Abstract
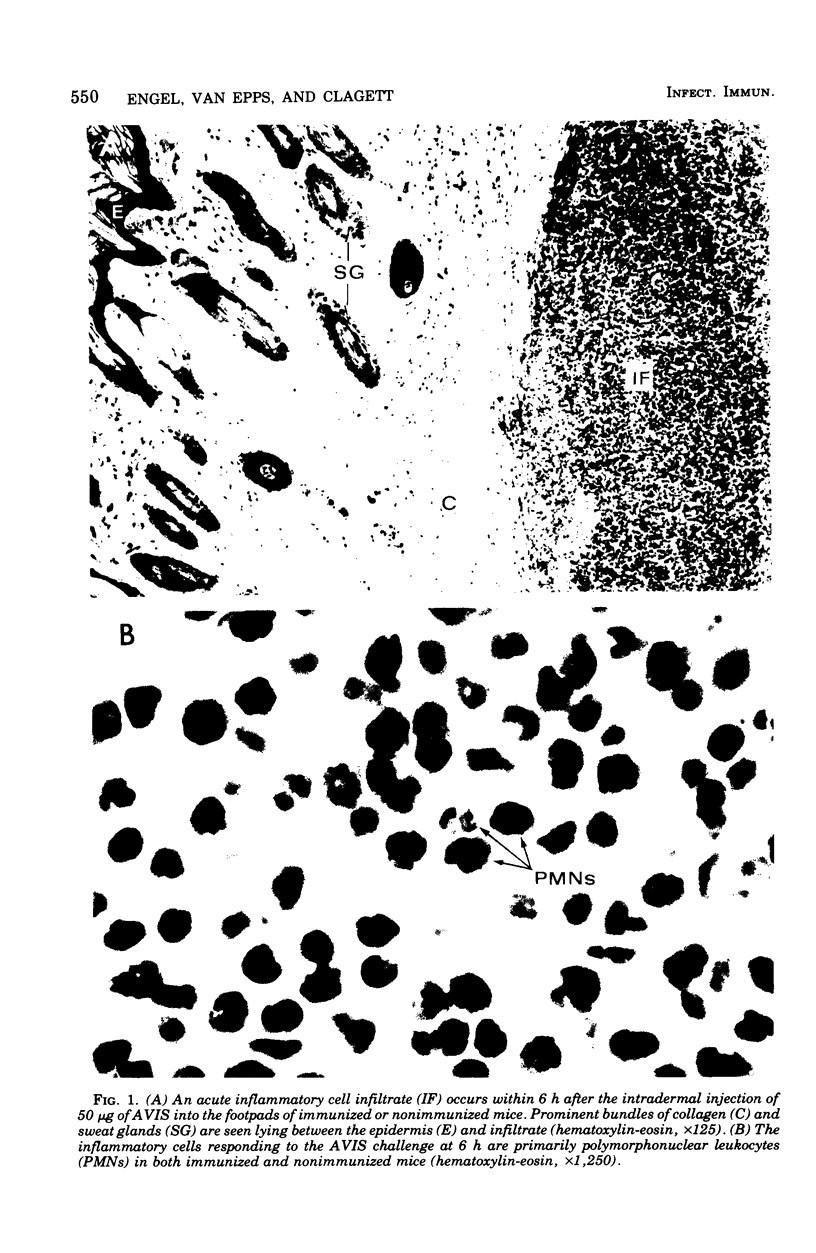
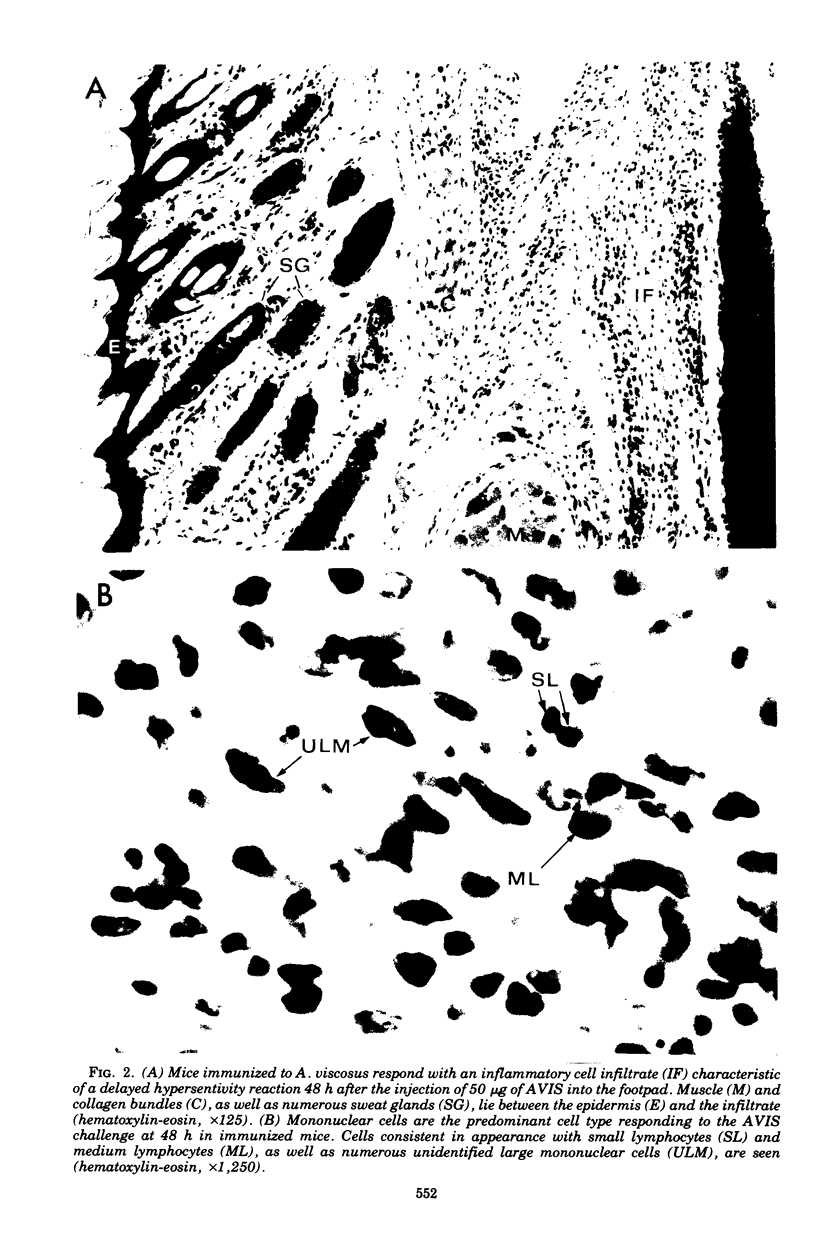
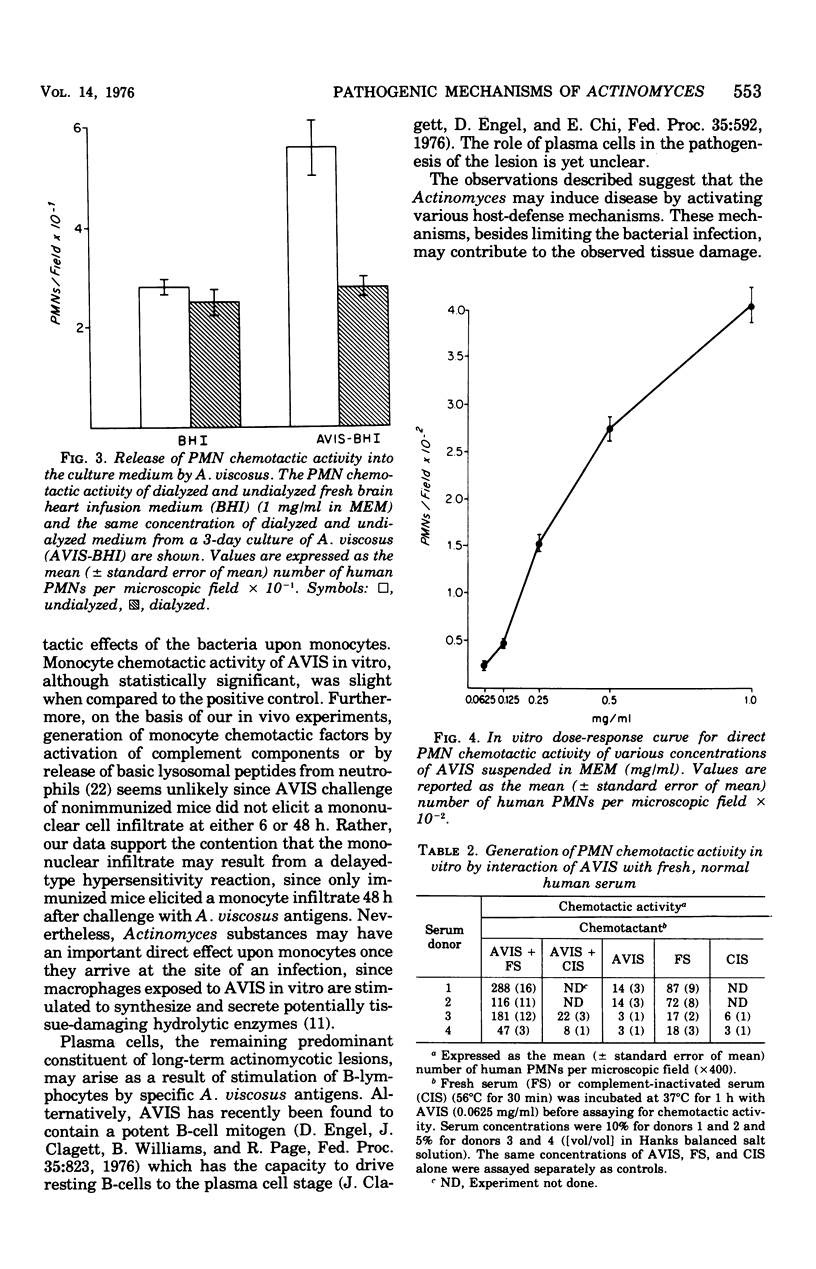
Actinomycotic infections are characterized by long-term inflammatory lesions containing large numbers of polymorphonuclear leukocytes (PMNs) and mononuclear cells. The pathogenic mechanisms involved in these lesions are not understood. Homogenates of Actinomyces viscosus (AVIS) induce an acute inflammatory response with a predominance of PMNs within 6 h after injection into the footpads of nonimmunized mice. These homogenates, when tested in vitro, contain potent chemotactic activity for human PMNs. In vitro chemotactic activity for human monocytes is weak but statistically significant (P less than 0.025). Doses of AVIS, which alone have little chemotactic activity, cause the generation of PMN chemotactic activity in fresh, but not complement-inactivated, serum. The injection of AVIS into the footpads of immunized mice induces an acute inflammatory response followed within 48 h by a mononuclear cell infiltrate, suggesting that factors affecting monocyte accumulation are generated by the immune host in response to challenge with the bacterial antigens. These findings indicate that the pathogenicity of the Actinomyces may result in part from (i) their direct chemotactic effect on PMNs, (ii) their cytotaxigenic effects on serum, and (iii) their ability to stimulate host immune cells to produce and release mediators of inflammation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYDEN