Abstract
Turritopsis nutricula (T. nutricula) is the one of the known reported organisms that can revert its life cycle to the polyp stage even after becoming sexually mature, defining itself as the only immortal organism in the animal kingdom. Therefore, the animal is having prime importance in basic biological, aging, and biomedical researches. However, till date, the genome of this organism has not been sequenced and even there is no molecular phylogenetic study to reveal its close relatives. Here, using phylogenetic analysis based on available 16s rRNA gene and protein sequences of Cytochrome oxidase subunit-I (COI or COX1) of T. nutricula, we have predicted the closest relatives of the organism. While we found Nemopsis bachei could be closest organism based on COX1 gene sequence; T. dohrnii may be designated as the closest taxon to T. nutricula based on rRNA. Moreover, we have figured out four species that showed similar root distance based on COX1 protein sequence.
Keywords: Turritopsis nutricula, immortal jellyfish, trans-differentiation, phylogeny, relativeness,
Background
Gerontologists and biologists reached a consensus “evolutionary theory of aging,” [1, 2] embedding aging research into the mainstream of biological research. T. nutricula is the one of the known hydrozoan in the animal kingdom that can revert back into the immature polyp stage after reaching sexual maturity, designating itself as the only immortal animal [3]. T. nutricula interplay with the polyp and sexual maturity stages by virtue of trans-differentiation process [4]. Theoretically, this process can go on indefinitely therefore, the organism can be considered as biologically immortal and does not experience aging. Hence, in the basic biology of aging research, the organism has found itself great importance [5]. If a cell or organism undergoes aging, there are two vital biological processes viz. negative regulation of cell division or proliferation and /or positive regulation of apoptosis may occur [6]. Furthermore, aging and neurodegeneration diseases are directly associated with these two biological processes [7]. While in cancer, aging does not happen and these two biological processes are inversely associated [8]. Thus, the organism can be a great tool in cancer, aging, and neurodegenerative related disorders. Although the transdifferentiation mechanism has been documented at physiological and cellular level [3, 4]; the molecular mechanism behind the T. Nutricula's trans-differentiation is not yet elucidated.
Although 16S rRNA based phylogenetic analysis is well established, COX 1 based analysis is not much reported. COX1 is one of the most conserved genes in almost all eukaryotes. It belong to the cytochrome oxidase subunit I protein family, which also includes mitochondrial encoded COX2 and COX3 those combine to form respiratory complex IV, a final enzyme of electron transport chain in mitochondrial oxidative phosphorylation [9]. COX1 exhibits the characteristics of a “housekeeping” gene and is constitutively expressed in almost all tissues. COX1 appears to be responsible for the production of prostaglandins [10]. Studies suggest that COX1 has its own importance in phylogenetic analysis. Its sequence is conserved among conspecifics and addition to this its mutation rate is also rapid enough to distinguish closely related species [11]. Additionally, in phylogenetic study, invasion patterns can be inferred from COX1 sequences [12]. Phylogenetic study on T. nutricula is not so far reported. Since the genome sequences or gene or protein expression profile or any kind of molecular data of T. nutricula is not available, we focused to identify its close relatives that may have similar physiology of immortality and may have molecular data so that, the organisms can be used to carry out aging and disease research. With these aims, at the very first step, here we tried to identify close relatives of T. nutricula using its only available Cytochrome Oxidase Subunit I (COI or COX1) and 16S rRNA sequences and bioinformatics based robust phylogenetic analysis.
Methodology
Gene and protein sequences:
All-over, only six nucleotide (nt) sequences (COI/COX1 from four different isolates and 16S rRNA form two different isolates) of T. nutricula are available in NCBI database. All the 4 isolates of COI/COX1 and 2 16s rRNA partial sequences have shown similar sequence comparatively. We used all the sequences for our phylogenetic analysis. However, COI/COX1 nt sequence of 621 bp from the isolate-1 (GenBank: JQ716082.1) and partial 16s rRNA sequence of the isolate WHO_1 (GenBank: EU624348.1) based analysis are represented here. In UniProt, only two COX1 protein sequences are available and both have similar homology. The COI/COX1 protein (UniProt: K4P7S9) of 207 amino acid (aa) based phylogenetic analysis is represented here.
Phylogenetic analysis:
We have started our analysis to identify the sequence homologs using NCBI BLASTn to identify nt sequence homologs and BLASTp to identify protein or aa based homologs with cut off values 97% identity (for aa) and 84% (for nt). Based on these cut off values, the species specific homologs were selected for multiple sequence alignment. Clustal Omega and ClustalW (http://www.clustal.org) were used for multiple sequence alignment. Molecular Evolutionary Genetic Analysis tool (MEGA) version 5.1 [13] was used for the construction of phylogeny. In MEGA 5.1, Neighbourhood joining method [14] was used for construction of nucleotide based phylogenetic trees whereas Poisson model [15] was used to build protein based phylogeny to estimate the number of amino acid replacements when species are closely related. Pairwise distances are calculated with scope as pairs of taxa using Maximum Composite likelihood method [16]. Rates among the sites were considered uniform, pattern of lineages were regarded as homogeneous, and gaps or missing data were treated as deletions.
