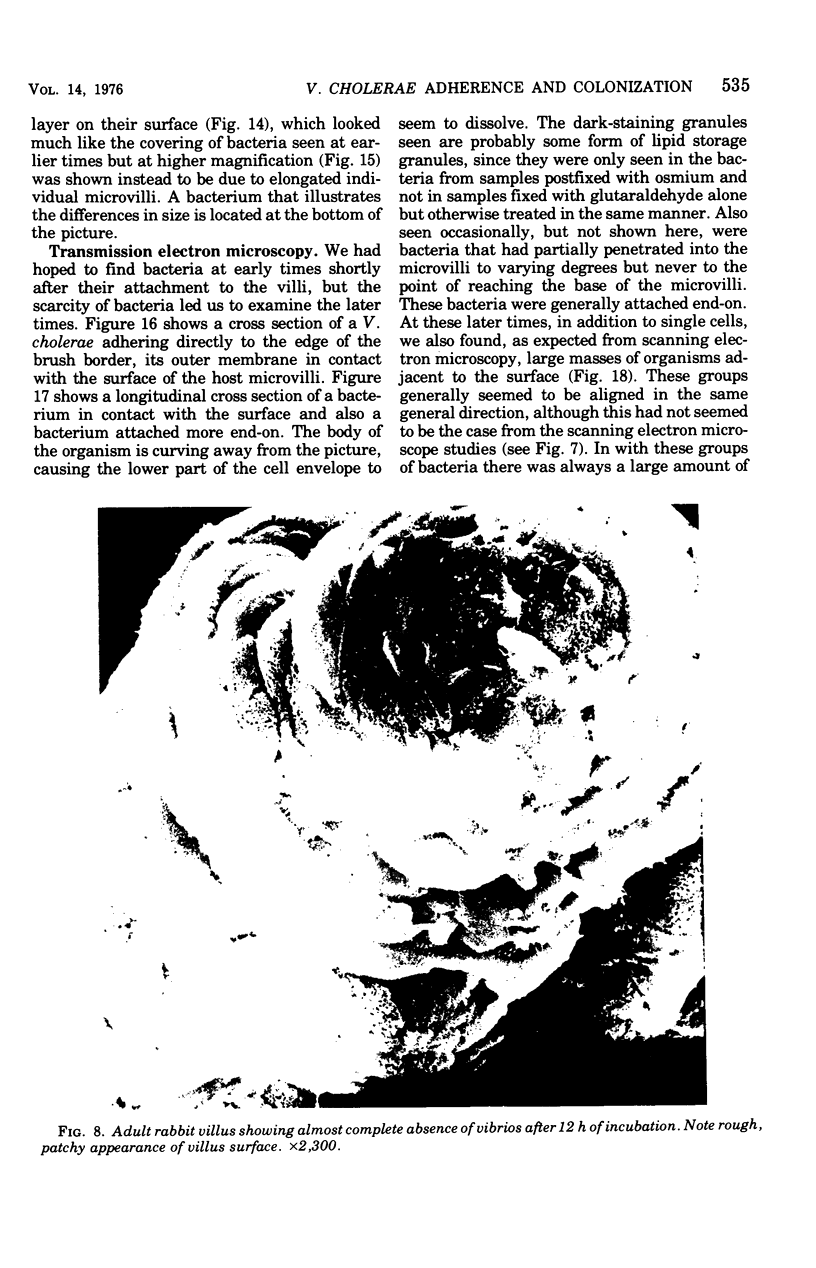
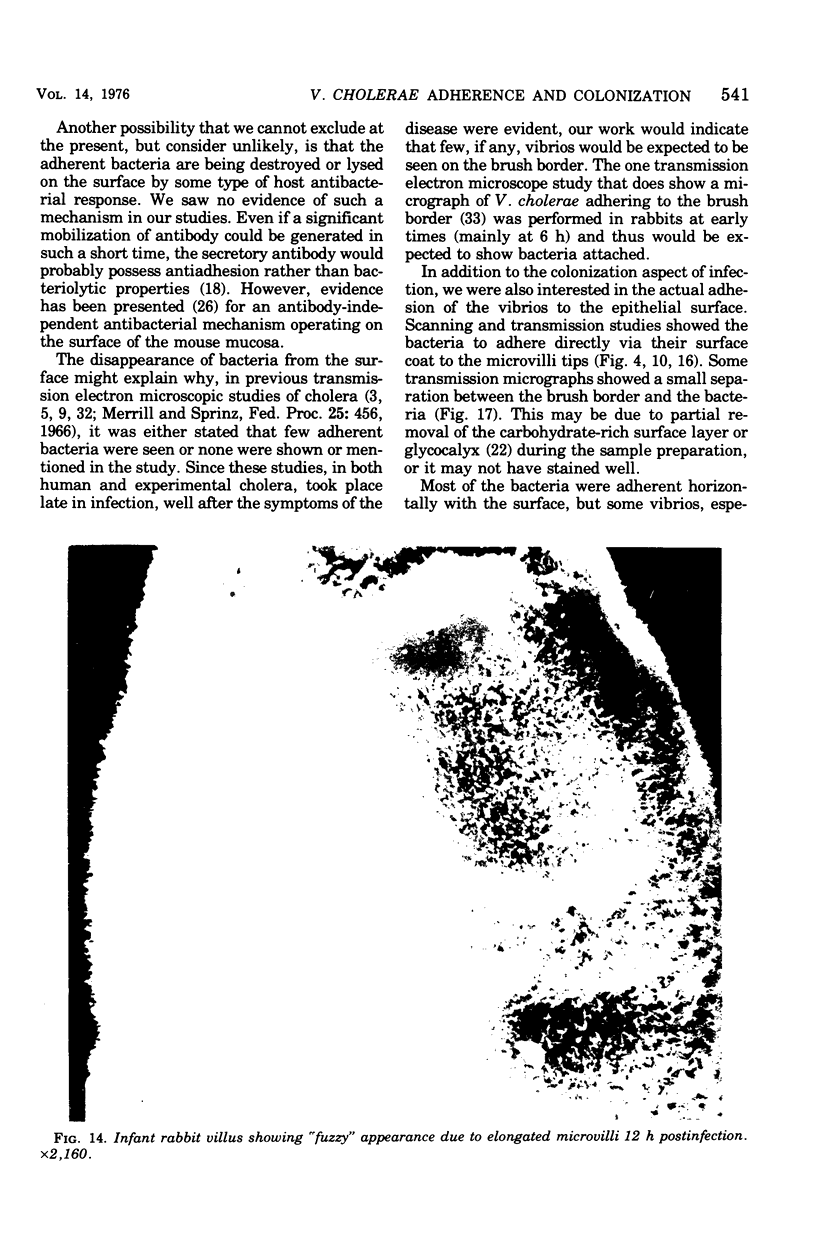
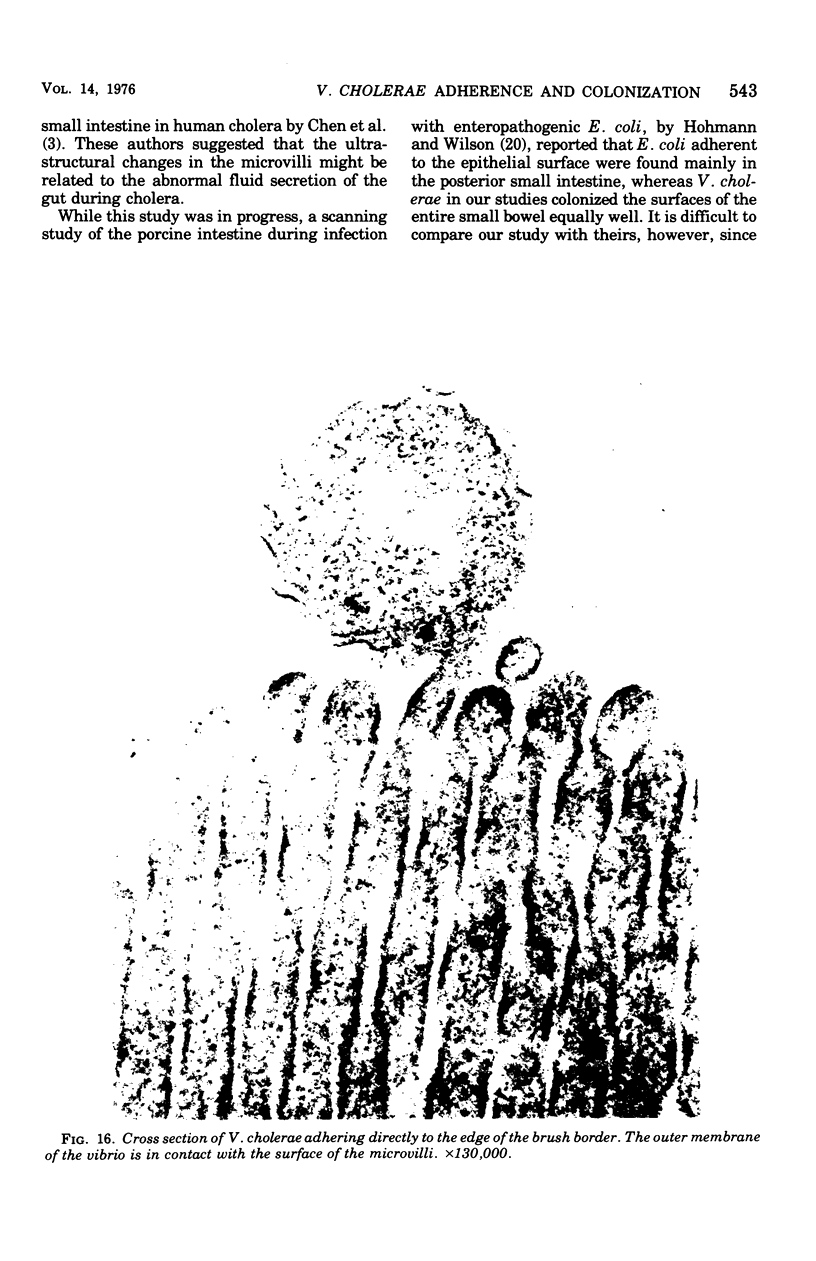
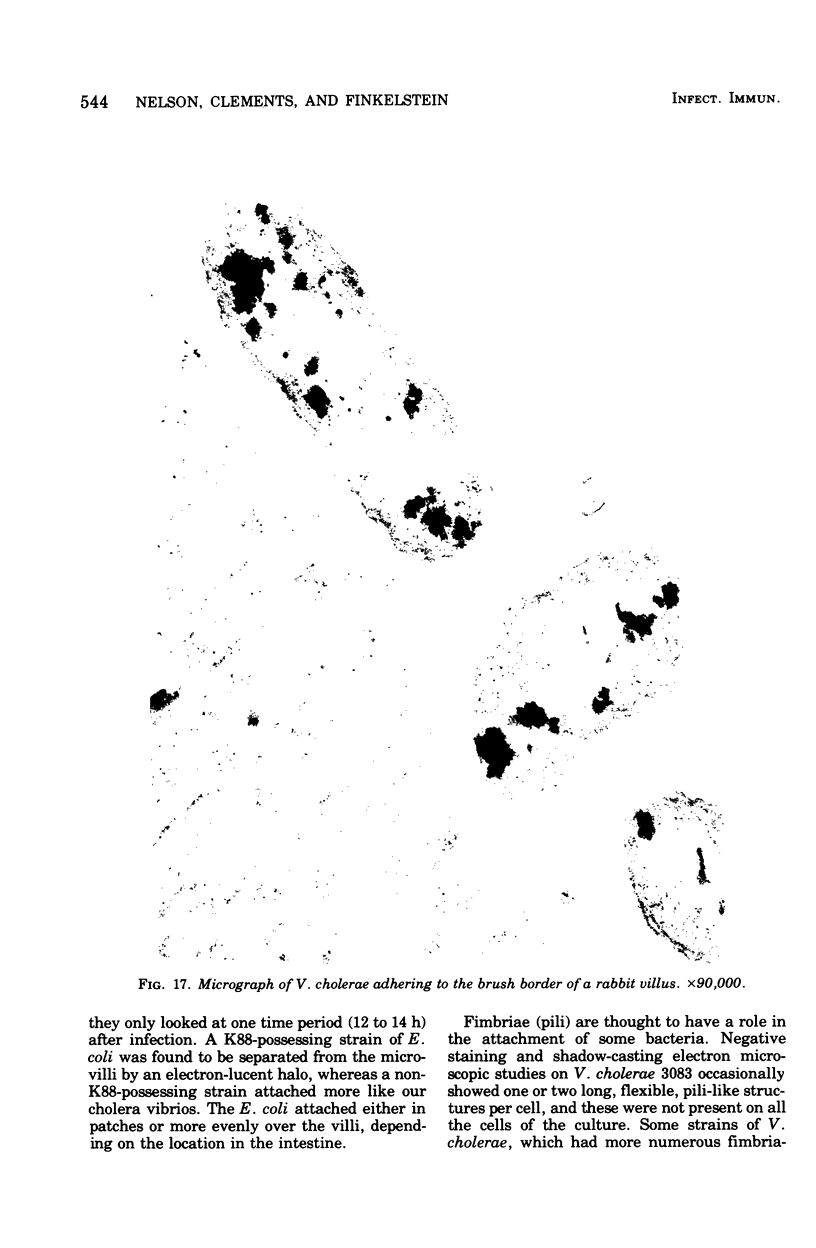
Colonization of the intestinal epithelium by Vibrio cholerae was examined in two model systems, in ligated ileal loops of adult rabbits and in the patent gut of infant rabbits, using both scanning and transmission electron microscopy. Time studies in the adult model showed a lag period of up to 1 h before the attachment of significant numbers of the vibrios. The bacteria appeared initially in small patches on the sides of the villi, predominantly along the transverse furrows. The number of adherent bacteria steadily increased, reaching a maximum between 4 and 7 h, when a dense mat of bacteria several layers thick covered much of the villi. After this time there was a rapid decline in the number of V. cholerae bound. By 12 to 16 h only a few bacteria could be seen on the surface of the villi, which had a rough, patchy appearance at these later times. Globular protrusions, with vibrios attached, may play a role in the clearance of bacteria. Colonization and clearance in the patent intestine of the infant rabbit occurred much as in the adult model. However, the bacteria adhered more uniformly and there was no lag in attachment. In both models the majority of bacteria were aligned horizontally with the epithelial surface, but some were attached in an end-on manner, with their