Abstract
The contents of working memory (WM) steer visual attention, but the extent of this guidance can be strategically enhanced or inhibited when WM content is reliably helpful or harmful to a visual task. Current understanding of the neural substrates mediating the cognitive control over WM biases is limited, however, by the correlational nature of functional MRI approaches. A recent fMRI study provided suggestive evidence for a functional lateralization of these control processes in posterior parietal cortex (PPC): activity in left PPC correlated with the presentation of WM cues that ought to be strategically enhanced to optimize performance, while activity in the right PPC correlated with the presentation of cues that ought to be inhibited to prevent detrimental attentional biases in a visual search. Here, we aimed to directly assess whether the left and right PPC are causally involved in the cognitive control of WM biases, and to clarify their precise functional contributions. We therefore applied 1Hz repetitive transcranial magnetic stimulation (rTMS) to left and right PPC (and a vertex control site) prior to administering a behavioral task assessing WM biasing control functions. We observed that the perturbation of left PPC eliminated the strategic benefit of predictably helpful WM cueing, while the perturbation of right PPC amplified the cost of unpredictable detrimental WM cueing. Left and right PPC thus play distinct causal roles in WM-attention interactions: left PPC to maximize benefits, and right PPC to minimize costs, of internally maintained content on visual attention.
Keywords: working memory, cognitive control, visual attention, parietal cortex
Representations in working memory can influence what we attend in the environment. This can be beneficial when those representations align with current goals (e.g., rehearsing your shopping list in the store helps you locate the apples) but detrimental when WM contents conflict with goals (e.g., rehearsing your shopping list in the car directs your eyes to a fruit stand, instead of the road). Accordingly, prior studies have found that items in WM can guide attention toward matching—but goal-irrelevant—items in a perceptual task (Soto, Hodsoll, Rotshtein, & Humphreys, 2008). When a visual search is performed during a WM delay, for instance, performance profits if a memory-matching item cues a search target (valid WM-benefit), and is impaired if a memory-matching item cues a distractor (invalid WM-cost). When the proportions of valid and invalid trials are manipulated, however, a high proportion of valid trials amplifies the WM-benefit, while a high proportion of invalid trials reduces the WM-cost (Carlisle & Woodman, 2011; Kiyonaga, Egner, & Soto, 2012). People, thus, use foreknowledge about WM validity to control the strength of WM biases of attention. This adaptive ability would be critical to optimizing the use of material that is currently on one’s mind, so that it can be exploited when it is beneficial to immediate demands in the environment, but kept in check when it is counter-productive.
How does the human brain implement this cognitive control over the linkage between WM and attention? We recently manipulated the predictability (i.e., proportion) of valid and invalid WM cues, during fMRI, to examine WM delay control signals involved in enhancing or inhibiting the influence of WM content in a visual search (Soto, Greene, Kiyonaga, Rosenthal, & Egner, 2012). Predictably helpful (i.e., valid) WM cues activated the left posterior parietal cortex (PPC), while predictably distracting (i.e., invalid) cues engaged the right PPC (both relative to a non-predictive baseline, Figure 1C). These findings suggest a neural dissociation of cognitive control processes associated with enhancement and inhibition of WM biases, giving rise to the intriguing hypothesis of a lateralization in human PPC according to the manner in which WM content is strategically employed to control attention.