S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962 Mar 1;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak U. B., Yamamoto H., Azuma R. Experimental actinomycotic abscess in mice of several strains. Natl Inst Anim Health Q (Tokyo) 1972 Winter;12(4):232–233. [PubMed] [Google Scholar]
- Brown J. R. Human actinomycosis. A study of 181 subjects. Hum Pathol. 1973 Sep;4(3):319–330. doi: 10.1016/s0046-8177(73)80097-8. [DOI] [PubMed] [Google Scholar]
- Brown J. R., Von Lichtenberg F. Experimental actinomycosis in mice. Study of pathogenesis. Arch Pathol. 1970 Nov;90(5):391–402. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Davenport A. A., Carter G. R., Schirmer R. G. Canine actinomycosis due to Actinomyces viscosus: report of six cases. Vet Med Small Anim Clin. 1974 Nov;69(11):1442, 1444-7. [PubMed] [Google Scholar]
- Guggenheim B., Schroeder H. E. Reactions in the periodontium to continuous antigenic stimulation in sensitized gnotobiotic rats. Infect Immun. 1974 Sep;10(3):565–577. doi: 10.1128/iai.10.3.565-577.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. Pathologic mechanisms in neutrophil-mediated injury. Am J Pathol. 1972 Sep;68(3):593–612. [PMC free article] [PubMed] [Google Scholar]
- Irving J. T., Socransky S. S., Heeley J. D. Histological changes in experimental periodontal disease in gnotobiotic rats and conventional hamsters. J Periodontal Res. 1974;9(2):73–80. doi: 10.1111/j.1600-0765.1974.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Keyes P. H., Bellack S. Periodontal lesions in hamsters and gnotobiotic rats infected with actinomyces of human origin. J Periodontal Res. 1972;7(1):21–28. doi: 10.1111/j.1600-0765.1972.tb00627.x. [DOI] [PubMed] [Google Scholar]
