Abstract
c-Jun N-terminal Kinase (JNK) is member of the Mitogen-Activated Protein Kinase (MAPK) family, activated through phosphorylation following cytokine exposure and stress. In this study, phosphorylation of JNK was examined in the urinary bladder with CYP-induced cystitis and the effects of SP600125, a selective inhibitor of phosphorylation of JNK, on urinary bladder function were assessed using conscious, open outlet, cystometry with continuous instillation of intravesical saline. We induced bladder inflammation in adult female Wistar rats by injecting CYP intraperitoneally to produce acute (150 mg/kg; 4 hr), intermediate (150 mg/kg; 48 hr) and chronic (75 mg/kg; every third day for 10 days) treatments. Western blotting of urinary bladder demonstrated a significant (p ≤ 0.01) increase (i.e., phosphorylation) in JNK activation with 4 hr and 48 hr CYP-induced cystitis. Immunohistochemistry and image analyses demonstrated a significant (p ≤ 0.01) increase in JNK activation in the urothelium with 4 hr and 48 hr CYP-induced cystitis. Blockade of JNK phosphorylation significantly (p ≤ 0.01) increased bladder capacity and intercontraction void intervals in CYP-treated rats (4 hr and 48 hr). Furthermore, blockade of JNK phosphorylation reduced (p ≤ 0.01) neuropeptide (substance P, calcitonin gene-related peptide) expression in the urinary bladder with CYP-induced cystitis (4 hr and 48 hr). In contrast, blockade of JNK phosphorylation was without effect on bladder function or neuropeptide expression in urinary bladder in control (no inflammation) rats. Blockade of JNK phosphorylation may represent a novel target for improving urinary bladder function with CYP-induced cystitis.
Keywords: micturition, western blot, cystometry, phosphorylation, IHC, neuropeptides
Introduction
c-Jun amino-terminal kinase (JNK)/stress activated protein kinase (SAPK) is a member of the mitogen-activated protein kinase (MAPK) family, a conserved group of protein kinase molecules, activated through phosphorylation from a variety of stressors including inflammatory cytokines, bacterial endotoxin, osmotic shock, ultraviolet radiation, and hypoxia (Doya et al., 2005; Peti and Page, 2013). JNK activation via phosphorylation of threonine and tyrosine residues (Markevich et al., 2004; Peti and Page, 2013) results in dimerization and subsequent translocation from cytoplasm to nucleus. These phosphorylation cascades transmit and amplify extracellular, receptor-mediated signals through the cytoplasm of the cell to activate nuclear transcription factors that bind and induce expression of target genes (Mehan et al., 2011). JNK/SAPK signaling molecules are linked to diverse cellular processes including inflammation, apoptosis and cancer (Mehan et al., 2011).
Bladder pain syndrome (BPS)/ interstitial cystitis (IC) is one type of chronic pain syndrome characterized by pain, pressure or discomfort perceived to be bladder related with at least one urinary symptom (Sant and Hanno, 2001). Although the etiology and pathogenesis of BPS/IC are unknown, numerous theories including infection, inflammation, autoimmune disorder, toxic urinary agents, urothelial dysfunction and neurogenic causes have been proposed (Driscoll and Teichman, 2001; Hanno and Sant, 2001; Sant and Hanno, 2001; Quillin and Erickson, 2012). Altered visceral sensations from the urinary bladder (i.e., pain at low or moderate bladder filling) that accompany BPS/IC (Driscoll and Teichman, 2001; Hanno and Sant, 2001; Sant and Hanno, 2001; Quillin and Erickson, 2012) may be mediated by many factors including changes in the properties of peripheral bladder afferent pathways such that bladder afferent neurons respond in an exaggerated manner to normally innocuous stimuli (allodynia). These changes may be mediated, in part, by inflammatory changes in the urinary bladder including neurotrophins (Schnegelsberg et al., 2010), cytokines (Gonzalez et al., 2013), chemokines (Arms et al., 2010; Arms et al., 2013) and neuropeptides (Vizzard, 2001; Merrill et al., 2013a). We have previously characterized the involvement of the classical mitogen activated protein (MAP) kinase (ERK1/2) in micturition reflexes in the context of urinary bladder inflammation (Corrow and Vizzard, 2007; 2009). The hypothesis for this project is that CYP-induced cystitis and the resulting bladder dysfunction also involve an additional kinase-based signaling cascade, the JNK/SAPK phosphorylation cascade. We specifically hypothesized that CYP-induced cystitis in female rats: (1) results in JNK activation (i.e., phosphorylation) in the urinary bladder and (2) pharmacological blockade of JNK phosphorylation at the level of the urinary bladder will improve urinary bladder function in CYP-treated rats. We also evaluated neuropeptide (i.e., substance P (Sub P), calcitonin gene-related peptide)(Vizzard, 2001; Merrill et al., 2013a) expression in urinary bladder in rats with CYP-induced cystitis in the presence and absence of JNK blockade with SP600125 (Bennett et al., 2001).
Materials and Methods
Cyclophosphamide (CYP)-induced cystitis in rats
Female Wistar rats (180-310 g; Charles River Canada, St. Constant, Canada) were randomly separated into 4 groups, each receiving a different concentration and/or duration of treatment with cyclophosphamide (CYP; Sigma ImmunoChemicals, St. Louis, MO, USA). Experimental groups received CYP-treatment (1) 4 hours (hr) before euthanasia (150 mg/kg, intraperitoneal, i.p.); (2) 48 hr before euthanasia (150 mg/kg, i.p.); (3) every third day over a 10 day period (75 mg/kg, i.p.) (Arms et al., 2010; Arms et al., 2013; Gonzalez et al., 2013). Control animals received no treatment. Rats were euthanized with isoflurane (4-5%) and thoracotomy. Efforts were taken to reduce the number of animals used in this study and to minimize pain and distress (Corrow and Vizzard, 2009; Corrow et al., 2010). The University of Vermont Institutional Animal Care and Use Committee approved all animal procedures.
Conscious Cystometry and effects of JNK phosphorylation blockade
Rats were anesthetized with isoflurane (3-4%), a lower midline abdominal incision was made, and polyethylene tubing (PE-50, Clay Adams, Parsippany, New Jersey) was inserted into the bladder dome and secured with a nylon purse-string sutures (6-zero) (Schnegelsberg et al., 2010; Gonzalez et al., 2013; Merrill et al., 2013b). The end of the PE tubing was heat flared, but the catheter did not extend into the bladder body or neck and it was not associated with inflammation or altered cystometric function (Schnegelsberg et al., 2010; Gonzalez et al., 2013, Merrill et al., 2013b). The distal end of the tubing was sealed, tunneled subcutaneously and externalized at the back of the neck out of reach of the animal (Schnegelsberg et al., 2010; Gonzalez et al., 2013; Merrill et al., 2013b). Abdominal and neck incisions were closed with nylon sutures (4-zero). Postoperative analgesics were given and animals were maintained for 72 to 96 hours after survival surgery to ensure recovery.
