Abstract
Background: Multiple abnormal metabolic traits are found together or “cluster” within individuals more often than is predicted by chance. The individual and combined role of adiposity and insulin resistance (IR) on metabolic trait clustering is uncertain. We tested the hypothesis that change in trait clustering is a function of both baseline level and change in these measures.
Methods: In 2616 nondiabetic Framingham Offspring Study participants, body mass index (BMI) and fasting insulin were related to a within-person 7-year change in a trait score of 0–4 Adult Treatment Panel III metabolic syndrome traits (hypertension, high triglycerides, low high-density lipoprotein cholesterol, hyperglycemia).
Results: At baseline assessment, mean trait score was 1.4 traits, and 7-year mean (SEM) change in trait score was +0.25 (0.02) traits, P<0.0001. In models with BMI predictors only, for every quintile difference in baseline BMI, the 7-year trait score increase was 0.14 traits, and for every quintile increase in BMI during 7-year follow-up, the trait score increased by 0.3 traits. Baseline level and change in fasting insulin were similarly related to trait score change. In models adjusted for age–sex–baseline cluster score, 7-year change in trait score was significantly related to both a 1-quintile difference in baseline BMI (0.07 traits) and fasting insulin (0.18 traits), and to both a 1-quintile 7-year increase in BMI (0.21 traits) and fasting insulin (0.18 traits).
Conclusions: Change in metabolic trait clustering was significantly associated with baseline levels and changes in both BMI and fasting insulin, highlighting the importance of both obesity and IR in the clustering of metabolic traits.
Introduction
Multiple abnormal metabolic traits are found together or “cluster” within individuals more often than is predicted by chance.1 Three or more abnormal metabolic traits are found in one-third of the general population.2 People who have this metabolic syndrome have a two- to four-fold higher risk for diabetes and cardiovascular disease (CVD) when compared to people without the syndrome.3,4
The underlying causes of metabolic trait clustering are thought to be adiposity5 and insulin resistance (IR).6,7 Although the impact on trait clustering of baseline measures of adiposity and IR, and prospective change in these exposures, has been examined in isolation or as paired exposures,1,5,7–30 the independent influence of these four exposures (baseline adiposity, baseline IR, change in adiposity, and change in IR) has not been studied.
In a population-based prospective cohort study, we tested the hypothesis that change in metabolic trait clustering over time is a function of baseline levels and prospective changes in both body mass index (BMI) and fasting insulin.
Methods
The Framingham Offspring Study (FOS) is a community-based prospective observational study of CVD and its risk factors.31 Offspring participants are white and of mixed European ancestry. Between 1991 and 1995, 3799 participants fasted overnight and had a standardized medical examination, including a 2-hr oral glucose tolerance test (OGTT). Seven years later (1998–2001), from 3799 participants, we excluded those who did not provide follow-up data (n=504), those with prevalent diabetes (n=259), and those with missing information on covariates (n=420), which left 2616 subjects for analysis. We defined abnormal levels for individual metabolic risk factors according to the current international definition.32
Clinical definitions and laboratory methods
We defined diabetes at the baseline exam as a fasting plasma glucose level ≥7.0 mmol/L, a 2-hr OGTT glucose of ≥11.1 mmol/L, or current use of hypoglycemic drug therapy. Over 99% of diabetes among Framingham Offspring is type 2 diabetes mellitus (T2DM).33 Impaired fasting glucose (IFG) was defined as a fasting plasma glucose level 5.6–6.9 mmol/L.
Adiposity was assessed by BMI34 or waist circumference (WC). IR was assessed using fasting insulin,35 or homeostasis model assessment of insulin resistance (HOMA-IR) [fasting plasma glucose (mmol/L)×fasting insulin (μU/mL)]/22.5, with higher values indicating IR.36,37 HOMA-IR formula values36 are highly correlated with computer-derived HOMA-IR model values38 in Framingham Offspring Study participants (r=0.98, P<0.0001), and therefore only HOMA-IR formula values are presented.