Figure 1.
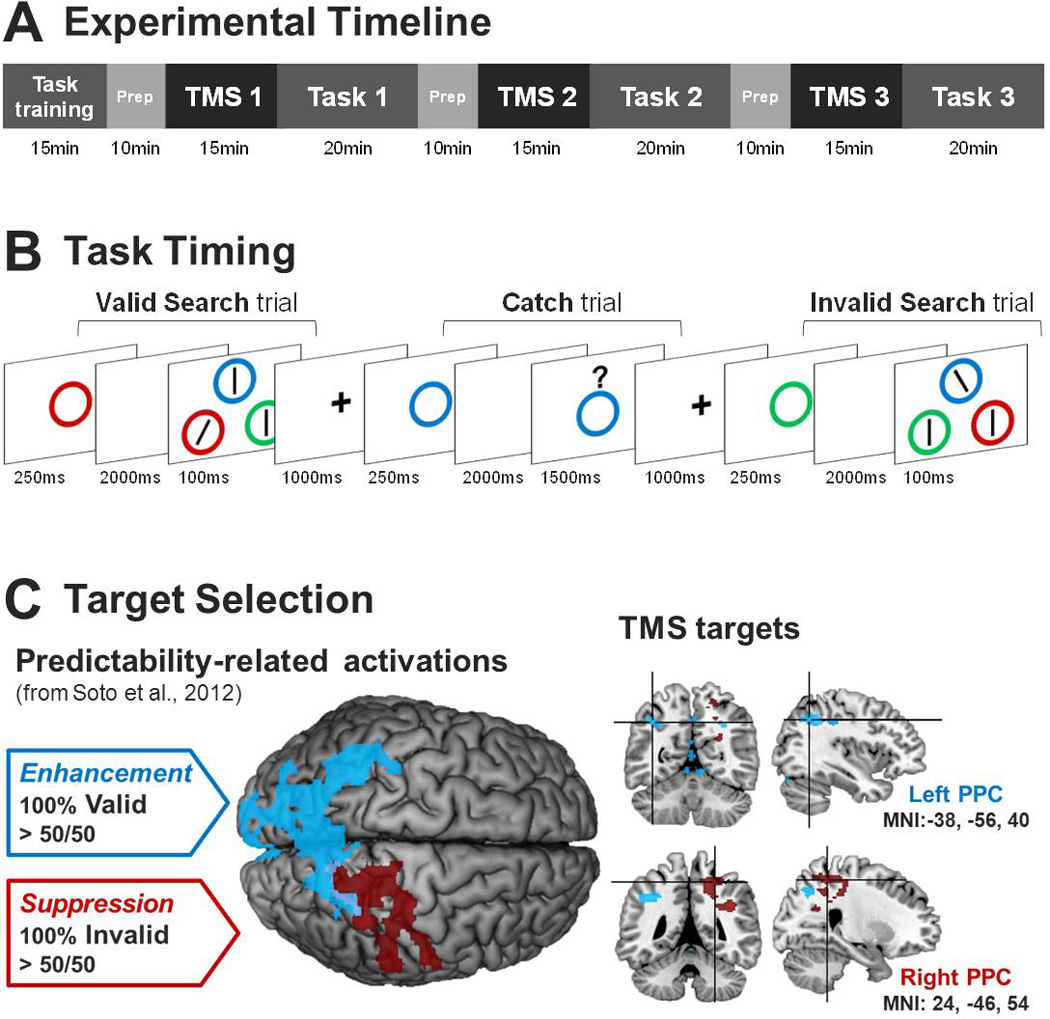
Experimental Methods. A) Before each task run, 1Hz rTMS was delivered to one of three targets locations—vertex, left PPC, or right PPC—in counterbalanced order. B) After each colored circle memory cue, participants saw either a visual search or a memory probe (i.e., catch trial). Blocks of trials were either 100% valid, 100% invalid, or 50% valid/50% invalid. C) Stimulation sites based on prior fMRI findings.
The PPC is a heterogenous region that has been implicated in the goal-oriented, flexible control of behavior to meet a variety of cognitive demands (e.g., Duncan, 2010). While there is some support for separable roles of left and right PPC in such functions as bottom-up attentional orienting either toward or away from salient stimuli (Mevorach, Humphreys, & Shalev, 2006), the evidence for hemispheric specialization of attentional processes has been mixed. Other studies have suggested, for instance, greater relative involvement of the left PPC in oculomotor attention, and right PPC in attentional orienting (Rushworth, Ellison, & Walsh, 2001), though not necessarily qualitatively distinct contributions from that of the contralateral hemisphere. Here we perturb normal brain activity, using repetitive transcranial magnetic stimulation (rTMS), to determine the specific and causal roles of left and right PPC in controlling the relationship between internal WM content and externally-geared visual attention.
We applied 1Hz rTMS to left and right PPC guided by activation peaks from the prior fMRI study (Soto et al., 2012). This rTMS protocol is generally found to temporarily reduce task-related cortical excitability in the targeted region (Pascual-Leone et al., 1998), and can have the behavioral consequence of hampering performance of tasks that rely on that region (Walsh & Cowey, 2000). Participants completed a combined WM cueing/visual search task—wherein we independently manipulated WM validity and predictability—after each stimulation session. If left and right PPC play causal and qualitatively dissociable roles in the cognitive control over WM biases of attention (i.e. enhancement and inhibition based on WM-validity foreknowledge), then left and right PPC rTMS ought to produce distinct modulation of visual search performance based on WM context.
Methods
Participants
Twenty naïve volunteers (10 female, mean age = 26, age range 21–43) gave written informed consent in accordance with the Duke University Health System Institutional Review Board, and were compensated with $20.00 per hour for their participation. Three participants were excluded—2 because of technical problems during their stimulation sessions, and 1 for chance performance on the behavioral task—leaving 17 participants in the final analysis.
Overview of Experiment
Participants were first trained on a composite WM-visual search task that we have previously used to examine cognitive control over WM biases of attention (Kiyonaga et al., 2012; Soto et al., 2012). Stimulation targets were based on group activation coordinates from Soto et al. (2012), which were mapped onto individual anatomical images. All participants underwent a 15 minute session of 1Hz rTMS for each of three target regions: left PPC, right PPC, and a vertex control site. The vertex was used to reproduce the sensations of real TMS and control for any non-specific effects of the stimulation (cf. Pitcher, Garrido, Walsh, & Duchaine, 2008). Order of stimulation was counterbalanced across participants, and each stimulation period was followed by a run of the behavioral task, which lasted between 15–20 minutes, resulting in approximately 30 minutes in between each round of stimulation (Figure 1A).