The effects of JNK phosphorylation blockade with SP600125, a selective inhibitor of phosphorylation of JNK (Bennett et al., 2001), on urinary bladder function in CYP-treated (4 hr and 48 hr; n = 6 each) rats and control rats (n = 6 each) were assessed using conscious, open outlet, cystometry with continuous instillation of intravesical saline (Schnegelsberg et al., 2010; Gonzalez et al., 2013; Merrill et al., 2013b). For intravesical administration of SP600125, rats were anesthetized with 2% isoflurane and SP600125 (<1.0 ml) was injected through the bladder catheter; the animals were maintained under anesthesia to prevent expulsion of SP600125 via a voiding reflex. In this procedure, SP600125 remained in the bladder for 30 min at which time, the drug was drained, the bladder washed with saline and animals recovered from anesthesia for 20 min before experimentation. The effectiveness of intravesical SP600125 (25 μM) administration was evaluated in control (no CYP treatment) rats and in rats treated 4 hr and 48 hr after a single injection of CYP (150 mg/kg, i.p.). These experiments were performed in the same CYP-treated rats before and after treatment with SP600125. The concentration (25 μM) of SP600125 used in these studies was based upon previous studies (Gao et al., 2010; Ikeda et al., 2012). Control groups of CYP-treated rats receiving intravesical administration of vehicle (0.1% DMSO; Sigma-Aldrich, St. Louis, MO) (n = 6) were also evaluated. For cystometry in conscious rats, an unrestrained animal was placed in a Plexiglas cage with a wire bottom. Before the start of the recording, the bladder was emptied and the catheter was connected via a T-tube to a pressure transducer (Grass Model PT300, West Warwick, RI) and microinjection pump (Harvard Apparatus 22, South Natick, MA). A Small Animal Cystometry Lab Station (MED Associates, St. Albans, VT) was used for urodynamic measurements (Schnegelsberg et al., 2010; Gonzalez et al., 2013; Merrill et al., 2013b). Saline solution was infused at room temperature into the bladder at a rate of 10 ml/h to elicit repetitive bladder contractions. At least four reproducible micturition cycles were recorded after the initial stabilization period of 25–30 min (Schnegelsberg et al., 2010; Gonzalez et al., 2013; Merrill et al., 2013b). To summarize, the experimental design involves administration of a one time, intravesical infusion of SP600125 (25 µM) with cystometric data collection occurring ~75 min after infusion. The following cystometric parameters were recorded in each animal: filling pressure (pressure at the beginning of the bladder filling), threshold pressure (bladder pressure immediately prior to micturition), micturition pressure, micturition interval (time between micturition events), bladder capacity, void volume, presence and amplitude of NVCs (Schnegelsberg et al., 2010; Gonzalez et al., 2013; Merrill et al., 2013b). In these rats, residual volume was less than 10 μl; therefore, voided volume and bladder capacity were similar. For the present study, NVCs were defined as increases in bladder pressure of at least 7 cm H2O without release of urine. At the conclusion of the experiment, the animal was euthanized (4% isoflurane plus thoracotomy), the urinary bladder was harvested and randomly assigned for use in one of the following procedures.
Western blotting for pJNK and total JNK
Bladders were harvested from rodents in control and experimental groups and were homogenized separately in tissue protein extraction agent (a proprietary detergent in 25 mM bicine and 150 mM sodium chloride, pH 7.6; T-PER, Roche, Indianapolis, IN) containing a protease inhibitor mix (16 μg/ml benzamidine, 2 μg/ml leupeptin, 50 μg/ml lima bean trypsin inhibitor, and 2 μg/ml pepstatin A; Sigma-Aldrich, St. Louis, MO) and phosphatase inhibitors (Sigma-Aldrich Inhibitor Cocktail II); aliquots were removed for protein assay as previously described (Corrow and Vizzard, 2009; Corrow et al., 2010). Samples (20 μg) were suspended in sample buffer for fractionation on gels and subjected to SDS-PAGE. Proteins were transferred to nitrocellulose membranes, and efficiency of transfer was evaluated. Membranes were blocked overnight in a solution of 5% milk and 3% bovine serum albumin in Tris-buffered saline with 0.1% Tween. For immunodetection, rabbit phospho-SAPK/JNK (1:200 in TBST/5% BSA; Cell Signaling Technology, Danvers, MA, USA) or SAPK/JNK (1:300 in TBST/5% BSA; Cell Signaling Technology) antibodies were used overnight at 4°C. Washed membranes were incubated in species-specific secondary antibodies for 2 hr at room temperature for enhanced chemiluminescence detection (Santa Cruz Biotechnology, Inc. Santa Cruz, CA, USA), exposed to Biomax film (Kodak, Rochester, NY, USA) and developed. Bands corresponding to pJNK and JNK were analyzed using un-Scan-It Gel software (Silk Scientific Corp., Orem, UT, USA) and the intensity of each band was determined separately. Background intensities were subtracted (Corrow and Vizzard, 2009; Corrow et al., 2010) and the mean value of bands was calculated and normalized with the loading control (total JNK). The average for the normalized density values of control experiments was determined and assigned a value of 100%. The normalized values of the CYP treatments were expressed as percentages relative to control experiments (Corrow and Vizzard, 2009; Corrow et al., 2010).
CGRP and Sub P enzyme-linked immunosorbent assays (ELISAs)
Tissue processing and ELISAs were performed as described previously (Vizzard, 2000). Briefly, rats from control (n = 6 each) and all experimental groups (n = 6 each) were deeply anesthetized (4% isoflurane), and a thoracotomy was performed. Individual rat bladders were dissected, weighed, and placed in Tissue Protein Extraction Reagent (1 g tissue/20 ml; Pierce Biotechnology, Woburn, MA) with Complete protease inhibitor cocktail tablets (Roche Applied Science, Mannheim, Germany), and stored at −80 °C. On the day of assay, individual bladders were disrupted with a Polytron homogenizer until homogeneous and centrifuged (10,000 rpm for 10 min) (Vizzard, 2000), and the supernatant was used for total protein estimation and CGRP and Sub P quantification. Total protein was determined by the Coomassie Plus Protein Assay Reagent Kit (Pierce) (Vizzard, 2000), and CGRP and Sub P were quantified using standard 96-well ELISA plates (Phoenix Pharmaceuticals, Burlingame, CA) according to the manufacturer's recommendations.
ELISAs for CRGP and Sub P expression in urinary bladder
The microtiter plates (Phoenix Pharmaceuticals) were coated with mouse anti-rat CGRP or anti-rat Sub P antibody. Sample and standard solutions were run in duplicate. Horseradish peroxidase-streptavidin conjugate was used to detect the antibody complex. Tetramethylbenzidine was the substrate, and the enzyme activity was measured by the change in optical density. The CRGP standard provided with this protocol generated a linear standard curve from 0 to 100 ng/ml (R2 = 0.998, P ≤ 0.0001) for bladder samples. The Sub P standard provided with this protocol generated a linear standard curve from 0 to 25 ng/ml (R2 = 0.998, P ≤ 0.0001) for bladder samples. The absorbance values of standards and samples were corrected by subtraction of the background value (absorbance due to nonspecific binding) (Vizzard, 2000; Merrill et al., 2013b). No samples were diluted and all samples had absorbance values that fell onto the linear portion of the standard curve. Curve fitting of standards and evaluation of CRGP or Sub P content of samples were performed using a least-squares fit.