flagella extending into the lumen. The bacteria adhered via their surface coats directly to the tips of the microvilli, except for a few vibrios that were partly embedded into the brush border. Some changes in the microvilli occurred as a consequence of the bacterial attachment.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balcerzak S. P., Lane W. C., Bullard J. W. Surface structure of intestinal epithelium. Gastroenterology. 1970 Jan;58(1):49–55. [PubMed] [Google Scholar]
- Chen H. C., Reyes V., Fresh J. W. An electron microscopic study of the small intestine in human cholera. Virchows Arch B Cell Pathol. 1971;7(2):236–259. doi: 10.1007/BF02892095. [DOI] [PubMed] [Google Scholar]
- DE S. N., CHATTERJE D. N. An experimental study of the mechanism of action of Vibriod cholerae on the intestinal mucous membrane. J Pathol Bacteriol. 1953 Oct;66(2):559–562. doi: 10.1002/path.1700660228. [DOI] [PubMed] [Google Scholar]
- DIXON J. M. The fate of bacteria in the small intestine. J Pathol Bacteriol. 1960 Jan;79:131–140. doi: 10.1002/path.1700790116. [DOI] [PubMed] [Google Scholar]
- De Jonge H. R. The response of small intestinal villous and crypt epithelium to choleratoxin in rat and guinea pig. Evidence against a specific role of the crypt cells in choleragen-induced secretion. Biochim Biophys Acta. 1975 Jan 13;381(1):128–143. doi: 10.1016/0304-4165(75)90195-6. [DOI] [PubMed] [Google Scholar]
