Abstract
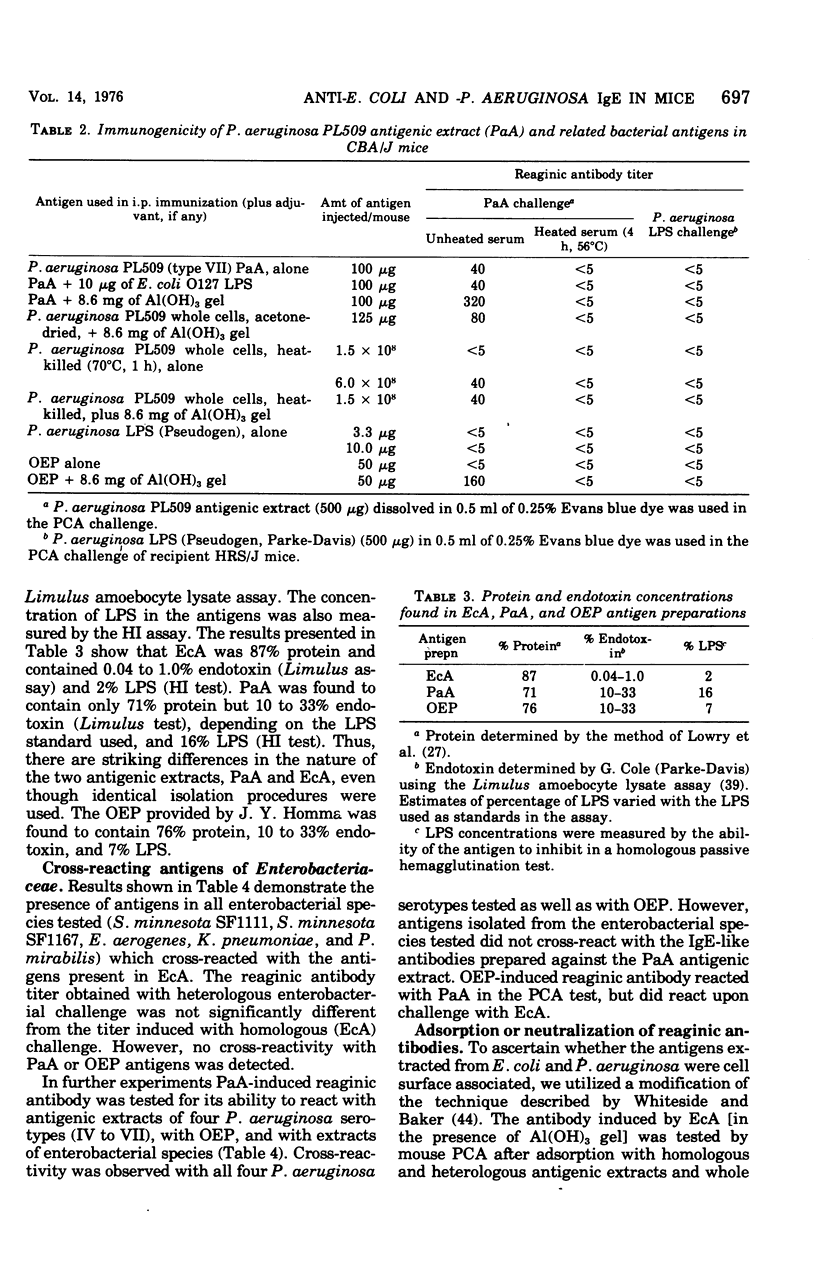
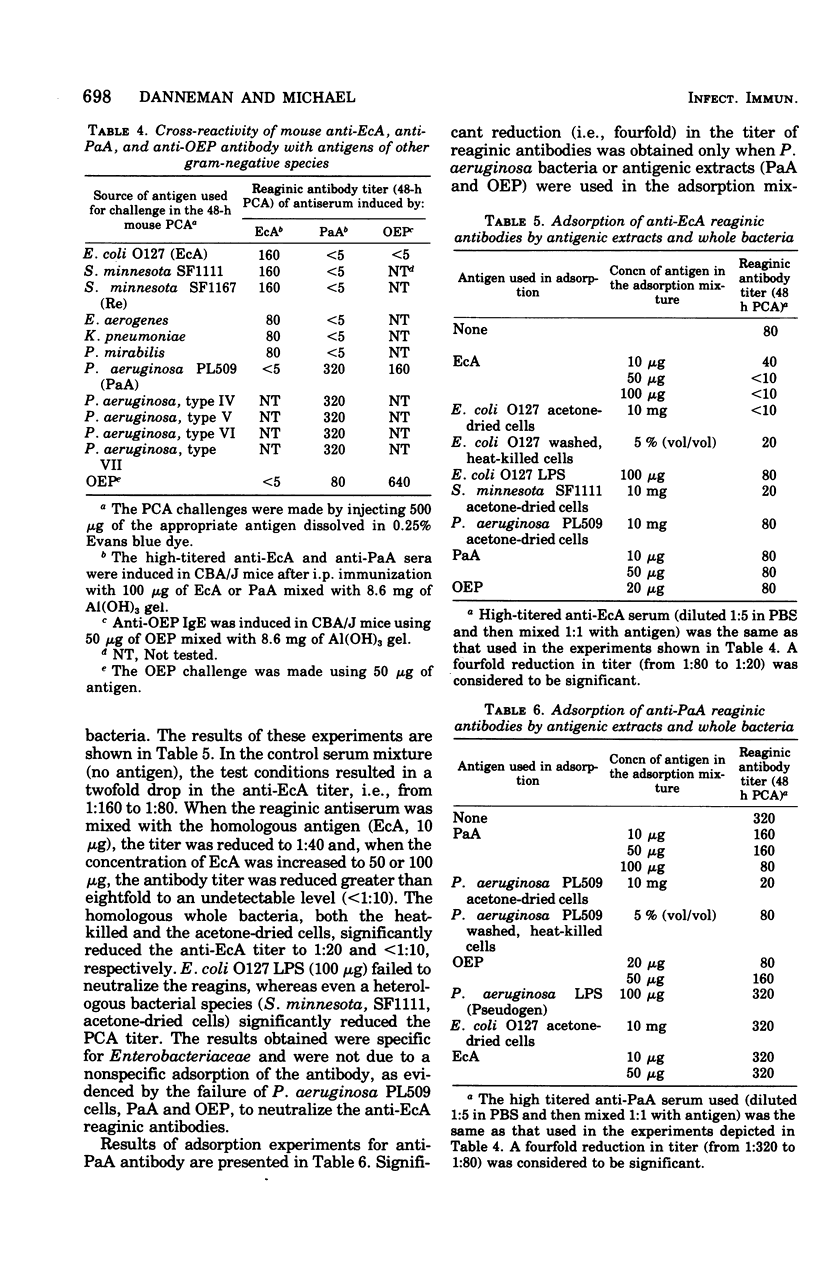
Water-soluble antigens isolated from acetone-dried, gram-negative bacteria elicited reaginic antibody formation in mice. Antibodies specific for Escherichia coli antigens reacted with antigens isolated from several enterobacterial species tested, but not with antigens isolated from Pseudomonas aeruginosa. Reaginic antibodies induced by antigens isolated from a P. aeruginosa strain reacted with antigens isolated from several P.aeruginosa serotypes as well as with a purified protein component of the envelope of P. aeruginosa. The anti-Pseudomonas reagins did not cross-react with enterobacterial antigens. Antigenicity of the bacterial extracts was destroyed by trypsin treatment and reduced by heating, which suggested that the antigens were protein in nature. Whole bacterial cells adsorbed out reaginic antibodies, indicating that the antigens are located at or near the surface of the bacteria.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe C., Shionoya H., Hirao Y., Okada K., Homma J. Y. Common protective antigen (OEP) of Pseudomonas aeruginosa. Jpn J Exp Med. 1975 Oct;45(5):355–359. [PubMed] [Google Scholar]
- Andersson J., Melchers F., Galanos C., Lüderitz O. The mitogenic effect of lipopolysaccharide on bone marrow-derived mouse lymphocytes. Lipid A as the mitogenic part of the molecule. J Exp Med. 1973 Apr 1;137(4):943–953. doi: 10.1084/jem.137.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmal A. C., Leary W. P. A study of the effects of 780SE on carbohydrate tolerance and plasma hormone and lipid profile in maturity-onset diabetes. Postgrad Med J. 1975;51 (Suppl 1):144–148. [PMC free article] [PubMed] [Google Scholar]
- Barber C., Eylan E., Keydar J. Sur les déterminants communs des Enterobacteriaceae. Pathol Microbiol (Basel) 1968;31(6):321–327. [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Danneman P. J., Michael J. G. Adjuvant and immunogenic properties of bacterial lipopolysaccharide in IgE and IgG antibody formation in mice. Cell Immunol. 1976 Mar 1;22(1):128–139. doi: 10.1016/0008-8749(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Deuschl H., Johansson S. G. Immunoglobulins in tracheo-bronchial secretion with special reference to IgE. Clin Exp Immunol. 1974 Mar;16(3):401–412. [PMC free article] [PubMed] [Google Scholar]