Urinary Bladder Preparation, pJNK immunohistochemistry and image analysis
Whole bladders from control (n = 6) and CYP treatment groups (n = 6 each) were postfixed in 4% paraformaldehyde, placed in ascending concentrations of sucrose (10–30%), frozen, sectioned (7 μm), and mounted on gelled (0.5%) microscope slides (Corrow and Vizzard, 2009; Corrow et al., 2010; Gonzalez et al., 2013). An on-slide processing technique was used to prepare bladder sections for pJNK1/2 immunostaining in control group and CYP-treatment group rats. Groups (n=6) of control animals and experimental animals were processed at the same time to minimize variation between tissues and animals. Bladder sections were incubated overnight with rabbit anti-phospho (p)JNK1/2 (pJNK; 1:1000; Cell Signaling Technology) in 1% goat serum and 0.1M KPBS (phosphate buffered solution with potassium) followed by washes (3 × 15 min) with 0.1M KPBS at pH 7.4. Tissue was incubated for 2 hr with Cy3-conjugated goat anti-rabbit IgG (1:500; Jackson ImmunoResearch) at room temperature and then washed (3 x 15 min) with 0.1M KPBS (Corrow and Vizzard, 2009; Corrow et al., 2010; Gonzalez et al., 2013). Tissues were then mounted on slides with Citifluor (Citifluor, London, UK) and coverslipped (Corrow and Vizzard, 2009; Corrow et al., 2010; Gonzalez et al., 2013). Methodological controls included tissues processed in the absence of primary or secondary antibody. Control sections incubated in the absence of primary or secondary antibody were also processed and evaluated for specificity or background staining levels. In the absence of primary antibody, no positive immunostaining was observed. Staining present in experimental tissue was then compared with staining observed in experiment-matched negative controls. Positively- stained tissue was determined to be tissue that exhibited immunoreactivity greater than the background observed in experiment- matched negative controls.
A Zeiss LSM 510 confocal scanning system (Carl Zeiss MicroImaging, Inc., Thornwood, NY, USA) attached to a Zeiss LSM 510 microscope using a plan Fluor 20 × objectives was used to examine tissue for Cy3 immunoreactivity using excitation wavelengths of 380 and 543 nm (Corrow and Vizzard, 2009; Corrow et al., 2010; Gonzalez et al., 2013). Tissue samples from bladders in the control, 4 hr and 48 hr treatment groups (6-9 per bladder) were photographed using a CCD camera (MagnaFire SP; Optronics; Optical Analysis Corp., Nashua, NH, USA) and LG-3 frame grabber attached to an Olympus microscope (Optical Analysis Corp) (Corrow and Vizzard, 2009; Corrow et al., 2010; Gonzalez et al., 2013). Exposure times were held constant when acquiring images from control and experimental animals processed and analyzed on the same day using MetaMorph image analysis software (Corrow and Vizzard, 2009; Corrow et al., 2010; Gonzalez et al., 2013).
Visualization and semi-quantitative analysis of pJNK-IR in urinary bladder sections
In this study, we have focused on pJNK-IR in the urothelium and detrusor smooth muscle. In preliminary studies using wholemount bladder preparations, no evidence of pJNK-IR in the suburothelial nerve plexus was observed. Five urinary bladder sections from each animal in the control (n = 6) and experimental groups (n = 6; 4 hr, 48 hr) were examined using an Olympus fluorescence photomicroscope (Optical Analysis, Nashua, NH) with a multiband filter set for visualization of the Cy3 fluorophore. Cy3 was visualized with a filter with an excitation range of 560–596 nm and an emission range from 610–655 nm (Corrow and Vizzard, 2009; Corrow et al., 2010; Gonzalez et al., 2013). Grayscale images acquired in tiff format here imported into MetaMorph image analysis software (version 4.5r4; Universal Imaging, Downingtown, PA) as previously described (Corrow and Vizzard, 2009; Corrow et al., 2010; Gonzalez et al., 2013). Images were calibrated for pixel size by applying a previously created calibration file to convert all measurements from pixels to square micrometers. The free-hand drawing tool was selected and the urothelium was drawn and measured in total pixels per area (Corrow and Vizzard, 2009; Corrow et al., 2010; Gonzalez et al., 2013). A threshold within an intensity range of 100–250 grayscale values was applied to the region of interest in the least brightly stained condition first. The same threshold was subsequently used for all images. Average intensity was calculated within the outlined area. The average intensity represents the average value of all of the pixels above the threshold value. Percent pJNK expression above threshold in the total area selected was then calculated and averaged for all samples from control (n = 6) and CYP-treated rats (n = 6 each). Percentage pJNK expression in CYP-treated rats was therefore expressed as a percentage of control.
Statistical Analyses
All values are mean ± SEM. Comparisons of pJNK protein concentration, pJNK-IR or urodynamic parameters among experimental groups were made using ANOVA. Percentage data from image analysis were arcsin transformed to meet the requirements of the statistical test. Animals, processed and analyzed on the same day, were tested as a block in the ANOVA. When F ratios exceeded the critical value (P ≤ 0.05), the Newman-Keul's post hoc test was used to compare experimental means among groups.
Figure Preparation
Digital images were obtained using a CCD camera (MagnaFire SP; Optronics; Optical Analysis, Nashua, NH) and LG-3 frame grabber attached to an Olympus microscope (Optical Analysis). Exposure times were held constant when acquiring images from control and experimental animals processed and analyzed on the same day. Images were imported into Adobe Photoshop 9.0 (Adobe Systems, San Jose, CA), where groups of images were assembled and labeled.
Results
Increased expression of pJNK1/2 in urinary bladders with CYP-induced cystitis
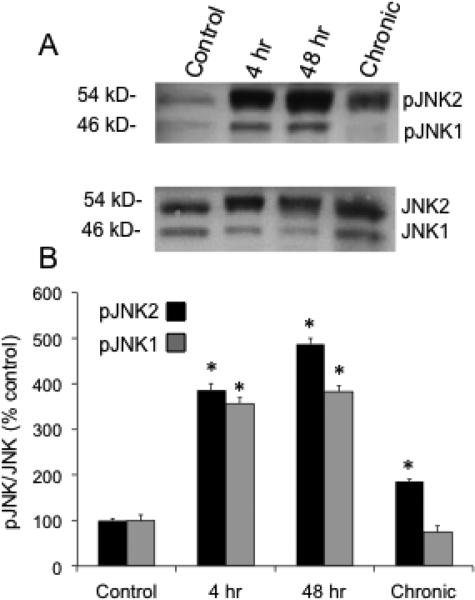
Both pJNK1 (46 kD) and pJNK2 (54 kD) expression was significantly (P ≤ 0.01) increased in the urinary bladder with CYP-treatment (Fig. 1). All durations (4 hr, 48 hr, chronic) of CYP-treatment increased pJNK2 expression in the urinary bladder. In contrast, pJNK1 expression was only significantly (P ≤ 0.01) increased in the urinary bladder with 4 hr and 48 hr CYP-treatment (Fig. 1). In subsequent experiments, we have focused on the 4 hr and 48 hr CYP treatment groups because of the increased pJNK1/2 expression demonstrated in the urinary bladder with Western blotting.
Figure 1.
JNK1 and JNK 2 activation with CYP-induced cystitis of varying duration. A: representative example of a Western blot of whole urinary bladder (20 μg) for pJNK expression in control rats and those treated with CYP for varying duration. Total JNK staining was used as a loading control. B: histogram of relative pJNK2 (54 kD) and pJNK1 (46 kD) band density in all groups examined normalized to total JNK in the same samples presented as a percentage of control JNK activation (pJNK expression). pJNK2 expression in urinary bladder is significantly increased at all durations of CYP treatment whereas pJNK1 expression in urinary bladder is significantly increased with 4 hr and 48 hr CYP-induced cystitis. *P ≤ 0.01. n = 6 for control and each CYP group in B.