Stimuli and task procedure
Each trial began with a 1000msec fixation, followed by a 500msec blank screen, then a 250msec colored circle memory cue. After a delay of 2000msec, a visual search display appeared for 100msec (Figure 1B). We used this brief display duration to discourage eye movements. The search display comprised three colored circles at the corners of an imaginary triangle. Each circle contained a line, two of which were vertical while one—the target—was tilted 16° to the left or right. The participants’ task was to indicate the orientation of the tilted line during a time window of 1000msec. To prevent participants from strategically using the search array to ‘perceptually resample’ the memory item (cf. Woodman & Luck, 2007), while also ensuring that they followed instructions to maintain the WM cue, memory probes were given on 20% of trials instead of a search display. On these “catch trials”, a colored circle memory probe was presented underneath a question mark for 1500msec, and was to be judged as a match or non-match to the initial memory cue (Figure 1B). All search target locations, as well as match and non-match probes, occurred equally often and in randomized order. All memory cue, probe, and search display circles were selected at random from one of four colors (red, blue, green or yellow), and only one circle per color appeared in a given search display. Individual search trials could be valid (memory cue reappeared surrounding the target) or invalid (memory cue reappeared surrounding a distractor). Blocks of trials were either always valid (100% valid), always invalid (100% invalid), or equally likely to be valid or invalid (50/50), and the condition of the impending block was indicated on an instruction screen during the rest between blocks. Participants completed 10 practice trials of each block condition. The experimental runs following TMS consisted of 2 blocks each of 100% valid and 100% invalid conditions, and 4 blocks of the 50/50 condition, the order of which was counterbalanced over participants. Each block consisted of 25 trials, for a total of 200 trials in each behavioral run.
Transcranial Magnetic Stimulation (TMS)
In Soto et al. (2012), the contrast of cue-related activation between 100% valid and 50/50 conditions revealed a primarily left-lateralized PPC cluster, while the contrast between 100% invalid and 50/50 revealed a right-lateralized PPC cluster (Figure 1C). TMS targets were selected within each of these two clusters based on the criteria that they be (1) among the top five local maxima for that contrast, (2) of roughly equivalent depth from the cortical surface, and (3) non-overlapping with any region of activation from the alternate contrast. The left PPC target was located at MNI: −38, −56, 40 (corresponding to the posterior portion of the supramarginal gyrus), and the right PPC target at MNI: 24, −46, 54 (corresponding to the base of the superior parietal lobule; Figure 1C). Each individual’s vertex, determined as the point midway between the inion and the nasion (at x = 0), was used as a control site, against which to compare the effects of stimulation to the left and right PPC regions. The Brainsight 2 frameless stereotaxic neuronavigation system (Rogue Research, Montreal, Canada) was used to co-register a normalized MNI brain to each individual’s T1-weighted structural MRI, to localize the MNI coordinates of the TMS targets in each participant, and to monitor the proper coil position and orientation throughout each stimulation session. Participants were seated in a reclining chair outfitted with a supportive neck rest and adjustable arms to limit head motion and stabilize the stimulation coil (Rogue Research).
Fifteen minutes of 1Hz rTMS was delivered to each target using a Magstim Rapid2 stimulator with a Magstim Double 70mm Air Film Coil. Stimulation of each target site totaled 900 pulses delivered at a fixed intensity of 60% of maximum stimulator output. Whereas many previous studies have calibrated stimulation intensity based on individual motor thresholds, we decided against this method in light of evidence that motor threshold is not necessarily a reliable indicator of the excitability of other brain regions (McConnell et al., 2001; Stewart, Walsh, & Rothwell, 2001). In keeping with parameters used in many prior studies (Pitcher et al., 2008; Pitcher, Walsh, Yovel, & Duchaine, 2007; Silvanto, Cattaneo, Battelli, & Pascual-Leone, 2008), therefore, we reasoned that a fixed intensity of stimulation would be the most consistent approach. To limit the duration of the testing session and the total number of TMS pulses, motor threshold was not assessed.
The goal of using rTMS was to perturb ongoing processing in the stimulated region of cortex. TMS induces a depolarization of neurons that temporarily changes neural spiking activity, and this alteration is reflected in both the hemodynamic response and phase relationships between neural signals (Allen, Pasley, Duong, & Freeman, 2007). While there is some evidence that TMS may actually suppress neural signals (Harris, Clifford, & Miniussi, 2007), it is widely considered to add random neural activity, or “noise” to the stimulated area, which can either interfere with or facilitate performance depending on the activation state and functional role of the stimulated region (e.g., Miniussi, Ruzzoli, & Walsh, 2010; O’Shea & Walsh, 2007; Sandrini, Umiltà, & Rusconi, 2011). When using 1Hz (i.e., low frequency) rTMS, the typical outcome of this perturbation is reduced task-related cortical excitability at the stimulated site (e.g., Robertson, Théoret, & Pascual-Leone, 2003). This 1Hz rTMS protocol has been demonstrated to produce effects in the stimulated area that can be expected to endure, after stimulation has stopped, for at least half of the duration of the TMS train (Robertson et al., 2003) and sometimes up to twice as long (Walsh & Cowey, 2000).
Results
Analysis strategy
Based on our prior study (Soto et al., 2012) we hypothesized that, compared to the vertex TMS control, 1Hz rTMS to the left PPC target should interfere with the performance benefits from foreknowledge of valid WM cues and attenuate the valid enhancement effect (but leave the influence of predictably invalid cues intact), whereas rTMS to the right PPC target should interfere with the influence from foreknowledge of invalid cues and attenuate the invalid inhibition effect (but leave the performance benefits from predictably valid cues intact). We therefore assessed effects of left and right PPC TMS on enhancement and inhibition of WM biases from foreknowledge of cue validity, relative to vertex TMS.