- Elliott H. L., Carpenter C. C., Sack R. B., Yardley J. H. Small bowel morphology in experimental canine cholera. A light and electron microscopic study. Lab Invest. 1970 Feb;22(2):112–120. [PubMed] [Google Scholar]
- FRETER R., SMITH H. L., Jr, SWEENEY F. J., Jr An evaluation of intestinal fluids in the pathogenesis of cholera. J Infect Dis. 1961 Jul-Aug;109:35–42. doi: 10.1093/infdis/109.1.35. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Sobocinski P. Z., Atthasampunna P., Charunmethee P. Pathogenesis of experimental cholera: identification of choleragen (procholeragen A) by disc immunoelectrophoresis and its differentiation from cholera mucinase. J Immunol. 1966 Jul;97(1):25–33. [PubMed] [Google Scholar]
- Finkelstein R. A., Vasil M. L., Holmes R. K. Studies on toxinogenesis in Vibrio cholerae. I. Isolation of mutants with altered toxinogenicity. J Infect Dis. 1974 Feb;129(2):117–123. doi: 10.1093/infdis/129.2.117. [DOI] [PubMed] [Google Scholar]
- Freter R. Interactions between mechanisms controlling the intestinal microflora. Am J Clin Nutr. 1974 Dec;27(12):1409–1416. doi: 10.1093/ajcn/27.12.1409. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Bacterial adherence to mucosal surfaces and its inhibition by secretory antibodies. Adv Exp Med Biol. 1974;45(0):315–325. doi: 10.1007/978-1-4613-4550-3_38. [DOI] [PubMed] [Google Scholar]
- Goldstein H. B., Merrill T. G., Sprinz H. Experimental cholera. Morphologic evidence of cytotoxicity. Arch Pathol. 1966 Jul;82(1):54–59. [PubMed] [Google Scholar]