Increased pJNK1/2 immunoreactivity (IR) in urothelium and detrusor smooth muscle with CYP-induced cystitis
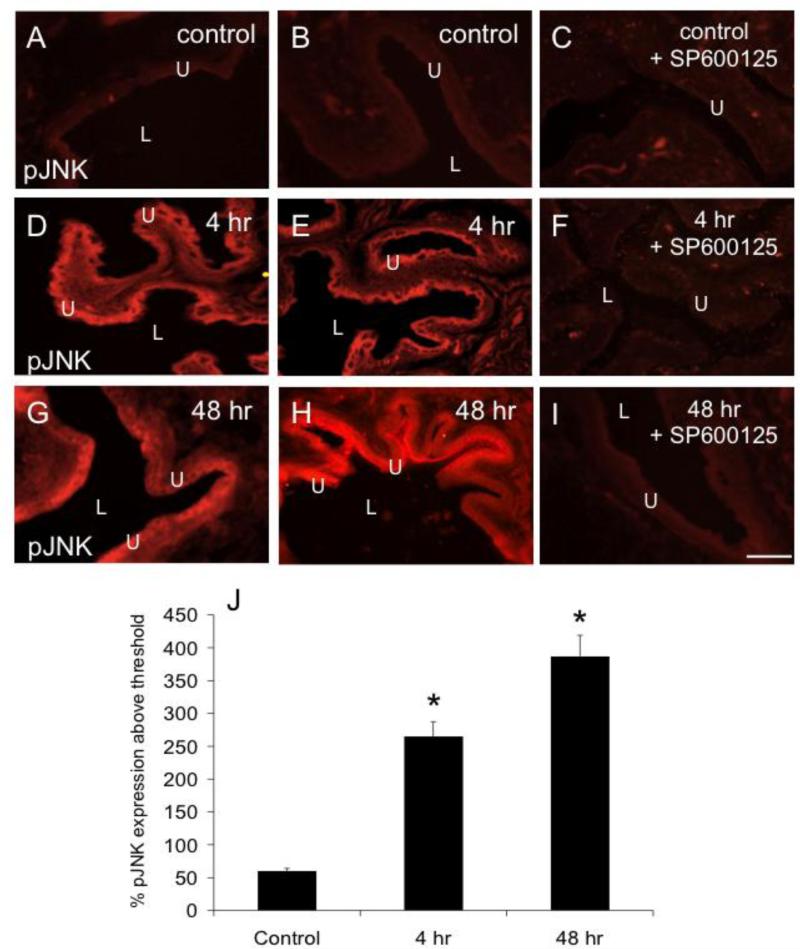
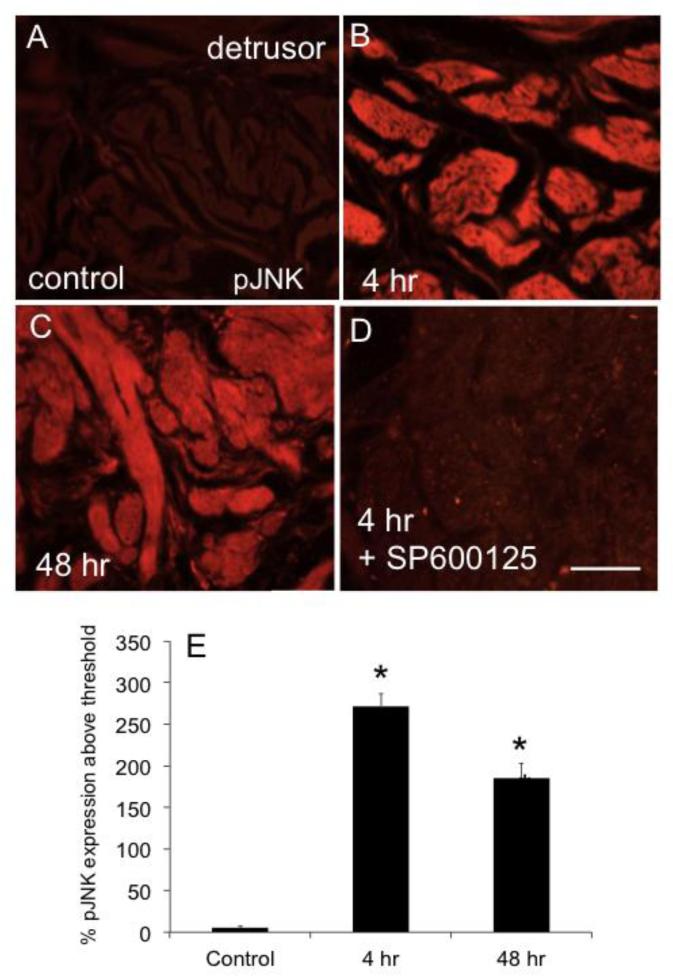
Urinary bladder sections from rats treated with CYP (4 hr, 48 hr) demonstrated significant (P ≤ 0.01) increases in pJNK1/2-IR in the urothelium (Fig. 2) and detrusor smooth muscle (Fig. 3). These immunohistochemical studies are consistent with results from Western blotting, which also demonstrated increased pJNK1/2 expression in the urinary bladder with 4 hr and 48 hr CYP treatment.
Figure 2.
CYP-induced cystitis increases pJNK expression in the urothelium (U). Fluorescence images of pJNK expression in urinary bladder sections of control (A, B), 4 hr (D, E), and 48 hr (G, H) CYP-treated rats. For all images, exposure times were held constant, and all tissues were processed simultaneously. In control rats, little if any pJNK expression was visible in the urothelium (U; A, B). CYP treatment (4 hr, D, E; 48 h, G, H) upregulated expression of pJNK in the urothelium in all layers (apical, intermediate and basal) of the urothelium. F, I: Intravesical instillation of SP600125 (25 μM) to block JNK phosphorylation reduced pJNK expression in the urothelium of rats treated with CYP (4 hr, F; 48 hr, I) but was without effect in control rats (C). J: summary histogram of pJNK expression in the urothelium with CYP treatment expressed a percentage of pJNK expression above threshold and averaged for all bladders from all conditions examined (n = 6). *P ≤ 0.01. L, lumen. Calibration bar represents 50 μm.
Figure 3.
CYP-induced cystitis increases pJNK expression in detrusor muscle of rat urinary bladder. Fluorescence images of pJNK expression in urinary bladder sections of control (A), 4 hr (B), and 48 hr (C) CYP-treated rats. For all images, exposure times were held constant, and all tissues were processed simultaneously. In control rats, little if any pJNK expression was visible in the detrusor (A). CYP treatment (4 hr, B; 48 h, C) upregulated expression of pJNK in the detrusor. D. Intravesical instillation of SP600125 (25 μM) to block JNK phosphorylation reduced pJNK expression in the detrusor of rats treated with CYP (4 hr). J: summary histogram of pJNK expression in the detrusor with CYP treatment expressed a percentage of pJNK expression above threshold and averaged for all bladders from all conditions examined (n = 6). *P ≤ 0.01. Calibration bar represents 50 μm.
