Abstract
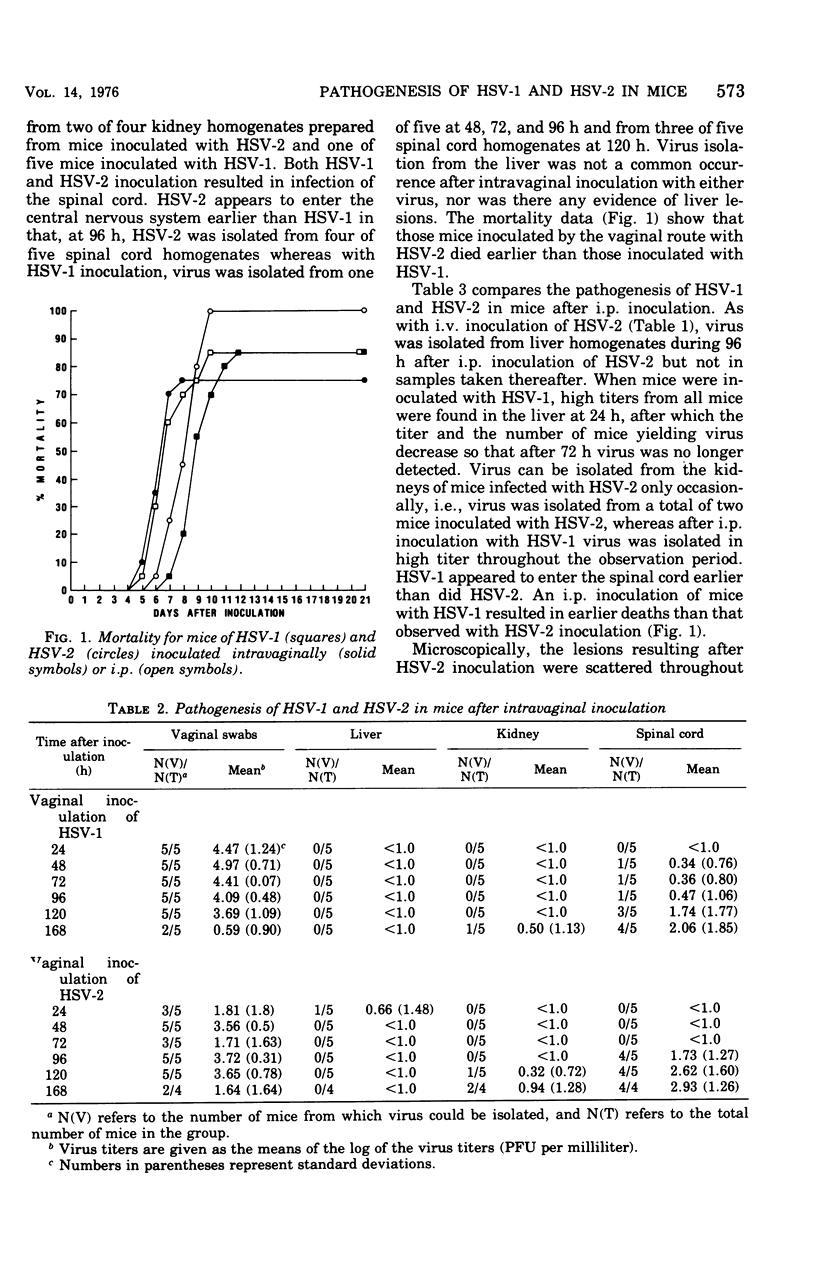
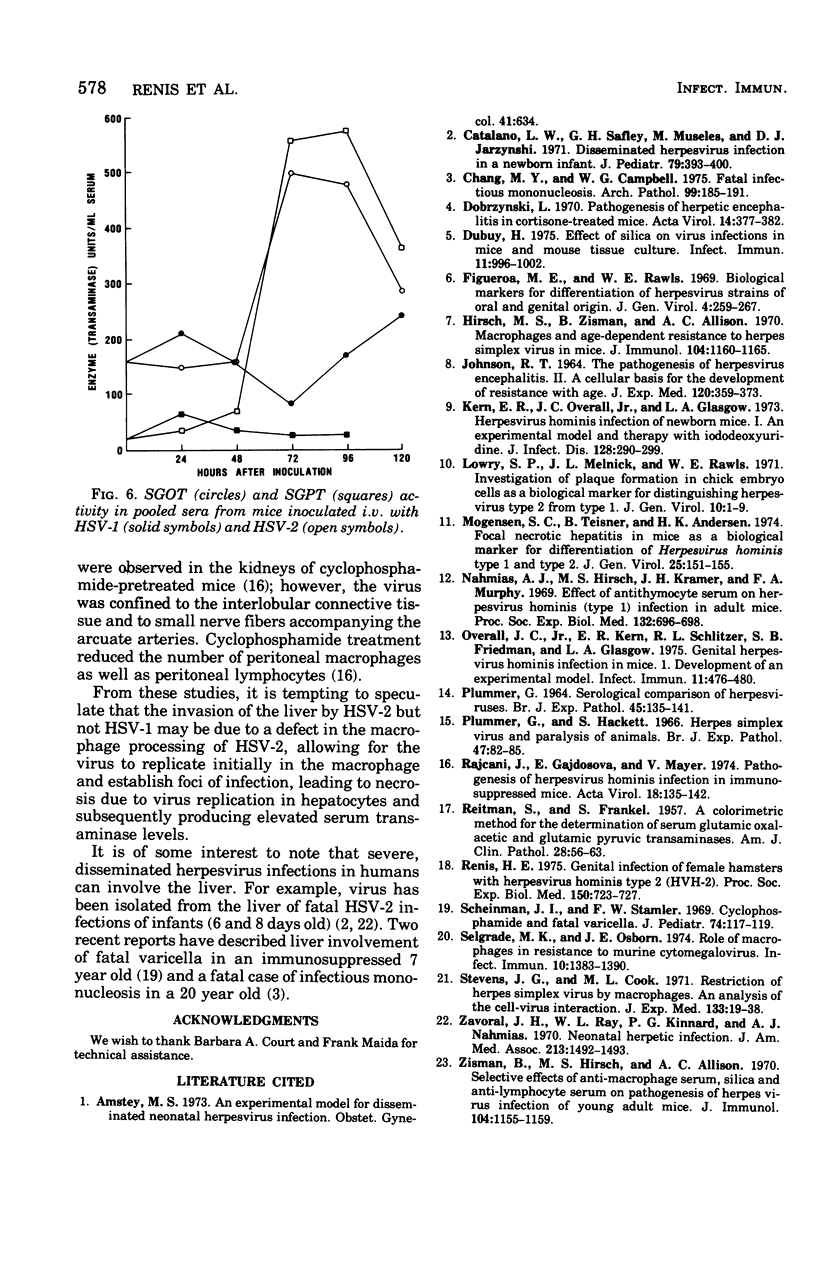
The pathogenesis of herpes simplex virus (HSV) types 1 and 2 was compared after inoculation of mice by different routes. Intravaginal inoculation of HSV-1 and HSV-2 produced a local infection, with virus recovery from the vagina through 5 days. Virus was recovered from the spinal cords 4 to 5 days after inoculation but not from liver, kidney, lung, spleen, or blood. Intravenous or intraperitoneal inoculation of HSV-2 produced a focal necrotic hepatitis similar to that described previously (S. C. Mogenson, B. Teisner, and H.K. Andersen, 1974). The viral etiology of the liver lesions was confirmed by virus isolation (through 4 days) and electron microscopy. No evidence of infection of the kidney, lung, blood, or spleen was observed, although virus was isolated from spinal cord homogenates 7 days after inoculation. HSV-1 inoculation by the intraperitoneal or intravenous route resulted in virus isolation from the kidney during the 7-day harvest period, without producing overt pathological changes. Virus was isolated from spinal cord homogenates 2 to 3 days after HSV-1 inoculation but not from homogenates prepared from spleen, lung, or blood. Increases in serum transaminase activity were observed after systemic (intravenous) inoculation of HSV-2 but not after HSV-1 inoculation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Catalano L. W., Jr, Safley G. H., Museles M., Jarzynski D. J. Disseminated herpesvirus infection in a newborn infant. Virologic, serologic, coagulation, and interferon studies. J Pediatr. 1971 Sep;79(3):393–400. doi: 10.1016/s0022-3476(71)80146-4. [DOI] [PubMed] [Google Scholar]