Plasma glucose was measured in fresh specimens with a hexokinase reagent kit (A-gent glucose test; Abbott, South Pasadena, CA). Glucose assays were run in duplicate; the intra-assay coefficient of variation (CV) was <3%. Fasting insulin levels were measured in plasma using different assays at baseline and follow-up, and were standardized to serum levels for reporting purposes. At baseline, the lower limit of sensitivity of the insulin assay was 8 pmol/L and the intra- and interassay CVs were 5.0%–10.0% (Coat-A-Count total immunereactive insulin, Diagnostic Products Corporation, Los Angeles, CA). At follow-up the lower limit of sensitivity was 12 pmol/L and the interassay CV was 6.1% (Human-specific insulin RIA, Linco, St Louis, MO).
The Institutional Review Board of Boston University approved the study protocol, and all subjects gave informed consent at each examination.
Statistical analysis
Outcome
Risk factor clustering was quantified by a “trait score” by counting (0–4) the number of abnormal metabolic traits [impaired fasting glucose, low high-density lipoprotein cholesterol (HDL-C), high triglycerides (TGs), hypertension] present in each subject. Trait score change was calculated as follow-up trait score minus baseline trait score, with a positive value indicating increased clustering and a negative value indicating decreased clustering.
Predictors
Baseline weight was defined by the baseline BMI quintile and change in weight by the change in BMI quintile during 7 years of follow-up. Similarly, baseline IR level was defined by the baseline fasting insulin quintile, and change in IR by the change in fasting insulin quintile. We adopted this method because the absolute change in insulin level between baseline and follow-up could not be determined since a different insulin assay had been used at baseline and follow-up.
Analysis
Because trait score change was approximately normally distributed (see Fig. S1) (Supplementary Data are available at www.liebertpub.com/met/), the relationships between the outcome (change in trait score) and the predictors (baseline BMI or/and fasting insulin or/and change in BMI or/and fasting insulin over time) were assessed using linear regression. Individuals were assigned values for predictor variables based on quintile values at baseline (values 1–5) and follow-up examinations (values 1–5). The change in BMI quintile and the change in fasting insulin quintile for each participant were calculated as [quintile at follow-up] minus [quintile at baseline] (values −4 to +4). The mean [95% confidence interval (CI)] change in trait score (outcome) associated with a 1-quintile difference in BMI or fasting insulin (predictor) at baseline was calculated from the slope of the regression line between outcome and predictor. Similarly, the mean (95% CI) difference in trait score (outcome) associated with a 1-quintile change over 7 years in BMI or fasting insulin (predictor) was calculated from the slope of the regression line between outcome and predictor. Predictors were modeled individually, in pairs (baseline and change in BMI or fasting insulin), and together (baseline and change in BMI and fasting insulin). All regression models were adjusted for age, sex, and baseline trait score to account for regression to the mean effects. We performed supplementary analysis using WC in place of BMI, and using HOMA-IR in place of fasting insulin (Table S1). We performed all analyses using SAS (SAS Institute, Cary, NC).
Results
Baseline data
There were 2616 subjects available for analysis [mean (standard deviation, SD): age, 54 (10) years; women, 54%; BMI, 27.1 (4.7) kg/m2; WC, 91.7 (14.0) cm or 36.1 (5.5) inches; fasting insulin 63 (47) pmol/L]. At baseline, approximately one-third of subjects had high levels of the four individual metabolic traits from which we derived the trait score (abnormal trait prevalence: hypertension, 43%; low HDL-C, 40%; high TGs, 32%; and impaired fasting glucose, 28%).
The baseline prevalence of elevated metabolic traits and the baseline trait score were positively related to the baseline BMI quintile and to the baseline fasting insulin quintile (Table 1).
Table 1.