Blockade of phosphorylation of JNK decreases pJNK-IR and CGRP and Sub P protein content in urinary bladder following CYP-induced cystitis
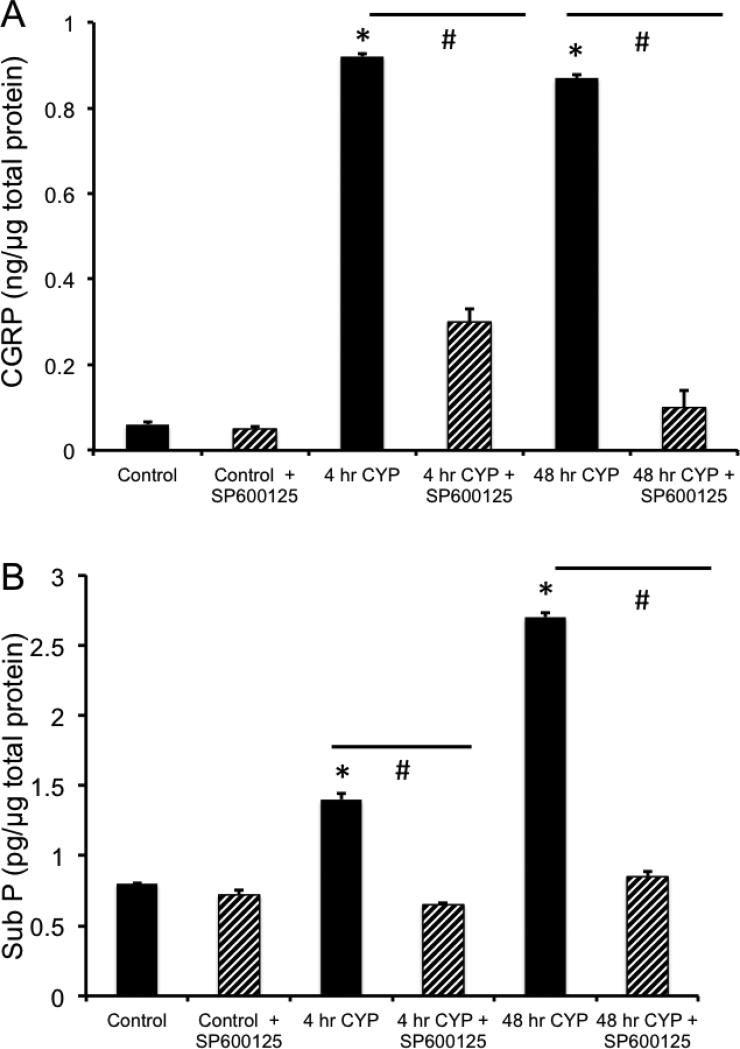
Intravesical instillation of SP600125 in rats treated with CYP (4 hr and 48 hr) prevented the increase in pJNK-IR in urothelium (Fig. 2) and detrusor smooth muscle (Fig. 3) demonstrated with CYP-induced cystitis. We have previously demonstrated increased expression of the neuropeptides, CGRP and Sub P, in micturition reflex pathways following CYP-induced cystitis (Vizzard, 2001). In the current studies using ELISAs, we demonstrate that CYP-induced cystitis is also associated with significant (P ≤ 0.01) increases in CGRP and Sub P content in the urinary bladder (Fig. 4). Intravesical instillation of SP600125 in rats treated with CYP (4 hr or 48 hr) prevented the increase in CGRP and Sub P protein content in the urinary bladder but had no effect on baseline CGRP or Sub P expression in urinary bladder of control (no inflammation) rats (Fig. 4).
Figure 4.
Blockade of JNK phosphorylation with SP600125 significantly decreases neuropeptide (CGRP, Sub P) expression in the urinary bladder with CYP-induced cystitis. A. Summary histogram of the effects of JNK phosphorylation blockade with SP600125 infusion (25 μM) on calcitonin gene-related peptide (CGRP) in control rats and those treated with CYP (4 hr and 48 hr). B. Summary histogram of the effects of JNK phosphorylation blockade with SP600125 infusion (25 μM) on Substance P (Sub P) in control rats and those treated with CYP (4 hr and 48 hr). n = 6 for control and each CYP group. *P ≤ 0.01 compared to control. #P ≤ 0.01 compared to the respective group (4 hr, 48 hr) without SP600125 treatment.
Blockade of phosphorylation of JNK improves urinary bladder function in CYP-treated rats
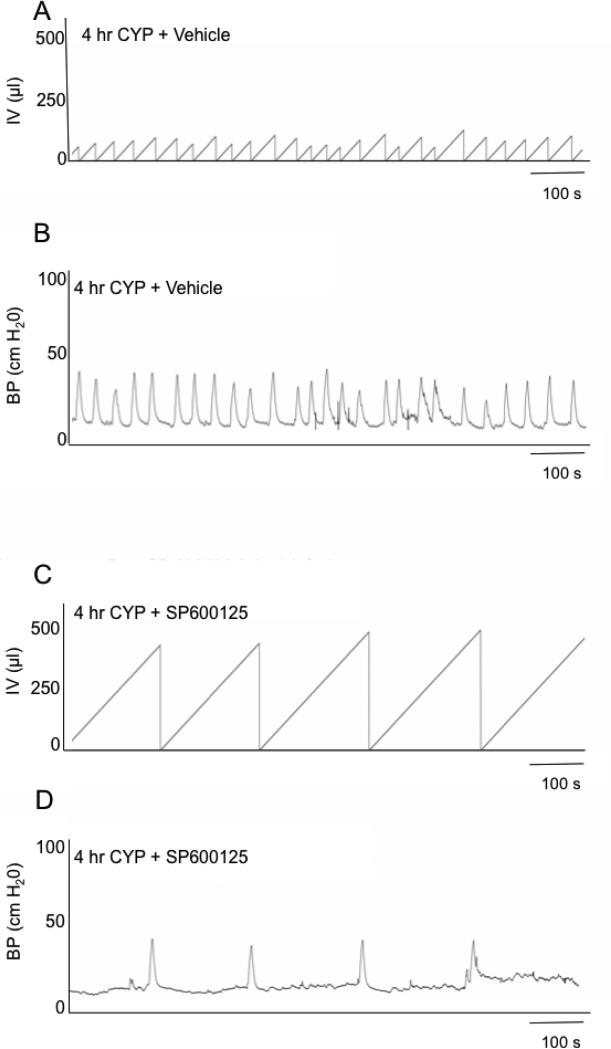
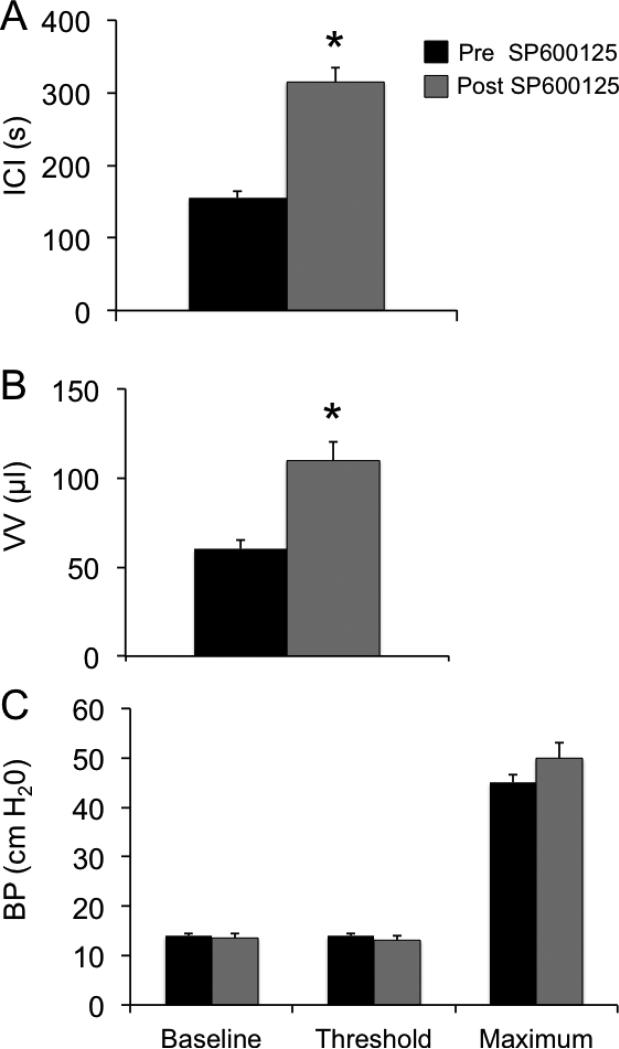
Consistent with previous studies (Corrow and Vizzard, 2007; Arms et al., 2010; Arms et al., 2013; Gonzalez et al., 2013), CYP-induced cystitis (4 hr or 48 hr) decreased (P ≤ 0.01) bladder capacity (75%) and the intercontraction interval (ICI) between voiding events. To evaluate whether increased pJNK expression and signaling participated in CYP-induced cystitis-associated bladder dysfunction, SP600125, was infused intravesically and the effects evaluated. Intravesical administration of SP600125 (25 μM) significantly increased void volume (VV) in 4 hr CYP-treated rats 1.9-fold (Fig. 5, 6; P ≤ 0.01) and the ICI (Fig. 5, 6). No changes in bladder pressure (BP) including baseline, threshold or maximum micturition pressure (Fig. 6) were observed with intravesical administration of SP600125. Effects of intravesical instillation of SP600125 on rats treated with CYP (48 hr) were similar to those observed in rats treated with CYP (4 hr). Intravesical instillation of SP600125 significantly (P ≤ 0.01) increased the VV (1.8-fold) and the ICI (1.8-fold) in rats treated with CYP (48 hr) with no changes in BP. Changes in bladder capacity and ICI observed in CYP-treated rats receiving SP600125 treatment lasted ~60 min. CYP-treated rats treated with vehicle (0.1% DMSO) showed no changes in bladder capacity, filling, threshold, or micturition pressure compared with CYP-treated groups (Fig. 5 and data not shown). Intravesical administration of SP600125 in control (no CYP treatment) rats had no effect on bladder function (data not shown).
Figure 5.
Intravesical administration of SP600125 (25 μM), an inhibitor of JNK phosphorylation, increased bladder capacity (reduced voiding frequency) after cyclophosphamide (CYP)-induced cystitis. Cystometrogram recordings from continuous instillation of room temperature saline in 4 hr CYP-treated rats + vehicle (0.1% DMSO) (A, B) and 4 hr CYP-treated rats + SP600125 (25 μM; C, D). As expected, CYP treatment decreased bladder capacity and increased voiding frequency. In 4 hr CYP-treated rats further treated with intravesical instillation of SP600125 (25 μM), bladder capacity was increased and voiding frequency reduced (C). The x-axis represents the time (s), and the y-axis represents the bladder pressure (BP, cm H2O) or infused volume (IV, μl).