- Domingue G., Salhi A., Rountree C., Little W. Prevention of experimental hematogenous and retrograde pyelonephritis by antibodies against enterobacterial common antigen. Infect Immun. 1970 Aug;2(2):175–182. doi: 10.1128/iai.2.2.175-182.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald G. W., Clyde W. A., Jr, Bienenstock J. Immunoglobulin-containing cells in lungs of hamsters infected with Mycoplasma pneumoniae. J Immunol. 1972 May;108(5):1400–1408. [PubMed] [Google Scholar]
- Gordon D. S., Hunter R. G., O'Reilly R. J., Conway B. P. Pseudomonas aeruginosa allergy and humoral antibody-mediated hypersensitivity pneumonia. Am Rev Respir Dis. 1973 Jul;108(1):127–131. doi: 10.1164/arrd.1973.108.1.127. [DOI] [PubMed] [Google Scholar]
- Gorzynski E. A., Ambrus J. L., Neter E. Effect of common enterobacterial antiserum on experimental Salmonella typhimurium infection of mice. Proc Soc Exp Biol Med. 1971 Sep;137(4):1209–1212. doi: 10.3181/00379727-137-35757. [DOI] [PubMed] [Google Scholar]
- Halegoua S., Hirashima A., Inouye M. Existence of a free form of a specific membrane lipoprotein in gram-negative bacteria. J Bacteriol. 1974 Dec;120(3):1204–1208. doi: 10.1128/jb.120.3.1204-1208.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. C., Weiss E. Protein fraction with immunogenic potential and low toxicity isolated from the cell wall of Neisseria meningitidis group B. Infect Immun. 1974 Sep;10(3):605–615. doi: 10.1128/iai.10.3.605-615.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma J. Y., Suzuki N. The protein moiety of the endotoxin of Pseudomonas aeruginosa. Ann N Y Acad Sci. 1966 Jun 30;133(2):508–526. doi: 10.1111/j.1749-6632.1966.tb52386.x. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Newcomb R. W. Presence of gammaE in nasal washings and sputum from asthmatic patients. J Allergy. 1970 Oct;46(4):197–204. doi: 10.1016/0021-8707(70)90023-7. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Martin A., Kenny C. P., Diena B. B. Cross-protective antigens of Neisseria meningitidis obtained from Slaterus group Y. Infect Immun. 1972 Apr;5(4):547–551. doi: 10.1128/iai.5.4.547-551.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns M. A., Whiteside R. E., Baker E. E., McCabe W. R. Common enterobacterial antigen. I. Isolation and purification from Salmonella typhose 0:901. J Immunol. 1973 Mar;110(3):781–790. [PubMed] [Google Scholar]