Baseline Prevalence of Abnormal Metabolic Syndrome Traits by Baseline BMI or Fasting Insulin Quintile
| Predictors: BMI or fasting insulin | Outcome: baseline prevelence of metabolic syndrome trait | Baseline BMI or fasting insulin qunitile | |||||
|---|---|---|---|---|---|---|---|
| Baseline BMI quintile | 1 | 2 | 3 | 4 | 5 | P value | |
| Hypertension (%) | 29 | 34 | 40 | 49 | 65 | <0.0001 | |
| High triglycerides (%) | 15 | 23 | 30 | 43 | 47 | <0.0001 | |
| Low HDL-cholesterol (%) | 22 | 33 | 41 | 47 | 55 | <0.0001 | |
| High fasting glucose (%) | 18 | 20 | 24 | 34 | 43 | <0.0001 | |
| Baseline trait score | 0.9 (1.0) | 1.1 (1.1) | 1.4 (1.1) | 1.7 (1.2) | 2.1 (1.2) | <0.0001 | |
| Baseline fasting insulin quintile | 1 | 2 | 3 | 4 | 5 | P value | |
| Hypertension (%) | 31 | 34 | 41 | 49 | 64 | <0.0001 | |
| High triglycerides (%) | 14 | 20 | 28 | 38 | 58 | <0.0001 | |
| Low HDL-cholesterol (%) | 20 | 29 | 36 | 48 | 67 | <0.0001 | |
| High fasting glucose (%) | 14 | 23 | 24 | 32 | 46 | <0.0001 | |
| Baseline trait score | 0.8 (0.9) | 1.1 (1.0) | 1.3 (1.1) | 1.7 (1.2) | 2.3 (1.2) | <0.0001 | |
Date are % or mean [standard deviation (SD)]. Trait score is calculated as the sum of abnormal metabolic syndrome traits.
BMI, body mass index; HDL, high-density lipoprotein.
Metabolic changes during follow-up
Adiposity was higher at follow-up than at baseline [mean (SD) BMI change 0.9 (0.0) kg/m2, and WC change 7.6 (0.3) cm (3.0 (0.1) inches), both P<0.0001]. The mean (SD) change in weight was 1.9 (6.3) kg, and the proportion of people losing weight in the whole cohort was 35%. After 7 years, a higher proportion of subjects was hypertensive (change, 11%, P<0.001) or had impaired fasting glucose (13%, P<0.001) when compared to baseline. The prevalence of hypertriglyceridemia was unchanged (change, −1%, P=0.24) and the prevalence of low HDL-C was lower after 7 years (change, −7%, P<0.001).
At follow-up, 25% of individuals gained ≥1 metabolic traits and 10% lost ≥1 metabolic traits. At follow-up, the proportion of individuals possessing <1 of the metabolic traits from which we derived the trait score was lower than at baseline, and the proportion with two or more of the four or traits was higher than at baseline (Fig. S2).
Trait score change was approximately normally distributed (Fig. S1). Unadjusted mean [standard error of the mean (SEM)] trait score change was 0.25 (0.02) units, and was unchanged after adjustment for age, sex, and trait score at baseline (both P<0.0001).
Individual predictors of trait score change over 7 years
Trait score change was negatively related to baseline age (β=−0.006, P=0.005) and baseline cluster score (β=−0.319, P<0.0001), and there was a weak nonsignificant relationship with male sex (β=0.054, P=0.16). All analyses of trait score change were adjusted for baseline age, sex and trait score.