Figure 6.
Effects of JNK phosphorylation blockade with SP600125 (25 μM) on intercontraction interval (ICI), void volume (VV) and bladder pressure (BP; baseline, threshold, maximum) determined using conscious cystometry with continuous instillation of saline in control and CYP-treated (4 hr) rats with an open outlet. A. Histogram demonstrating significant increases in the duration of the ICI with intravesical instillation of SP600125 (25 μM) in rats treated with CYP (4 hr). B. Histogram demonstrating significant increases in the VV of CYP treated (4 hr) rats with intravesical instillation of SP600125 (25 μM). C. No changes in BP were demonstrated in CYP-treated (4 hr) rats with intravesical instillation of SP600125. n = 6 for control and CYP group. *P ≤ 0.01.
Discussion
The current studies demonstrate several novel findings with respect to the role of JNK activation (i.e., phosphorylation) in micturition reflex pathways following inflammation induced by cyclophosphamide (CYP). Immunohistochemical and western blotting studies demonstrated significant increases in pJNK expression in the urinary bladder including the urothelium and detrusor smooth muscle. Blockade of JNK phosphorylation improved urinary bladder function in rats treated with CYP. Intravesical administration of SP600125 also prevented the increase in pJNK-IR in the urothelium and detrusor in rats treated with CYP. In addition, SP600125 also reduced the increase in CGRP and Sub P protein content in the urinary bladder following CYP-induced cystitis. These results suggest that blockade of JNK pathways at the level of the urinary bladder may be a useful target to improve urinary bladder function in the context of urinary bladder inflammation.
c-Jun Amino-terminal kinase (JNK) is a member of the mitogen-activated protein kinase (MAPK) that becomes activated (i.e., phosphorylated) in response to environmental stressors (Doya et al., 2005). There are three distinct JNK genes, including Jnk1 and Jnk2 ubiquitously expressed in eukaryotes and Jnk3 with a more restricted distribution in the brain, heart and testes (Davis, 2000; Peti and Page, 2013). Genes in the MAPK family can undergo alternative splicing, mutually exclusive exon utilization and a region of variable amino acid composition near the end of the C-terminal lobe to yield ten splice variants (Peti and Page, 2013). The mutually exclusive utilization of two exons yields two differently sized protein products, 46 kD (JNK1) and 54 kD (JNK2); however, no significant functional differences have been demonstrated between these two variants (Davis, 2000). In the current studies, Western blotting experiments demonstrated that phosphorylation of JNK1 and JNK2 was significantly increased in whole urinary bladder with 4 hr and 48 hr CYP-induced cystitis. However, chronic CYP-induced cystitis was only associated with increased activation of JNK2 in the urinary bladder. The current studies extend previous studies that examined JNK activation in the urinary bladder following CYP-induced cystitis of shorter duration (2 hr, 8 hr, 48 hr) (Chung et al., 2010). In addition, the current studies demonstrate significant and robust JNK activation in the urinary bladder with 48 hr CYP treatment. In contrast, previous studies demonstrated peak JNK activation with 8 hr CYP treatment (Chung et al., 2010). Differences in the time course of JNK activation (Chung et al., 2010) may be attributed to strain and gender differences in rats. The involvement of the JNK1/2 signaling cascade in other components of the micturition reflex pathways (e.g., dorsal root ganglia (DRG), spinal cord) is not known but can be addressed in future studies. Previous studies have demonstrated involvement of ERK1/2 pathways in multiple components of micturition reflexes including the urinary bladder, spinal cord and DRG following CYP-induced cystitis (Corrow and Vizzard, 2007; 2009).