- Chang M. Y., Campbell W. G., Jr Fatal infectious mononucleosis. Association with liver necrosis and herpes-like virus particles. Arch Pathol. 1975 Apr;99(4):185–191. [PubMed] [Google Scholar]
- Dobrzyński L. Pathogenesis of herpetic encephalitis in cortisone-treated mice. Acta Virol. 1970 Sep;14(5):377–382. [PubMed] [Google Scholar]
- Figueroa M. E., Rawls W. E. Biological markers for differentiation of herpes-virus strains of oral and genital origin. J Gen Virol. 1969 Mar;4(2):259–267. doi: 10.1099/0022-1317-4-2-259. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern E. R., Overall J. C., Jr, Glasgow L. A. Herpesvirus hominis infection in newborn mice. I. An experimental model and therapy with iododeoxyuridine. J Infect Dis. 1973 Sep;128(3):290–299. doi: 10.1093/infdis/128.3.290. [DOI] [PubMed] [Google Scholar]
- Lowry S. P., Melnick J. L., Rawls W. E. Investigation of plaque formation in chick embryo cells as a biological marker for distinguishing herpes virus type 2 from type 1. J Gen Virol. 1971 Jan;10(1):1–9. doi: 10.1099/0022-1317-10-1-1. [DOI] [PubMed] [Google Scholar]
- Mogensen S. C., Teisner B., Andersen H. K. Focal necrotic hepatitis in mice as a biological marker for differentiation of Herpesvirus hominis type 1 and type 2. J Gen Virol. 1974 Oct;25(1):151–155. doi: 10.1099/0022-1317-25-1-151. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Hirsch M. S., Kramer J. H., Murphy F. A. Effect of antithymocyte serum on herpesvirus hominis (type 1) infection in adult mice. Proc Soc Exp Biol Med. 1969 Nov;132(2):696–698. doi: 10.3181/00379727-132-34290. [DOI] [PubMed] [Google Scholar]