- HOWARD M. B., LANKFORD C. E. Minimum nutritional requirements for mucinase synthesis by Vibrio cholerae. Tex Rep Biol Med. 1960;18:612–619. [PubMed] [Google Scholar]
- Hohmann A., Wilson M. R. Adherence of enteropathogenic Escherichia coli to intestinal epithelium in vivo. Infect Immun. 1975 Oct;12(4):866–880. doi: 10.1128/iai.12.4.866-880.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S. Structure and function of the glycocalyx. Fed Proc. 1969 Jan-Feb;28(1):12–25. [PubMed] [Google Scholar]
- Knop J., Rowley D. Antibacterial mechanisms in the intestine. Elimination of V. cholerae from the gastrointestinal tract of adult mice. Aust J Exp Biol Med Sci. 1975 Apr;53(2):137–146. [PubMed] [Google Scholar]
- Knop J., Rowley D. Antibacterial mechanisms in the intestine. Elimination of V. cholerae from the intestines of infant mice and the role of antibody. Aust J Exp Biol Med Sci. 1975 Apr;53(2):147–154. [PubMed] [Google Scholar]
- Knop J., Rowley D. Protection against cholera. A bactericidal mechanism on the mucosal surface of the small intestine of mice. Aust J Exp Biol Med Sci. 1975 Apr;53(2):155–165. [PubMed] [Google Scholar]
- LANKFORD C. E. Factors of virulence of Vibrio cholerae. Ann N Y Acad Sci. 1960 Nov 21;88:1203–1212. doi: 10.1111/j.1749-6632.1960.tb20111.x. [DOI] [PubMed] [Google Scholar]