JNK is activated through the duel phosphorylation of threonine and tyrosine residues on a Thr-Pro-Tyr motif within kinase subdomain VIII (Markevich et al., 2004) resulting in dimerization and translocation from the cytoplasm to the nucleus. SP600125, a small molecule, anthrapyrazolone inhibitor of JNK1, JNK2, and JNK3, is a reversible ATP-competitive inhibitor with >20-fold selectivity vs. a range of kinases and enzymes tested (Bennett et al., 2001). A combination of cell culture and animal studies demonstrated that SP600125 dose dependently inhibited the phosphorylation of c-Jun, the expression of inflammatory genes (e.g., COX-2, IL-2, TNF-α), prevented the activation and differentiation of primary human CD4 cell cultures and blocked lipopolysaccharide-induced expression of TNF-α (Bennett et al., 2001). In the current studies, intravesical administration of SP600125 prevented pJNK expression in the urothelium and detrusor smooth muscle in CYP-treated rats. Previous studies from our laboratory have demonstrated increased expression of neuropeptides, including CGRP and Sub P, in bladder afferent cells in lumbosacral dorsal root ganglia following CYP-induced cystitis (Vizzard, 2001; Merrill et al., 2013a). We extend these observations with the demonstration of increased CGRP and Sub P expression in the urinary bladder following CYP-induced cystitis. It is our contention that CGRP and Sub P are synthesized in lumbosacral DRG cells bodies and transported in an anterograde manner to the suburothelial nerve plexus in the urinary bladder (Merrill et al., 2013a). Blockade of phosphorylation of JNK at the level of the urinary bladder significantly reduced the expression of CGRP and Sub P in the urinary bladder of rats treated with CYP (4 hr and 48 hr) but had no effect in control (no CYP treatment) rats. Previous studies demonstrated that increased CGRP expression observed in rat trigeminal ganglion with organ culture is regulated via MAPK pathways (Lei et al., 2012). Blockade of phosphorylation of ERK1/2, P38, or JNK signaling cascades markedly reduced CGRP expression in the cultured rat trigeminal ganglion (Lei et al., 2012). The current studies demonstrate that blockade of JNK phosphorylation at the level of the urinary bladder reduced Sub P and CGRP protein content in the urinary bladder, which could also contribute to the improved urinary bladder function in rats treated with CYP and SP600125.
Intravesical instillation of SP600125 improved urinary bladder function in rats treated with CYP by reducing voiding frequency and increasing the interval between micturition events but produced no changes in urinary bladder pressure. In the current studies, we evaluated the effects of JNK phosphorylation blockade following 4 hr and 48 hr CYP-induced cystitis because these time points exhibited the most robust in JNK1 and JNK2 activation with Western blotting. SP600125 reduced JNK phosphorylation and the duration of effects on bladder function was approximately 60 minutes suggesting that JNK phosphorylation is a dynamic, ongoing process induced by CYP-induced cystitis. Consistent with this suggestion, previous studies have demonstrated sustained JNK activation in Parkinson's and Alzheimer's diseases (Wang et al., 2014) and rheumatoid arthritis (de Launay et al., 2012). Previous studies from our laboratory have evaluated other components of MAPK pathways including the effects of blockade of phosphorylation of ERK1/2 and P38 signaling cascades on micturition reflex function (Corrow and Vizzard, 2007; 2009). Intravesical administration of U0126, a potent and selective MEK inhibitor (Corrow and Vizzard, 2007; 2009), significantly increased bladder capacity and tended to decrease nonvoiding bladder contractions in CYP-treated rats. In the current study, few nonvoiding bladder contractions were observed in control or CYP-treated rats in the presence or absence of SP600125 and were therefore not evaluated. It would be of interest in future studies to determine if treatment with a combination of intravesical blockers of phosphorylation of JNK, ERK1/2 and/or P38 would be additive or of no additional benefit to urinary bladder function (Corrow and Vizzard, 2007; 2009). Future studies could also determine the effects on bladder function with different routes of administration including intrathecal and intravenous delivery.
Downstream target genes modulated by the JNK pathway are numerous and include cytokines, growth factors, immunoglobulins, inflammatory enzymes, and matrix metalloproteinases (Mercer and D'Armiento, 2006; Mehan et al., 2011). Many of these downstream targets have also been implicated in urinary bladder dysfunction in preclinical animal models of BPS/IC (Arms et al., 2010; Schnegelsberg et al., 2010; Arms et al., 2013; Gonzalez et al., 2013) as well as in individuals with BPS/IC. Given the number and diversity of downstream targets modulated by JNK signaling (Mercer and D'Armiento, 2006), the potential for MAP kinase inhibitors is another promising area of research. MAP kinase inhibitors including SP600125 have already demonstrated efficacy in reducing apoptosis, inflammation, cytokine production, fibrosis, and MMP expression (Bennett et al., 2001; Mercer and D'Armiento, 2006). Future studies should evaluate the ability of MAP kinase inhibitors including SP600125 to reduce bladder dysfunction in additional preclinical models of BPS/IC. Compared to ERK and p38, much less is known about how JNK regulates pain. The JNK pathway is preferentially activated in spinal astrocytes important for the maintenance of neuropathic pain (Gao et al., 2010). Intrathecal infusion of the JNK inhibitor SP600125 attenuates neuropathic pain in the spinal nerve ligation model (Obata et al., 2004; Zhuang et al., 2006), spinal cord injury model (Lee et al., 2013) and in a diabetic model (Daulhac et al., 2006). The ability of MAP kinase inhibitors to reduce pelvic sensitivity should also be evaluated.
Previous studies have demonstrated a role for NGF in the activation of MAPK pathways including JNK in the urinary bladder following CYP-induced cystitis (Chung et al., 2010). NGF neutralization in CYP-treated rats reduced the activation of JNK and also reduced the induction of type I collagen and urinary bladder weight. Our previous studies (Schnegelsberg et al., 2010) revealed that urothelium-specific overexpression of NGF in the urinary bladder of transgenic mice (1) stimulates neuronal sprouting or proliferation in the urinary bladder; (2) produces local inflammatory changes in the urinary bladder; (3) results in increased voiding frequency; and (4) results in increased referred somatic hypersensitivity. Examination of MAPK pathways in urinary bladder of the NGF-OE mouse model will be important first steps in further understanding the potential regulation of MAPK pathways by NGF.
In summary, these studies demonstrate activation (i.e., phosphorylation) of JNK in the urinary bladder following CYP-induced cystitis using immunohistochemical and Western blotting approaches. Functional studies demonstrate that blockade of JNK phosphorylation reduces voiding frequency and neuropeptide content in the urinary bladder with CYP-induced cystitis. The JNK pathway may be a useful target for improving urinary bladder function in the context of urinary bladder inflammation.
ACKNOWLEDGEMENTS
This work was funded by National Institutes of Health (NIH) grants DK051369 (MAV), DK060481 (MAV) and DK065989 (MAV). This publication was also supported by grants from the National Center for Research Resources (5 P30 RR 032135) and the National Institute of General Medical Sciences (8 P30 GM 103498) from the NIH. The authors gratefully acknowledge the technical expertise and support provided by the VT Cancer Center DNA Analysis Facility.