Because RTs for the visual search and memory catch trials were non-normally distributed (Search: skewness = .6, std error = .023; Catch: skewness = .8, std. error = .023), we performed a square root transformation to achieve normality (Hildebrand, 1986). Raw mean of correct RT and accuracy for all conditions are reported in Table 1. The impact of TMS on visual search performance was first evaluated using a 3 × 2 × 2 repeated measures ANOVA with Stimulation Site (Vertex, Left PPC, Right PPC), Validity (Valid vs. Invalid), and Predictability (100% vs. 50/50) as within-subjects factors. Then separate 2 × 2 × 2 ANOVAs compared each experimental TMS target to the vertex control, to test our hypotheses about the unique contributions of each PPC region. As WM catch trials themselves can be categorized as neither valid nor invalid, the impact of TMS on catch trial performance was evaluated using separate 2 × 3 repeated measures ANOVAs with Stimulation Site (Vertex vs. Left or Right PPC) and Block Condition (100% valid, 100% invalid, 50/50) as within-subjects factors.
Table 1.
Means (and s.e.m) of raw RT and accuracy for all conditions
| TMS target |
Block Predictability |
Trial Validity |
Visual Search Trials | WM Catch Trials | ||
|---|---|---|---|---|---|---|
| Raw RT (msec) |
Accuracy (% correct) |
Raw RT (msec) |
Accuracy (% correct) |
|||
| Vertex Control |
100% | Valid | 554 (18) | 95.8 (1.4) | 838 (31) | 95.2 (2.1) |
| Invalid | 628 (23) | 93.9 (1.5) | 871 (25) | 92.6 (2.4) | ||
| 50/50 | Valid | 576 (21) | 95.8 (1.6) | 860 (31) | 92.3 (1.2) | |
| Invalid | 637 (23) | 93.5 (1.6) | ||||
| Left PPC |
100% | Valid | 573 (26) | 95.8 (1.3) | 827 (27) | 93.3 (2.4) |
| Invalid | 619 (20) | 94.3 (1.4) | 843 (29) | 93.9 (2.6) | ||
| 50/50 | Valid | 568 (18) | 96.8 (1.0) | 849 (27) | 94.6 (1.0) | |
| Invalid | 630 (19) | 92.9 (2.0) | ||||
| Right PPC |
100% | Valid | 566 (23) | 96.0 (0.9) | 858 (34) | 93.8 (2.6) |
| Invalid | 624 (20) | 95.5 (1.4 | 856 (37) | 93.8 (1.8) | ||
| 50/50 | Valid | 575 (21) | 96.0 (1.0) | 843 (32) | 91.9 (2.0) | |
| Invalid | 649 (21) | 92.3 (1.8) | ||||
Note: WM catch trials themselves are neither valid nor invalid, but occur in a context where the validity of the visual search trials is known (100%) or unknown (50/50).
Control site (vertex) stimulation replicates standard behavioral effects
In order to first ensure that vertex served as an effective control target, accuracy and RT were analyzed for the behavioral task run following vertex stimulation, using a 2 (Validity: Valid vs. Invalid) × 2 (Predictability: 100% vs. 50/50) ANOVA. Participants were faster for valid trials, F(1, 16) = 90.8, p < .001, η2p = .85, faster during 100% predictable blocks, F(1, 16) = 10.5, p < .01, η2p = .39, and there was a marginal Validity × Predictability interaction F(1, 16) = 3.8, p < . 1, η2p = .19, (Figure 2, middle panel). These findings reflect both the capture of attention by WM content (validity effects), and its modulation by cognitive control (predictability effects). These data replicate previous investigations using this task (Kiyonaga et al., 2012; Soto et al., 2012), confirming that vertex stimulation yielded standard behavioral performance. Accuracy on search trials was high overall (95%), and marginally better on valid trials, F(1, 16) = 3.5, p < . 1, η2p = .18, but showed no other main effects or interactions (all p > .1).
Figure 2.
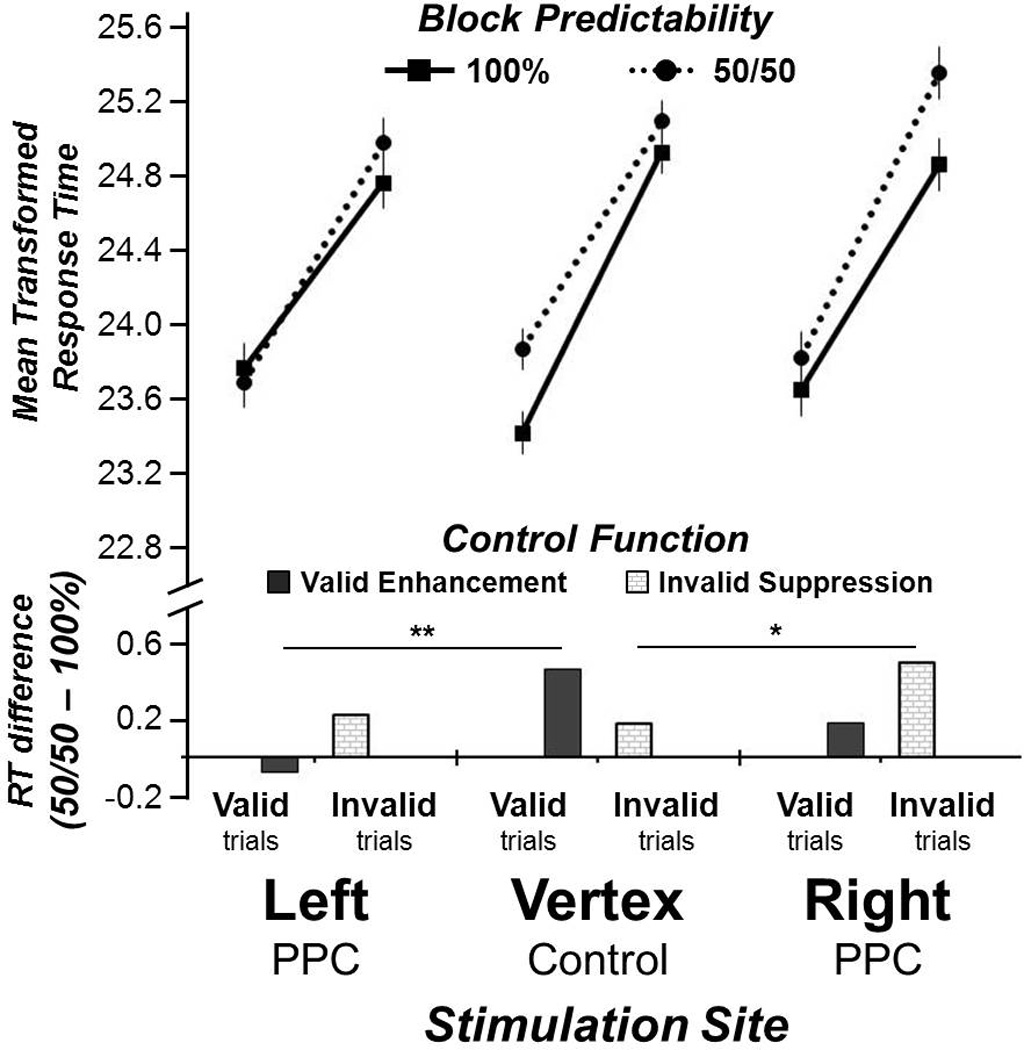