Results
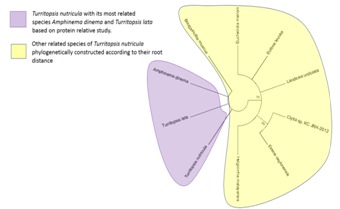
Homologous sequence similarity search provided number of related species from which best homologous sequences were selected according to the cut-off values Table 1 (see supplementary material). Nemopsis bachei is found to be closest relative of T. nutricula in COI/COX1 gene based study that exhibits the shortest root distance of 0.174 (Figure 1 & Figure 2) (Table 1)with a bootstrapping percentage of 88. The 16S rRNA based analysis Table 2 (see supplementary material) revealed that the immortal jellyfish is genetically close to T. dohrnii [17, 18] (Root distance: 0.034) followed by T. rubra [17, 18] (Root distance: 0.099) (Figure 3 & Figure 4) (Table 1)with the bootstrapping percentages of 100 and 99.In contrast to the nucleotide or gene based analysis, the COI/COX1 protein based analysis Table 3 (see supplementary material) showed some different findings. The result demonstrates that T. lata, Amphinema dinema, Eucheilota menoni, and Eutima levuka have similar root distance of 0.029 (Figure 5 & Figure 6) (Table 1). These species have shown a low bootstrapping percentage of 70 when compared to the gene and 16s rRNA bootstrapping results.
Figure 1.
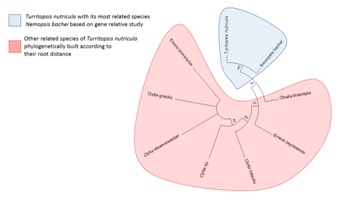
Phylogram representing Turritopsis nutricula and its COX1 nt sequence based relatives according to specific root distance. Turritopsis nutricula and Nemopsis bachei are emerging from the same node.
Figure 2.
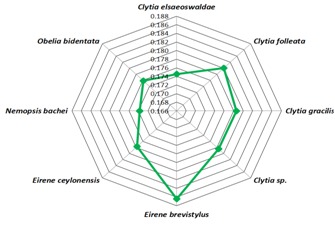
Web Graphical view of root distances of relative's species verses Turritopsis nutricula. Root distance of Nemopsis bacheis showing low root distance to Turritopsis nutricula than other relatives.
Figure 3.
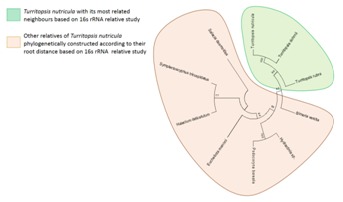
Phylogram representing Turritopsis nutricula and its 16s rRNA based relatives according to root distance. In this Phylogram Turritopsis dohrnii and Turritopsis nutricula are shown to be emerging together.
Figure 4.
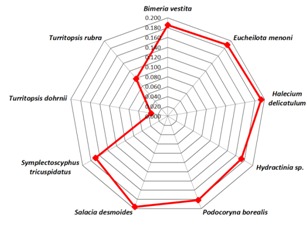
Web Graphical view of root distances showing relative species verses Turritopsis nutricula. Centre point of the web refers to Turritopsis nutricula. The distance of Turritopsisdohrnii followed by Turritopsis rubra are nearer to Turritopsis nutricula according to their root distances based on 16s rRNA relative study.
Figure 5.
Phylogram representing Turritopsis nutricula and its COX1 amino acid based relatives according to specific root distance.
Figure 6.
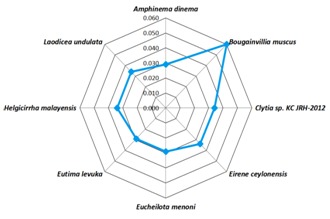
Web Graphical view of root distances of relative's species verses Turritopsis nutricula. Except Bougainvillia muscus almost all species are have shown similar root distance of ~0.030 towards Turritopsis nutricula.
Discussion
Phylogenetic analysis is a powerful tool for precise taxonomic classification as well as predicting origin or relatives of a species [19]. Attempts have been made in systematic classification of the genus Turritopsis [20, 21]. However, the closest taxa based on these studies are not reported to have features of immortality similar to the T. nutricula. Since our aim is to identify closest organism of T. nutricula having possible immortality; we discussed the results focusing on this specific aspect. In T. nutricula trans-differentiation directly allows functionally differentiated cells to switch to entirely new functions. Theoretically, this process can go on indefinitely therefore, the organism can be considered as biologically immortal and does not experience aging [8]. Based on the wellestablished 16S rRNA phylogeny, our predicted close relatives of T. nutricula are T. dohrniiand T. rubra where T. dohrnii is reported to undergo reverse development [17, 18]. COX1 is recently used for phylogenetic analysis [11, 12]. While considering the association of COX with aging and immortality, recent study on C. elegans illustrates that two COX assembly factors are involved in energy metabolism, reproduction, and aging [22]. Genomic level studies on Podospora anserina support the idea that, immortality can be acquired by the lack of active cytochrome c oxidase [23]. In Drosophila, COX is down regulated during aging [24] and in male wistar rats an inter-link between muscle-fiber trans-differentiation and mitochondrial respiratory chain was observed [25]. Therefore, based on these aging and trans-differentiation association of Cytochrome C Oxidase, we used T. nutricula available COI/COX1 gene and protein sequences for this phylogenetic study. Based on the COI/COX1 gene sequence, our predicted closest taxa of T. nutricula is Nemopsis bachei. However, we have observed some deviation in COX1 amino acid based analysis. Most of the predicted relatives are from different genus except T. lata; although few of these genus are jellyfish and as per our analysis they are close relatives. As per out limited knowledge, the life cycle of these identified possible close relatives of T. nutricula are not studied in depth. Therefore, we are not sure if these animals are having the features of immortality like the T. nutricula. Since our prediction demonstrates close relativeness of these animals with T. nutricula, they might have immortal features and hence the biology of these animals may be explored to find possible identification of new members of immortal animal.