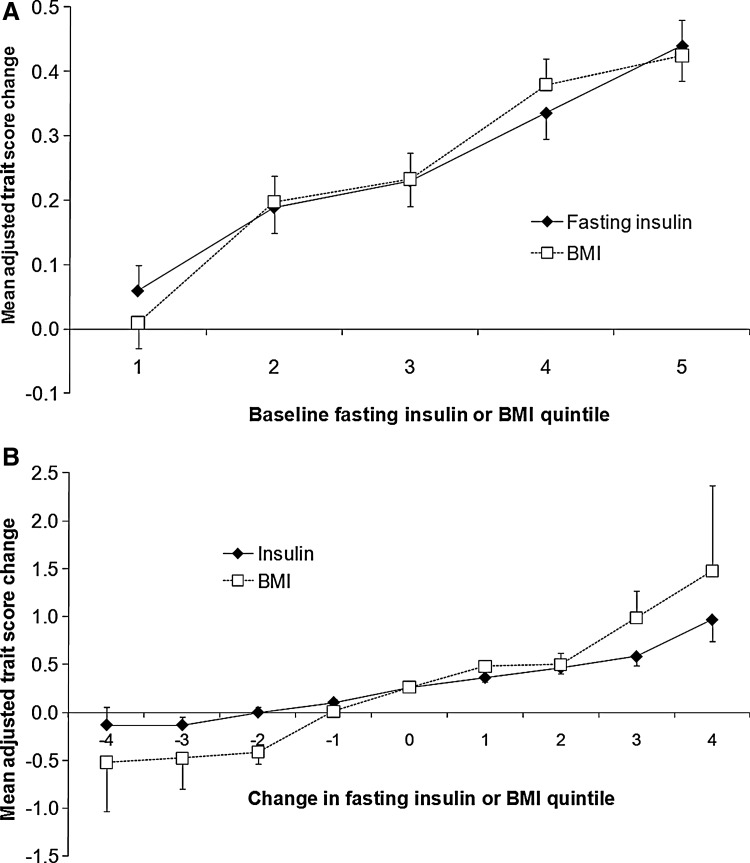
Adjusted trait score changes were associated with baseline BMI and the baseline fasting insulin quintile (Fig. 1A). Adjusted trait score changes appeared to be more strongly related to change in the BMI quintile than to change in the fasting insulin quintile (Fig. 1B). The individuals who experienced a 2–3 quintile reduction in fasting insulin or BMI during follow-up were also more likely to experience declustering—in other words have a reduction in trait score (Fig. 1B).
FIG. 1.
(A) Trait score change shows positive relationships with baseline fasting insulin and baseline body mass index (BMI). (B) Trait score change shows positive relationships with change in fasting insulin and change in BMI. Mean (SE) values are adjusted for age, sex, and baseline trait score.
Adjusted mean trait score change was positively related to a 1-quintile difference in baseline BMI or fasting insulin, or a 1-quintile change in BMI or fasting insulin in models that included these variables individually (Table 2, see data columns 1–4). Multivariable-adjusted trait score changes appeared to be more strongly related to quintile changes in BMI than to fasting insulin [Table 2, see comparison of mean (95% CI) data in columns 2 and 4].
Table 2.
Multivariable-Adjusted Mean (95% CI) Trait Score Change Associated with a 1-Quintile Baseline Difference in BMI or/and Fasting Insulin, or/and a 1-Quintile Change in BMI or/and Fasting Insulin over 7-years
| Outcome: multivariable-adjusted mean (95% CI) trait score change | ||||||||
|---|---|---|---|---|---|---|---|---|
| Predictor variables | 1-quintile baseline difference or/and 1-quintile 7-year change in predictor variable considered | All predictors modeled individually in these columns | Predictors modeled in pairs | All 4 predictors modeled together | ||||
| BMI | Baseline difference | 0.10 (0.08–0.13) | 0.14 (0.12–0.17) | 0.07 (0.04–0.10) | ||||
| 7-year change | 0.23 (0.19–0.28) | 0.30 (0.25–0.34) | 0.21 (0.16–0.26) | |||||
| Fasting insulin | Baseline difference | 0.09 (0.06–0.12) | 0.22 (0.19–0.25) | 0.18 (0.15–0.21) | ||||
| 7-year change | 0.12 (0.10–0.15) | 0.23 (0.20–0.25) | 0.18 (0.15–0.21) | |||||
All trait score change P values<0.0001 for the test that trait score change=0.
All models are adjusted for age, sex, and baseline cluster score.
In models that included individual predictors, the trait score change assoicated with a 1-quintile difference in baseline BMI was 0.10 traits, and with a 1-quintile 7-year change in BMI was 0.23 traits, and with a 1-quintile difference in fasting insulin was 0.09 traits, and with a 1-quintile change in fasting insulin was 0.12 traits. In a model that included both baseline BMI and 7-year change in BMI, the trait score change associated with a 1-quintile difference in baseline BMI was 0.14 traits and for 7-year change in BMI was 0.3 traits. Similarly, in a model that included both baseline fasting insulin and 7-year change in fasting insulin, the trait score change associated with a 1-quintile difference in baseline fasting insulin was 0.22 traits and for 7-year change in fasting insulin was 0.23 traits. In a model that included 1-quintile baseline differences and 1-quintile prospective changes in BMI and fasting insulin, all predictors were positively assoicated with trait score change (BMI difference: 0.07 traits, BMI change: 0.21 traits, fasting insulin difference: 0.18 traits and fasting insulin change: 0.18 traits).