- Kaijser B. Immunological studies of an antigen common to many gram-negative bacteria with special reference to E. coli. Characterization and biological significance. Int Arch Allergy Appl Immunol. 1975;48(1):72–81. doi: 10.1159/000231293. [DOI] [PubMed] [Google Scholar]
- Kaltreider H. B., Salmon S. E. Immunology of the lower respiratory tract. Functional properties of bronchoalveolar lymphocytes obtained from the normal canine lung. J Clin Invest. 1973 Sep;52(9):2211–2217. doi: 10.1172/JCI107406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Takahashi T., Goto S., Maeda K., Kuwabara G., Uehara T., Shibuya T. Role of infection in bronchial asthma. J Asthma Res. 1972 Dec;10(2):87–107. doi: 10.3109/02770907209108770. [DOI] [PubMed] [Google Scholar]
- LANDY M., JOHNSON A. G., WEBSTER M. E., SAGIN J. F. Studies on the O antigen of Salmonella typhosa. II. Immunological properties of the purified antigen. J Immunol. 1955 Jun;74(6):466–478. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine B. B., Vaz N. M. Effect of combinations of inbred strain, antigen, and antigen dose on immune responsiveness and reagin production in the mouse. A potential mouse model for immune aspects of human atopic allergy. Int Arch Allergy Appl Immunol. 1970;39(2-3):156–171. doi: 10.1159/000230343. [DOI] [PubMed] [Google Scholar]
- McCabe W. R., Greely A. Common enterobacterial antigen. II. Effect of immunization on challenge with heterologous bacilli. Infect Immun. 1973 Mar;7(3):386–392. doi: 10.1128/iai.7.3.386-392.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe W. R. Immunization with R mutants of S. Minnesota. I. Protection against challenge with heterologous gram-negative bacilli. J Immunol. 1972 Mar;108(3):601–610. [PubMed] [Google Scholar]
- Reynolds H. Y., Thompson R. E., Devlin H. B. Development of cellular and humoral immunity in the respiratory tract of rabbits to Pseudomonas lipopolysaccharide. J Clin Invest. 1974 May;53(5):1351–1358. doi: 10.1172/JCI107683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZUKI T., WHANG H. Y., GORZYNSKI E. A., NETER E. INHIBITION BY LIPOPOLYSACCHARIDE (ENDOTOXIN) OF ANTIBODY RESPONSE OF RABBIT TO COMMON ANTIGEN OF ENTEROBACTERIACEAE. Proc Soc Exp Biol Med. 1964 Dec;117:785–789. doi: 10.3181/00379727-117-29698. [DOI] [PubMed] [Google Scholar]