- Page R. C., Davies P., Allison A. C. Participation of mononuclear phagocytes in chronic inflammatory diseases. J Reticuloendothel Soc. 1974 May;15(5):413–438. [PubMed] [Google Scholar]
- Pantalone R. M., Page R. C. Lymphokine-induced production and release of lysosomal enzymes by macrophages. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2091–2094. doi: 10.1073/pnas.72.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S. M., Carpenter C. B., Merrill J. P. Cellular immunity in the mouse. II. Correlation of in vivo and in vitro phenomena. Cell Immunol. 1972 Oct;5(2):249–263. doi: 10.1016/0008-8749(72)90051-2. [DOI] [PubMed] [Google Scholar]
- Socransky S. S., Hubersak C., Propas D. Induction of periodontal destruction in gnotobiotic rats by a human oral strain of Actinomyces naeslundii. Arch Oral Biol. 1970 Oct;15(10):993–995. doi: 10.1016/0003-9969(70)90095-6. [DOI] [PubMed] [Google Scholar]
- Tempel T. R., Snyderman R., Jordan H. V., Mergenhagen S. E. Factors from saliva and oral bacteria, chemotactic for polymorphonuclear leukocytes: their possible role in gingival inflammation. J Periodontol. 1970 Feb;41(2):71–80. doi: 10.1902/jop.1970.41.2.71. [DOI] [PubMed] [Google Scholar]