CI, confidence interval; BMI, body mass index.
Influence of baseline level and follow-up changes in BMI or fasting insulin on cluster score change
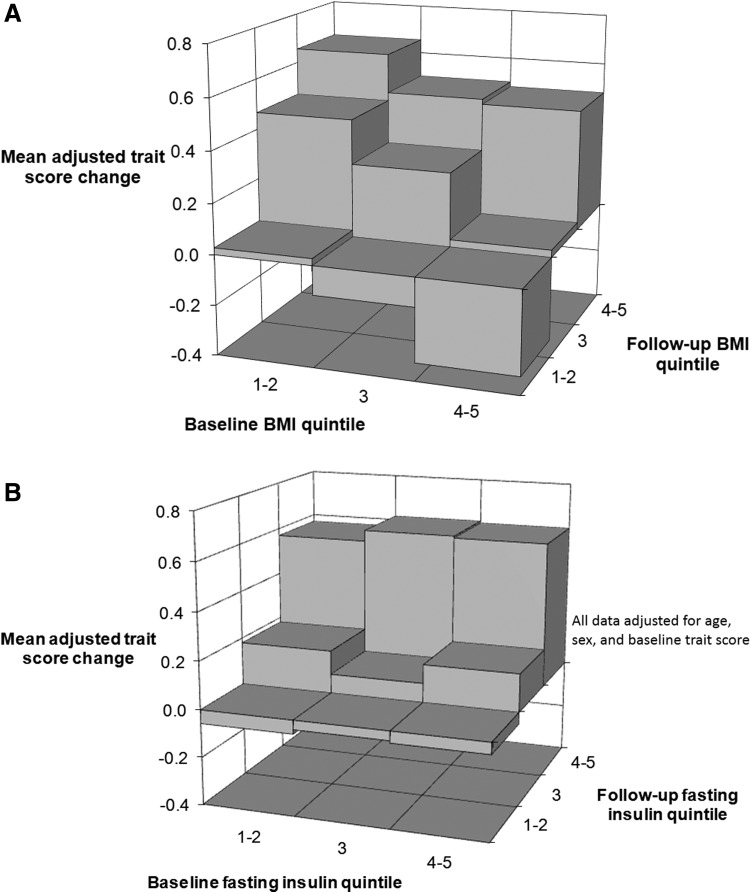
The adjusted trait score change was positively associated with baseline and follow-up BMI quintiles (Fig. 2A). Adjusted trait score change appeared to be more strongly related to quintile changes in BMI during follow-up than to quintile differences in BMI at baseline (Table 2, see data column 5).
FIG. 2.
(A) Subjects moving to a higher body mass index (BMI) quintile or those maintaining the same BMI quintile experience the greatest increase in trait score. Moving to a lower BMI quintile is associated with a lower trait score at follow-up. (B) Subjects becoming or remaining insulin resistant experience the greatest increase in trait score. All data are adjusted for age, sex, and baseline trait score.
The adjusted trait score change was positively related to the baseline fasting insulin quintile and to the follow-up insulin quintile (Fig. 2B). Quintile changes in fasting insulin and quintile differences in baseline fasting insulin were independently and equally related to the adjusted trait score change (Table 2, see data column 6).