- Schwartz H. A., Levine B. B. The molecular classes of two homocytotropic antibodies in the mouse. J Immunol. 1973 Jun;110(6):1638–1641. [PubMed] [Google Scholar]
- Smith J., Holmgren J., Ahlstedt S., Hanson L. A. Local antibody production in experimental pyelonephritis: amount, avidity, and immunoglobulin class. Infect Immun. 1974 Sep;10(3):411–415. doi: 10.1128/iai.10.3.411-415.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Ishizaka K. Distribution of gamma E-forming cells in lymphoid tissues of the human and monkey. J Immunol. 1970 Feb;104(2):377–387. [PubMed] [Google Scholar]
- Turner M. W., Johansson S. G., Barratt T. M., Bennich H. Studies on the levels of immunoglobulins in normal human urine with particular reference to IgE. Int Arch Allergy Appl Immunol. 1970;37(4):409–417. doi: 10.1159/000230803. [DOI] [PubMed] [Google Scholar]
- WHITESIDE R. E., BAKER E. E. Antigenic analysis of Salmonella typhosa and related salmonellas. J Immunol. 1962 May;88:650–660. [PubMed] [Google Scholar]
- Waldman R. H., Henney C. S. Cell-mediated immunity and antibody responses in the respiratory tract after local and systemic immunization. J Exp Med. 1971 Aug 1;134(2):482–494. doi: 10.1084/jem.134.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman R. H., Virchow C., Rowe D. S. IgE levels in external secretions. Int Arch Allergy Appl Immunol. 1973;44(2):242–248. doi: 10.1159/000230933. [DOI] [PubMed] [Google Scholar]
- Wesley J., Fisher A., Fisher M. W. Immunization against Pseudomonas in infection after thermal injury. J Infect Dis. 1974 Nov;130 (Suppl)(0):S152–S158. doi: 10.1093/infdis/130.supplement.s152. [DOI] [PubMed] [Google Scholar]
- Whang H. Y., Mayer H., Schmidt G., Neter E. Immunogenicity of the common enterobacterial antigen produced by smooth and rough strains. Infect Immun. 1972 Oct;6(4):533–539. doi: 10.1128/iai.6.4.533-539.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin E. T., Galanos C., Kinsky S., Bradshaw R. A., Wessler S., Lüderitz O., Sarmiento M. E. Picogram-sensitive assay for endotoxin: gelation of Limulus polyphemus blood cell lysate induced by purified lipopolysaccharides and lipid A from Gram-negative bacteria. Biochim Biophys Acta. 1972 Jan 28;261(1):284–289. doi: 10.1016/0304-4165(72)90340-6. [DOI] [PubMed] [Google Scholar]