- Norris H. T., Majno G. On the role of the ileal epithelium in the pathogenesis of experimental cholera. Am J Pathol. 1968 Aug;53(2):263–279. [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., Blumershine R. V. Surface-surface associations in microbial communities populating epithelial habitats in the murine gastrointestinal ecosystem: scanning electron microscopy. Infect Immun. 1974 Jul;10(1):240–250. doi: 10.1128/iai.10.1.240-250.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrank G. D., Verwey W. F. Distribution of cholera organisms in experimental Vibrio cholerae infections: proposed mechanisms of pathogenesis and antibacterial immunity. Infect Immun. 1976 Jan;13(1):195–203. doi: 10.1128/iai.13.1.195-203.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C. J., Kimberg D. V., Ware P. Adenylate cyclase in intestinal crypt and villus cells: stimulation by cholera enterotoxin and prostaglandin E1. Gastroenterology. 1975 Jan;68(1):94–104. [PubMed] [Google Scholar]
- Trier J. S., Rubin C. E. Electron microscopy of the small intestine: a review. Gastroenterology. 1965 Nov;49(5):574–603. [PubMed] [Google Scholar]
- Weiser M. M., Quill H. Intestinal villus and crypt cell responses to cholera toxin. Gastroenterology. 1975 Aug;69(2):479–482. [PubMed] [Google Scholar]
- Yardley J. H., Bayless T. M., Luebbers E. H., Halsted C. H., Hendrix T. R. Goblet cell mucus in the small intestine. Findings after net fluid production due to cholera toxin and hypertonic solutions. Johns Hopkins Med J. 1972 Jul;131(1):1–10. [PubMed] [Google Scholar]