Visual Search Response Time (RT). The line graph displays RT (square-root transformed) for all conditions after stimulation to each target. The bar graph displays the same data as subtractions between the 50/50 and 100% predictable blocks (i.e., difference between dotted and solid lines), to illustrate the effects of enhancement and inhibition. ** p < .01, * p < .05, one-tailed.
Performance measures for the memory catch trials were submitted to an ANOVA with the three-level factor of Block Condition (100% valid, 100% invalid, 50/50). Participants were slowest to respond to memory probes in 100% invalid blocks, and fastest in 100% valid blocks, F(2, 32) = 3.34, p < .05, η2p = .17, again replicating previous findings (Kiyonaga et al., 2012). Catch trial accuracy was high overall (93%), and did not differ by Block Condition (p > .1), confirming that participants maintained the WM cues as instructed.
Disruption of left PPC abolishes the strategic enhancement of predictably valid WM cues
When all three stimulation targets were included in the analysis, participants were faster overall on valid trials, F(1, 16) = 185.9, p < .001, η2p = .92, and faster during 100% predictable blocks, F(1, 16) = 9.7, p < .01, η2p = .38. There was also a three-way Stimulation Site × Validity × Predictability interaction, F(2, 32) = 3.8, p < .05, η2p = .20, which was explored further with separate hypothesis-driven analyses comparing each experimental site to the vertex. There were no other significant main effects or interactions (all p > .1).
The RT comparison between Vertex and Left PPC rTMS revealed faster search performance for valid trials, F(1, 16) = 160.2, p < .001, η2p = .90, a marginal Stimulation Site × Predictability interaction, F(1, 16) = 3.4, p < .1, η2p = .17, and critically, a three-way Stimulation Site × Validity × Predictability interaction, F(1, 16) = 4.8, p < .05, η2p = .23. Two-tailed t-tests compared the enhancement effect from foreknowledge of valid cues (50/50 valid – 100% valid), and the inhibition effect from foreknowledge of invalid cues (50/50 invalid – 100% invalid) between the vertex and left PPC stimulation conditions, to disambiguate the three-way interaction. The magnitude of the valid enhancement effect was significantly smaller after left PPC stimulation compared to vertex, t(16) = 2.2, p < .05, whereas the invalid inhibition effect was unchanged, t(16) = .6, p > .1. Confirming our hypothesis, stimulation to the left PPC eliminated the attention benefit associated with valid WM foreknowledge but left the inhibition of consistently invalid cues unaffected (compare left and middle panels of Figure 2). There were no other main effects or interactions (all p > .1). Left PPC, thus, makes a necessary and specific contribution to the strategic enhancement of beneficial WM content. Visual search accuracy was high overall (95%), and better for valid trials, F(1, 16) = 11.9, p < .01, η2p = .43, but was not affected by any other factors (all p > .1).
Catch trial RT varied with block condition, F(2, 32) = 5.9, p < .01, η2p = .27, and accuracy was high after stimulation to both sites (94%), but neither were affected by stimulation site or an interaction between factors (all p > .1). Therefore, a failure to remember the WM content cannot explain that left PPC stimulation interfered with the strategic enhancement of predictably helpful WM content. The left PPC’s role does not appear to be in maintaining WM content, but in wielding it in the service of attentional goals—specifically by amplifying its impact.