- Overall J. C., Jr, Kern E. R., Schlitzer R. L., Friedman S. B., Glasgow L. A. Genital herpesvirus hominis infection in mice. I. Development of an experimental model. Infect Immun. 1975 Mar;11(3):476–480. doi: 10.1128/iai.11.3.476-480.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLUMMER G. SEROLOGICAL COMPARISON OF THE HERPES VIRUSES. Br J Exp Pathol. 1964 Apr;45:135–141. [PMC free article] [PubMed] [Google Scholar]
- Plummer G., Hackett S. Herpes simplex virus and paralysis of animals. Br J Exp Pathol. 1966 Feb;47(1):82–85. [PMC free article] [PubMed] [Google Scholar]
- REITMAN S., FRANKEL S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957 Jul;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Renis H. E. Genital infection of female hamsters with herpesvirus hominis type 2 (HVH-2). Proc Soc Exp Biol Med. 1975 Dec;150(3):723–727. doi: 10.3181/00379727-150-39114. [DOI] [PubMed] [Google Scholar]
- Scheinman J. I., Stamler F. W. Cyclophosphamide and fatal varicella. J Pediatr. 1969 Jan;74(1):117–119. doi: 10.1016/s0022-3476(69)80018-1. [DOI] [PubMed] [Google Scholar]
- Selgrade M. K., Osborn J. E. Role of macrophages in resistance to murine cytomegalovirus. Infect Immun. 1974 Dec;10(6):1383–1390. doi: 10.1128/iai.10.6.1383-1390.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Restriction of herpes simplex virus by macrophages. An analysis of the cell-virus interaction. J Exp Med. 1971 Jan 1;133(1):19–38. doi: 10.1084/jem.133.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavoral J. H., Ray W. L., Kinnard P. G., Nahmias A. J. Neonatal herpetic infection. A fatal consequence of penile herpes in a serviceman. JAMA. 1970 Aug 31;213(9):1492–1493. doi: 10.1001/jama.213.9.1492. [DOI] [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]
- duBuy H. Effect of silica on virus infections in mice and mouse tissue culture. Infect Immun. 1975 May;11(5):996–1002. doi: 10.1128/iai.11.5.996-1002.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]