Influence of baseline level or/and follow-up changes in BMI and fasting insulin on cluster score change
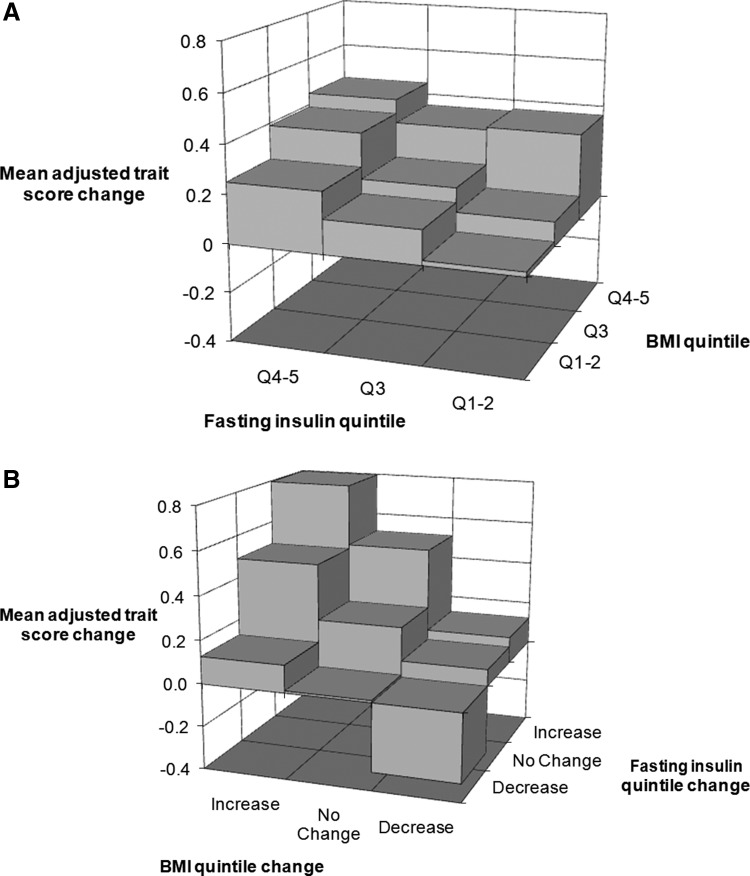
The adjusted trait score change was positively related to quintile differences in BMI and fasting insulin at baseline (Fig. 3A) and to quintile changes in BMI and fasting insulin in an analysis that also adjusted for the baseline BMI and fasting insulin quintile (Fig. 3B).
FIG. 3.
(A) Subjects with high body mass index (BMI) and high fasting insulin at baseline experience the greatest increase in trait score. All data are adjusted for age, sex, and baseline trait score. (B) Subjects moving to both a higher BMI quintile and insulin resistance (IR) quintile experienced the greatest increase in trait score; simultaneous lowering of BMI quintile and IR quintile are linked to a decrease in trait score. All data are adjusted for age, sex, baseline trait score, baseline BMI quintile, and baseline fasting insulin quintile.
The adjusted trait score change was independently related to quintile differences and quintile changes in BMI and fasting insulin [Table 2, see mean (95% CI) data column 7]. Of the four predictor variables in this analysis, the baseline BMI quintile appeared to be least strongly associated with the adjusted trait score change.
Influence of WC or/and HOMA-IR on cluster score change
Results were virtually identical when WC replaced BMI and HOMA-IR replaced fasting insulin in the analyses (see Table S1).
Discussion
Main findings
Our novel findings are that change in the clustering of metabolic traits is related to both BMI and IR at baseline and also to changes in BMI and IR over time. IR appears to account for some of the effect of BMI on changes in trait clustering, but BMI accounts for little of the effect of IR. We have shown for the first time that declustering is related to both a decrease in BMI quintile and to a decrease over time in the IR quintile (Fig. 1B).
Prior studies
Cross-sectional studies
Our cross-sectional data confirm the results of several cross-sectional studies that have shown consistent positive relationships between metabolic traits and measures of adiposity8–14 or IR.7–10,12–16 Our data are also in keeping with the studies that assessed both adiposity and IR and showed positive independent relationships with prevalent metabolic traits8–10,12–14 or prevalent metabolic trait clustering,8–10,13,14 including a study that assessed IR by hyperinsulinemic euglycemic clamp.13
Baseline BMI and IR in relation to metabolic trait change over time
A smaller number of studies have assessed how adiposity5,17–23 or IR5,17,19–21,23,24,30 or both5,17,19–21,23 measured at baseline are related to the development of abnormal metabolic traits18,20–23,30 or metabolic trait clustering17,19 over time.