Disruption of right PPC amplifies the cost of unpredictable invalid WM cues
The RT comparison between vertex and right PPC revealed that performance was faster for valid trials, F(1, 16) = 112.7, p < .001, η2p = .87, and for 100% predictable blocks, F(1, 16) = 28.9, p < .001, η2p = .65, but there was also a three-way Stimulation Site × Validity × Predictability interaction, F(1, 16) = 4.6, p <.05, η2p = .23. There were no other main effects or interactions (all p > .1). To determine the source of this 3-way interaction, two-tailed t-tests compared the enhancement and inhibition effects from WM-validity foreknowledge between the vertex control and right PPC stimulation conditions. The modulation of the invalid inhibition effect, though only a trend, t(16) = 2.0, p = .06, was numerically greater than the valid enhancement effect, t(16) = 1.2, p > .1. Intriguingly, the direction of this effect was opposite to what was anticipated from the previously-observed fMRI activation pattern (Soto et al., 2012), which linked BOLD signals in right PPC to strategic inhibitory processes during predictably invalid contexts (relative to when WM validity was unpredictable). Hence TMS ought to have slowed 100% invalid trials and reduced the performance difference between 50/50 and 100% invalid trials; instead, that difference was increased following right PPC TMS (compare middle and right panels of Figure 2). This magnified inhibition effect was characterized by slightly faster performance of 100% predictable invalid trials after right PPC TMS compared to vertex (4msec), as well as slower unpredictable 50/50 invalid trials (12msec). There was, however, a trend for this this slowing on 50/50 invalid trials to be greater than the speeding on 100% invalid trials, t(16) = 2.0, p < .06, two-tailed, implying that the increase in the magnitude of the inhibition effect after right PPC TMS is due to greater attentional capture by the unexpected 50/50 invalid WM cues. The TMS results indicate, therefore, that right PPC is selectively and causally involved in the inhibition process, but is not necessary to strategically curb the impact of predictably harmful WM content. Rather, right PPC appears essential to handling invalid cues when encountered unexpectedly, possibly via rapid disengagement or re-orienting away from these distractors. Visual search accuracy was high overall (93%), and better for valid trials, F(1, 16) = 6.2, p < .05, η2p = .28, but was not affected by any other factors (all p > .1).
Neither memory catch trial RT nor accuracy varied with block condition, stimulation site, or an interaction between factors (all p > .1). The right PPC stimulation that modified the inhibition of harmful WM content in the visual search, thus, did not alter the ability to recall the WM item when probed. Instead, catch trial accuracy was high in all conditions, confirming that participants consistently maintained the memory cues as instructed.
In sum, our TMS results document a double-dissociation of the functional roles of left vs. right PPC in the linkage between WM content and attention: left PPC TMS modulated the strategic enhancement of predictably valid WM cues, but did not affect invalid conditions, whereas right PPC stimulation modulated the cost of unpredictable invalid WM cues, but did not affect valid WM cue conditions.
Discussion
A recent fMRI study suggested that strategic cognitive control over WM biases of visual attention relied mostly on left PPC when WM content was reliably advantageous to attention goals, and on right PPC when it was reliably detrimental (Soto et al., 2012). Using rTMS, here we found causal evidence that left and right PPC do indeed play distinct roles in controlling the impact of internal WM content on attention. In particular, the perturbation of left PPC eliminated the strategic benefit of predictable (100%) valid WM cueing (which had been robust after vertex stimulation), but had no impact on the inhibition of predictably invalid WM content (which was identical to that after vertex control stimulation). On the other hand, perturbation of right PPC amplified the cost of unpredictable (50/50) invalid cueing, but had no impact on valid WM cueing trial conditions.
PPC has been implicated in a range of attention and cognitive control functions, and is considered part of a “multiple-demand” network, that responds to diverse cognitive challenges (Duncan, 2010). Our findings indicate that the PPC can nevertheless be fractionated in terms of the subregions that are necessary for particular functional operations in the interplay between WM and attention. For instance, earlier studies have demonstrated that left PPC is involved in endogenous orienting to spatially cued locations (Du, Chen, & Zhou, 2012), but our results extend these findings to specify that left PPC is critical in exerting strategic control over the WM-attention relationship according to trial validity. Stimulating left PPC did not produce a general impact on effects of validity or predictability but, as anticipated, revealed that one of the region’s specific functions is to voluntarily boost the impact of WM content on visual attention when it is known to be helpful.
The direction of right PPC TMS effects, on the other hand, was notably opposite to what was predicted based on the fMRI activations. Because the targeted area of right PPC was more active during 100% invalid blocks (compared to 50/50), it was expected that the introduction of neural noise to the region would impair performance on predictable 100% invalid trials, and reduce the inhibition effect. Instead, right PPC TMS produced slowing on unpredictable 50/50 invalid trials, and hence an increase in the difference between the 100% and 50/50 invalid conditions. The engagement of the right PPC, thus, seems to signal something other than strategic inhibition, per se. In keeping with the characterization of the PPC as a multiple-demand region, the right PPC target did share some overlap with an area that was overall responsive to invalid > valid trials (Soto et al., 2012, Figure 2b), suggesting that the right PPC is activated by task-adverse WM-matching stimuli, in general, but one of its functionally essential roles is the expedient disengagement or re-orienting from such stimuli when they arise unexpectedly (cf. Corbetta, Kincade, Ollinger, McAvoy, & Shulman, 2000). The TMS modulation of right PPC, therefore, provided a critical glimpse into the causal function of this region, which was not readily foreseeable from the fMRI activation pattern. Taken together, these results are consistent with a distinction between “proactive” and “reactive” mechanisms of cognitive control (Braver, 2012): left PPC appears essential to proactively enhancing valid WM content, while right PPC appears essential to reactively reorienting attention when it has been captured by invalid WM content.