Conclusion
Predicted seven species to be closely related to immortal jellyfish T. nutricula based on COX1 and 16S rRNA sequences. Among these seven, three (T. dohrnii, T. rubraand T. lata) belongs to same genus Turritopsis, two (Nemopsis bachei and Amphinema dinema) belongs to same suborder Folifera, while the rest two (Eucheilota menoni and Eutima levuka) belongs to same order Leptothecata. Precisely, these all species including T. nutricula are grouped under a common order Leptothecata [26]. Limited studies have been done on those animals and none of these organisms is so far reported to be immortal similar to T. nutricula. There is high probability that these animals may have features of immortality. Detail physiology and molecular studies of these animals along with T. nutricula will shed light on aging biology and therefore the knowledge may be translated and would be applied to biomedical sciences especially in proliferative, degenerative, and aging related disorders.
Conflict of Interest
The authors are no conflict of interest of this article
Supplementary material
Acknowledgments
The authors thank DST-SERB for financial support in young scientist award scheme and Vasco Azevedo is sponsored by CNPq and UFMG
Footnotes
Citation:Devarapalli et al, Bioinformation 10(9): 586-591 (2014)
References
- 1.Williams GC. Evolution. 1957;11:398. [Google Scholar]
- 2.Kirkwood TBL, et al. Cell. 2005;120:437. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Piraino S, et al. Biol Bull. 1996;190:302. doi: 10.2307/1543022. [DOI] [PubMed] [Google Scholar]
- 4.Carlà EC, et al. Tissue Cell. 2003;35:213. doi: 10.1016/s0040-8166(03)00028-4. [DOI] [PubMed] [Google Scholar]
- 5.Rochelle B, et al. Humana Press. 2008;499:506. [Google Scholar]
- 6.Cristofalo VJ, et al. SurgClin North Am. 1994;74:1. doi: 10.1016/s0039-6109(16)46225-0. [DOI] [PubMed] [Google Scholar]
- 7.Hung CW, et al. Ageing Res Rev. 2010;9:S36. doi: 10.1016/j.arr.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Campisi J. Annu Rev Physiol. 2013;75:685. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keilin D, Hartree EF. Biological Sciences. 1939;127:167. [Google Scholar]
- 10.Crofford LJ. The Journal of rheumatology. 1997;49:15. [PubMed] [Google Scholar]
- 11.Hebert PD, et al. Biological Sciences. 2003;270:S96. doi: 10.1098/rsbl.2003.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackie JA, et al. Marine Biology. 2006;149:285. [Google Scholar]
- 13.Tamura K, et al. Mol Biol Evol. 2011;28:2731. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones DT, et al. Comput Appl Biosci. 1992;8:275. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 15.Bishop MJ, et al. Biol Sci. 1985;226:271. [Google Scholar]
- 16.Tamura K, et al. Proc Natl Acad Sci USA. 2004;101:11030. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. http://www.vliz.be/en/ims?refid=208159.
- 18.Schmich J, et al. Int J Dev Biol. 2007;51:45. doi: 10.1387/ijdb.062152js. [DOI] [PubMed] [Google Scholar]
- 19.Cavalli-Sforza LL, Edwards AW. Am J Hum Genet. 1967;19:233. [PMC free article] [PubMed] [Google Scholar]
- 20.Miglietta MP, et al. J ZoolSystEvol Res. 45:11. [Google Scholar]
- 21.Miglietta MP, Lessios HA. Biol Invasions. 2009;11:825. [Google Scholar]
- 22.Maxwell S, et al. Longevity & Healthspan. 2013;2:9. doi: 10.1186/2046-2395-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begel O, et al. Mol Cell Biology. 1999;19:4093. doi: 10.1128/mcb.19.6.4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calleja M, et al. J Biol Chem. 1993;268:18891. [PubMed] [Google Scholar]
- 25.Venhoff N, et al. Arthritis Research Ther. 2012;14:R233. doi: 10.1186/ar4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. http://www.marinespecies.org at VLIZ. Accessed 2013-11- 28.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