We showed that BMI and IR assessed at baseline were each related to prospective changes in metabolic trait clustering. Our prospective data is in keeping with the results of two large population-based studies,5,17 but most studies have indicated that baseline IR is less important than baseline adiposity.19–21,23 The most important of these was a large study from Mauritius and Australia in which insulin sensitivity, assessed by homeostatic model assessment of insulin sensitivity (HOMA-S), was related to progression of individual metabolic traits in a univariate analysis, but not in a multivariate analysis that adjusted for all other features of the metabolic syndrome. Although the authors took precautions to avoid co-linearity in their multivariable statistical models, it is possible that the direct effects of IR on changes in metabolic traits were negated by their multivariable modeling procedure.
Change in BMI and IR in relation to metabolic trait change over time
Although there have been several studies that have assessed how a change in BMI is related to prospective changes in metabolic traits,1,25–28 only one study has assessed the relationship of a change in IR, with or without a change in BMI, to metabolic trait clustering.29
Previously, we showed in the Framingham Heart Study that baseline weight in women and weight gain in men and women was related to increased metabolic trait clustering over time.1 In that study, we showed that weight loss was associated with declustering. Our current study adds to these observations by showing the additional relationship of IR with clustering and declustering of metabolic traits.
A dominant influence of change in BMI on changes on metabolic traits was shown in a community-based study of 937 individuals.29 In this study, principal component analysis revealed that prospective change in BMI was the variable most strongly related to prospective change in other metabolic risk factors, including IR. Our study extends this work by showing that changes in risk factor clustering are independently related both to baseline levels and to change in BMI or/and IR.
Pathophysiology
All definitions of the metabolic syndrome include a measure of adiposity as a core component. There are compelling reasons to believe that obesity could cause the other features of the syndrome through mechanisms that may involve IR. For example, adipose tissue and ectopic fat in liver and skeletal muscle is a rich source of adipokines such as tumor necrosis factor-α (TNFα), which can inhibit insulin signaling leading to IR and hyperglycemia.39 There is good evidence that IR can give rise to the so-called diabetic dyslipidemia by increasing hepatic TG synthesis and reducing circulating HDL-C levels.40 Obesity can cause hypertension directly through increasing cardiac output and by inducing a renal-pressure natriuresis.41 However, obesity could also cause hypertension by causing IR and hyperinsulinemia, which could influence sodium retention,42 the sympathetic nervous system,43 cell membrane cation transport, and smooth muscle proliferation.6,44
The idea that IR might mediate some of the metabolic effects of obesity is relevant because it is entirely consistent with the results of our analysis, which suggest that IR accounts for some of the effect of obesity on metabolic trait clustering. Conversely, we showed that BMI accounts for little of the effect of IR on trait clustering. Gerald Reaven first popularized the idea that IR has a central role in the etiology of the syndrome,6 but more recent biological arguments cast doubt on this view.45–47 However, the lack of stronger arguments in favor of IR may simply reflect our limited understanding of the relevant pathophysiology. Our data suggest that IR is related to metabolic syndrome clustering at least as strongly as BMI.
Strengths and limitations
Our study has several strengths. We assessed changes in the metabolic trait clustering in relation to baseline levels and to prospective changes in BMI and IR in a large population-based sample. All models in our analysis adjusted for baseline cluster score to correct for regression to the mean effects.
Our study has some limitations. First, we did not use directly measured IR. Second, we used different insulin assays at baseline and follow-up assessment, but we addressed this by using fasting insulin quintiles defined by subjects without diabetes at each exam. Third, our analysis may have overestimated the influence of within-person increase in BMI or IR on trait clustering because population mean values for both BMI and IR may have increased during follow-up, and our analysis was unable to adjust for this. Similarly, because of the potential for having a shifting (rising) BMI and IR baseline, our analysis may underestimate the influence of reducing BMI or reducing IR quintile on risk factor declustering. Fourth, we cannot exclude the influence of unmeasured confounders such as inflammation,48 diet, exercise, and medication on the observed relationships. Fifth, we had limited power to perform sex-stratified analysis, which might be important because BMI could have a stronger influence on metabolic trait clustering in women than in men.1,17 Last, our findings may have limited generalizability to non-white ethnic groups such as blacks, who may demonstrate less metabolic risk factor clustering than in whites.17,21
Clinical implications
Our study did not evaluate any intervention but the results support promotion of dietary and lifestyle interventions that encourage weight loss and lower IR. Reduction in BMI or IR could be potential targets to reduce the risk of diabetes and CVD embodied in trait clustering.