- Van Epps D. E., Andersen B. R. Streptolysin O inhibition of neutrophil chemotaxis and mobility: nonimmune phenomenon with species specificity. Infect Immun. 1974 Jan;9(1):27–33. doi: 10.1128/iai.9.1.27-33.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van D. E., Paxton L. L. Chemotaxis as a preparative technique for human polymorphonuclear leukocytes. J Lab Clin Med. 1975 Aug;86(2):309–314. [PubMed] [Google Scholar]
- Wahl L. M., Wahl S. M., Mergenhagen S. E., Martin G. R. Collagenase production by lymphokine-activated macrophages. Science. 1975 Jan 24;187(4173):261–263. doi: 10.1126/science.163038. [DOI] [PubMed] [Google Scholar]
- Ward P. A. Chemotoxis of mononuclear cells. J Exp Med. 1968 Nov 1;128(5):1201–1221. doi: 10.1084/jem.128.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A. Leukotaxis and leukotactic disorders. A review. Am J Pathol. 1974 Dec;77(3):520–538. [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Zurier R. B., Hoffstein S. Leukocytic proteases and the immunologic release of lysosomal enzymes. Am J Pathol. 1972 Sep;68(3):539–564. [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. C., O'Neill G. J., Wapshaw K. G. Role of anaerobic coryneforms in specific and non-specific immunological reactions. II. Production of a chemotactic factor specific for macrophages. Immunology. 1973 Jun;24(6):997–1006. [PMC free article] [PubMed] [Google Scholar]
- Williams B. L., Pantalone R. M., Sherris J. C. Subgingival microflora and periodontitis. J Periodontal Res. 1976 Feb;11(1):1–18. doi: 10.1111/j.1600-0765.1976.tb00045.x. [DOI] [PubMed] [Google Scholar]