Bilateral PPC is known to play an important role in the control of spatial attention (Colby & Goldberg, 1999; Corbetta & Shulman, 2002) and has, furthermore, been implicated in visual WM capacity and control (Todd & Marois, 2004; Xu & Chun, 2006). Evidence for hemispheric specialization of attentional function in this area, however, has been mixed (Corbetta et al., 2000; Mevorach et al., 2006; M. F. Rushworth et al., 2001). The TMS data reported herein augment our understanding of this heterogeneous region by showing that left and right PPC clearly play distinct, yet complimentary, roles in control over WM content.
It should be noted, however, that the chosen targets were not directly contralateral to one another so it may not strictly be lateralization of function that is reflected here, but rather distinct roles of the subdivisions of parietal cortex. The more superior and anterior area of the PPC that was stimulated on the right, for instance, corresponds to a region that has been shown, with neuroimaging, to be functionally correlated with activity in the dorsal premotor cortex (Mars et al., 2011), and is activated by the updating of visuo-motor contingencies (Rushworth, Paus, & Sipila, 2001), which aligns with its role here in overcoming WM-matching visual distractors and responding to targets instead. The more inferior and posterior area that was stimulated on the left, however, has been shown to be functionally connected with the parahippocampal gyrus and anterior prefrontal cortex (Mars et al., 2011), and associated with endogenous spatial orienting (Du et al., 2012; Thiel, Zilles, & Fink, 2004), consistent with its role here in strategically attending WM-matching targets. The distinct functional and anatomical connections of the stimulated subcomponents of left and right PPC may underlie their unique contributions to the interaction between WM content and visual attention. While our task comprised very brief display durations that discouraged eye movement, furthermore, PPC is also known to play an important role in encoding intentions to make saccades (Andersen, Snyder, Bradley, & Xing, 1997), and future investigations employing eye-tracking might illuminate the extent to which TMS to PPC influences control over both overt and covert attention.
The current TMS data also indicate a singular role for the enhancement of helpful WM content that is separable, at the neural level, from inhibition of harmful WM content: enhancement can be thwarted without any impact on inhibition. Studies of cognitive control have produced mixed conclusions as to whether enhancement and inhibition control functions are in fact qualitatively distinct operations (see Aron, 2007 for review)—presumably reliant on distinct neural processes—or simply two ends of the same spectrum, with inhibition of irrelevant information being a natural consequence of the enhancement of relevant material in a biased competition framework (Desimone & Duncan, 1995; Egner & Hirsch, 2005). Here we have demonstrated that enhancement and inhibition control operations over WM content are mediated by distinct neural mechanisms.
By using TMS to induce neural activity, and thereby interfere with normal processing, we have produced novel evidence for a double dissociation of the causal roles of left and right PPC in the linkage between internal WM content and visual attention. An area of left PPC is causally involved in maximizing the use of internal representations when they are compatible with current task goals. An area of right PPC is causally involved in minimizing the distracting impact of internal representations when they unexpectedly obstruct those goals. Thus, although bilateral PPC is recruited for a diversity of tasks, it is possible, within this region, to distinguish areas of causal function in controlling WM biasing of attention.
Acknowledgements
This research was funded by National Institute of Mental Health Award R01MH087610 to T.E. and a grant from the Medical Research Council (UK, 89631) to D.S.. We thank Joseph King and Amelia Abbott-Frey for help with data collection.
Footnotes
The authors declare no competing financial interests.
References
- Allen EA, Pasley BN, Duong T, Freeman RD. Transcranial Magnetic Stimulation Elicits Coupled Neural and Hemodynamic Consequences. Science. 2007;317(5846):1918–1921. doi: 10.1126/science.1146426. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Snyder LH, Bradley DC, Xing J. Multimodal Representation of Space in the Posterior Parietal Cortex and Its Use in Planning Movements. Annual Review of Neuroscience. 1997;20(1):303–330. doi: 10.1146/annurev.neuro.20.1.303. [DOI] [PubMed] [Google Scholar]
- Aron AR. The Neural Basis of Inhibition in Cognitive Control. The Neuroscientist. 2007;13(3):214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends in Cognitive Sciences. 2012;16(2):106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle NB, Woodman GF. Automatic and strategic effects in the guidance of attention by working memory representations. Acta Psychologica. 2011;137(2):217–225. doi: 10.1016/j.actpsy.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CL, Goldberg ME. Space and Attention in Parietal Cortex. Annual Review of Neuroscience. 1999;22(1):319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience. 2000;3(3):292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews. Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural Mechanisms of Selective Visual Attention. Annual Review of Neuroscience. 1995;18(1):193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Du X, Chen L, Zhou K. The role of the left posterior parietal lobule in top-down modulation on space-based attention: A transcranial magnetic stimulation study. Human Brain Mapping. 2012;33(10):2477–2486. doi: 10.1002/hbm.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends in Cognitive Sciences. 2010;14(4):172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nature Neuroscience. 2005;8(12):1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Harris JA, Clifford CWG, Miniussi C. The Functional Effect of Transcranial Magnetic Stimulation: Signal Suppression or Neural Noise Generation? Journal of Cognitive Neuroscience. 2007;20(4):734–740. doi: 10.1162/jocn.2008.20048. [DOI] [PubMed] [Google Scholar]