References
- Arms L, Girard BM, Malley SE, Vizzard MA. Expression and function of CCL2/CCR2 in rat micturition reflexes and somatic sensitivity with urinary bladder inflammation. Am. J. Physiol. Renal Physiol. 2013;305:F111–122. doi: 10.1152/ajprenal.00139.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arms L, Girard BM, Vizzard MA. Expression and function of CXCL12/CXCR4 in rat urinary bladder with cyclophosphamide-induced cystitis. Am. J. Physiol. Renal Physiol. 2010;298:F589–600. doi: 10.1152/ajprenal.00628.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. (U.S.A.) 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CW, Zhang QL, Qiao LY. Endogenous nerve growth factor regulates collagen expression and bladder hypertrophy through Akt and MAPK pathways during cystitis. J. Biol. Chem. 2010;285:4206–4212. doi: 10.1074/jbc.M109.040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrow K, Girard BM, Vizzard MA. Expression and response of acid-sensing ion channels in urinary bladder to cyclophosphamide-induced cystitis. Am. J. Physiol. Renal Physiol. 2010;298:F1130–1139. doi: 10.1152/ajprenal.00618.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrow KA, Vizzard MA. Phosphorylation of extracellular signal-regulated kinases in urinary bladder in rats with cyclophosphamide-induced cystitis. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2007;293:R125–134. doi: 10.1152/ajpregu.00857.2006. [DOI] [PubMed] [Google Scholar]
- Corrow KA, Vizzard MA. Phosphorylation of extracellular signal-regulated kinases in bladder afferent pathways with cyclophosphamide-induced cystitis. Neuroscience. 2009;163:1353–1362. doi: 10.1016/j.neuroscience.2009.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daulhac L, Mallet C, Courteix C, Etienne M, Duroux E, Privat AM, Eschalier A, et al. Diabetes-induced mechanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia via N-methyl-D-aspartate-dependent mechanisms. Mol. Pharmacology. 2006;70:1246–1254. doi: 10.1124/mol.106.025478. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- de Launay D, van de Sande MG, de Hair MJ, Grabiec AM, van de Sande GP, Lehmann KA, Wijbrandts CA, et al. Selective involvement of ERK and JNK mitogen-activated protein kinases in early rheumatoid arthritis (1987 ACR criteria compared to 2010 ACR/EULAR criteria): a prospective study aimed at identification of diagnostic and prognostic biomarkers as well as therapeutic targets. Annals Rheumatic Dis. 2012;71:415–423. doi: 10.1136/ard.2010.143529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doya H, Ohtori S, Fujitani M, Saito T, Hata K, Ino H, Takahashi K, et al. c-Jun N-terminal kinase activation in dorsal root ganglion contributes to pain hypersensitivity. Biochem. Biophys. Res. Com. 2005;335:132–138. doi: 10.1016/j.bbrc.2005.07.055. [DOI] [PubMed] [Google Scholar]
- Driscoll A, Teichman JM. How do patients with interstitial cystitis present? J. Urol. 2001;166:2118–2120. [PubMed] [Google Scholar]
- Gao YJ, Xu ZZ, Liu YC, Wen YR, Decosterd I, Ji RR. The c-Jun N-terminal kinase 1 (JNK1) in spinal astrocytes is required for the maintenance of bilateral mechanical allodynia under a persistent inflammatory pain condition. Pain. 2010;148:309–319. doi: 10.1016/j.pain.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez EJ, Girard BM, Vizzard MA. Expression and function of transforming growth factor-beta isoforms and cognate receptors in the rat urinary bladder following cyclophosphamide-induced cystitis. Am. J. Physiol. Renal Physiol. 2013;305:F1265–1276. doi: 10.1152/ajprenal.00042.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanno PM, Sant GR. Clinical highlights of the National Institute of Diabetes and Digestive and Kidney Diseases/Interstitial Cystitis Association scientific conference on interstitial cystitis. Urology. 2001;57:2–6. doi: 10.1016/s0090-4295(01)01112-8. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Kiritoshi T, Murase K. Contribution of microglia and astrocytes to the central sensitization, inflammatory and neuropathic pain in the juvenile rat. Mol. Pain. 2012;8:43. doi: 10.1186/1744-8069-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Choi DC, Oh TH, Yune TY. Analgesic effect of acupuncture is mediated via inhibition of JNK activation in astrocytes after spinal cord injury. PloS one. 2013;8:e73948. doi: 10.1371/journal.pone.0073948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Yuan X, Wang S, Zhang F, Han Y, Ning Q, Luo G, et al. Mitogen-activated protein kinase pathways are involved in the upregulation of calcitonin gene-related peptide of rat trigeminal ganglion after organ culture. J. Mol. Neurosci. 2012;48:53–65. doi: 10.1007/s12031-012-9772-y. [DOI] [PubMed] [Google Scholar]
- Markevich NI, Hoek JB, Kholodenko BN. Signaling switches and bistability arising from multisite phosphorylation in protein kinase cascades. J. Cell Biol. 2004;164:353–359. doi: 10.1083/jcb.200308060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehan S, Meena H, Sharma D, Sankhla R. JNK: a stress-activated protein kinase therapeutic strategies and involvement in Alzheimer's and various neurodegenerative abnormalities. J. Mol. Neurosci. 2011;43:376–390. doi: 10.1007/s12031-010-9454-6. [DOI] [PubMed] [Google Scholar]
- Mercer BA, D'Armiento JM. Emerging role of MAP kinase pathways as therapeutic targets in COPD. Intl. J. Chronic Obstructive Pulmonary Dis. 2006;1:137–150. doi: 10.2147/copd.2006.1.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill L, Girard B, Arms L, Guertin P, Vizzard MA. Neuropeptide/Receptor expression and plasticity in micturition pathways. Current Pharmaceutical Design. 2013a;19:4411–4422. doi: 10.2174/1381612811319240008. [DOI] [PubMed] [Google Scholar]
- Merrill L, Malley S, Vizzard MA. Repeated variate stress in male rats induces increased voiding frequency, somatic sensitivity, and urinary bladder nerve growth factor expression. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2013b;305:R147–156. doi: 10.1152/ajpregu.00089.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, et al. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J. Neurosci. 2004;24:10211–10222. doi: 10.1523/JNEUROSCI.3388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peti W, Page R. Molecular basis of MAP kinase regulation. Protein Sci. 2013;22:1698–1710. doi: 10.1002/pro.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillin RB, Erickson DR. Management of interstitial cystitis/bladder pain syndrome: a urology perspective. Urol. Clin. N. Am. 2012;39:389–396. doi: 10.1016/j.ucl.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Sant GR, Hanno PM. Interstitial cystitis: current issues and controversies in diagnosis. Urology. 2001;57:82–88. doi: 10.1016/s0090-4295(01)01131-1. [DOI] [PubMed] [Google Scholar]
- Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, et al. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2010;298:R534–547. doi: 10.1152/ajpregu.00367.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp. Neurol. 2000;161:273–284. doi: 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J. Chem. Neuroanatomy. 2001;21:125–138. doi: 10.1016/s0891-0618(00)00115-0. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang C, Sheng X, Zhang X, Wang B, Zhang G. Peripheral expression of MAPK pathways in Alzheimer's and Parkinson's diseases. J Clin Neurosci. 2014 doi: 10.1016/j.jocn.2013.08.017. in press. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, Decosterd I, et al. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J. Neurosci. 2006;26:3551–3560. doi: 10.1523/JNEUROSCI.5290-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]