- Hildebrand DK. Statistical Thinking for Behavioral Scientists. Duxbury Press; 1986. [Google Scholar]
- Kiyonaga A, Egner T, Soto D. Cognitive control over working memory biases of selection. Psychonomic Bulletin & Review. 2012;19(4):639–646. doi: 10.3758/s13423-012-0253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Jbabdi S, Sallet J, O’Reilly JX, Croxson PL, Olivier E, Rushworth MFS. Diffusion-Weighted Imaging Tractography-Based Parcellation of the Human Parietal Cortex and Comparison with Human and Macaque Resting-State Functional Connectivity. The Journal of Neuroscience. 2011;31(11):4087–4100. doi: 10.1523/JNEUROSCI.5102-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell KA, Nahas Z, Shastri A, Lorberbaum JP, Kozel FA, Bohning DE, George MS. The transcranial magnetic stimulation motor threshold depends on the distance from coil to underlying cortex: a replication in healthy adults comparing two methods of assessing the distance to cortex. Biological Psychiatry. 2001;49(5):454–459. doi: 10.1016/s0006-3223(00)01039-8. [DOI] [PubMed] [Google Scholar]
- Mevorach C, Humphreys GW, Shalev L. Opposite biases in salience-based selection for the left and right posterior parietal cortex. Nature Neuroscience. 2006;9(6):740–742. doi: 10.1038/nn1709. [DOI] [PubMed] [Google Scholar]
- Miniussi C, Ruzzoli M, Walsh V. The mechanism of transcranial magnetic stimulation in cognition. Cortex. 2010;46(1):128–130. doi: 10.1016/j.cortex.2009.03.004. [DOI] [PubMed] [Google Scholar]
- O’Shea J, Walsh V. Transcranial magnetic stimulation. CURR BIOL. 2007;17(6):R196–R199. doi: 10.1016/j.cub.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Cañete C, Catalá MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. Journal of Clinical Neurophysiology: Official Publication of the American Electroencephalographic Society. 1998;15(4):333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- Pitcher D, Garrido L, Walsh V, Duchaine BC. Transcranial Magnetic Stimulation Disrupts the Perception and Embodiment of Facial Expressions. The Journal of Neuroscience. 2008;28(36):8929–8933. doi: 10.1523/JNEUROSCI.1450-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D, Walsh V, Yovel G, Duchaine B. TMS Evidence for the Involvement of the Right Occipital Face Area in Early Face Processing. Current Biology. 2007;17(18):1568–1573. doi: 10.1016/j.cub.2007.07.063. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Théoret H, Pascual-Leone A. Studies in Cognition: The Problems Solved and Created by Transcranial Magnetic Stimulation. Journal of Cognitive Neuroscience. 2003;15(7):948–960. doi: 10.1162/089892903770007344. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Ellison A, Walsh V. Complementary localization and lateralization of orienting and motor attention. Nature Neuroscience. 2001;4(6):656–661. doi: 10.1038/88492. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Paus T, Sipila PK. Attention Systems and the Organization of the Human Parietal Cortex. The Journal of Neuroscience. 2001;21(14):5262–5271. doi: 10.1523/JNEUROSCI.21-14-05262.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini M, Umiltà C, Rusconi E. The use of transcranial magnetic stimulation in cognitive neuroscience: A new synthesis of methodological issues. Neuroscience & Biobehavioral Reviews. 2011;35(3):516–536. doi: 10.1016/j.neubiorev.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Cattaneo Z, Battelli L, Pascual-Leone A. Baseline Cortical Excitability Determines Whether TMS Disrupts or Facilitates Behavior. Journal of Neurophysiology. 2008;99(5):2725–2730. doi: 10.1152/jn.01392.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto D, Greene CM, Kiyonaga A, Rosenthal CR, Egner T. A Parieto-Medial Temporal Pathway for the Strategic Control over Working Memory Biases in Human Visual Attention. The Journal of Neuroscience. 2012;32(49):17563–17571. doi: 10.1523/JNEUROSCI.2647-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto D, Hodsoll J, Rotshtein P, Humphreys GW. Automatic guidance of attention from working memory. Trends in Cognitive Sciences. 2008;12(9):342–348. doi: 10.1016/j.tics.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Stewart L, Walsh V, Rothwell J. Motor and phosphene thresholds: a transcranial magnetic stimulation correlation study. Neuropsychologia. 2001;39(4):415–419. doi: 10.1016/s0028-3932(00)00130-5. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Zilles K, Fink GR. Cerebral correlates of alerting, orienting and reorienting of visuospatial attention: an event-related fMRI study. NeuroImage. 2004;21(1):318–328. doi: 10.1016/j.neuroimage.2003.08.044. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428(6984):751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Walsh V, Cowey A. Transcranial magnetic stimulation and cognitive neuroscience. Nature Reviews Neuroscience. 2000;1(1):73–80. doi: 10.1038/35036239. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Do the contents of visual working memory automatically influence attentional selection during visual search? Journal of Experimental Psychology: Human Perception and Performance. 2007;33(2):363. doi: 10.1037/0096-1523.33.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440(7080):91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]