Conclusions
We conclude that trait clustering is independently associated with baseline levels and changes in BMI and IR, highlighting the importance of both obesity and IR. We showed that IR accounts for some of the effect of BMI, but BMI accounts for little of the effect of IR. These observations are in keeping with our current understanding of the molecular mechanisms linking IR and obesity to trait clustering.
Our results need to be replicated in other cohorts, and, if confirmed, the mechanisms directly linking IR with change in metabolic trait clustering should be explored because this may have important therapeutic implications.
Supplementary Material
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with Boston University (contract no. N01-HC-25195), and the National Institute for Diabetes and Digestive and Kidney Diseases (K24 DK080140) (J.B.M.). M.K.R. is supported by the Higher Education Funding Council for England (Clinical Senior Lecturer Award). The research was facilitated by the Manchester Biomedical Research Centre and the NIHR Greater Manchester Clinical Research Network.
We acknowledge the support of Peter Shrader, MS, who performed the statistical analysis.
M.K.R., L.M.S., C.S.F., P.W.F.W., R.S.V., R.B.D'A., and J.B.M. were involved in the conception and design of the study. D.M.N. was involved in the acquisition of data. M.K.R. wrote the first draft of the manuscript. All authors were involved with the interpretation of data, revision of the article, and the approval of the final draft.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Wilson PW, Kannel WB, Silbershatz H, et al. Clustering of metabolic factors and coronary heart disease. Arch Intern Med 1999;159:1104–1109 [DOI] [PubMed] [Google Scholar]
- 2.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care 2005;28:2745–2749 [DOI] [PubMed] [Google Scholar]
- 3.Hanley AJ, Karter AJ, Williams K, et al. Prediction of type 2 diabetes mellitus with alternative definitions of the metabolic syndrome: The Insulin Resistance Atherosclerosis Study. Circulation 2005;112:3713–3721 [DOI] [PubMed] [Google Scholar]
- 4.Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol 2010;56:1113–1132 [DOI] [PubMed] [Google Scholar]
- 5.Han TS, Williams K, Sattar N, et al. Analysis of obesity and hyperinsulinemia in the development of metabolic syndrome: San Antonio Heart Study. Obes Res 2002;10:923–931 [DOI] [PubMed] [Google Scholar]
- 6.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988;37:1595–1607 [DOI] [PubMed] [Google Scholar]
- 7.Ferrannini E, Haffner SM, Mitchell BD, et al. Hyperinsulinaemia: The key feature of a cardiovascular and metabolic syndrome. Diabetologia 1991;34:416–422 [DOI] [PubMed] [Google Scholar]
- 8.Schmidt MI, Watson RL, Duncan BB, et al. Clustering of dyslipidemia, hyperuricemia, diabetes, and hypertension and its association with fasting insulin and central and overall obesity in a general population. Atherosclerosis Risk in Communities Study Investigators. Metabolism 1996;45:699–706 [DOI] [PubMed] [Google Scholar]
- 9.Schmidt MI, Duncan BB, Watson RL, et al. A metabolic syndrome in whites and African-Americans. The Atherosclerosis Risk in Communities baseline study. Diabetes Care 1996;19:414–418 [DOI] [PubMed] [Google Scholar]
- 10.Meigs JB, D'Agostino RB, Sr, Wilson PW, et al. Risk variable clustering in the insulin resistance syndrome. The Framingham Offspring Study. Diabetes 1997;46:1594–1600 [DOI] [PubMed] [Google Scholar]
- 11.Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: Evidence in support of current National Institutes of Health guidelines. Arch Intern Med 2002;162:2074–2079 [DOI] [PubMed] [Google Scholar]
- 12.Lin WY, Yang WS, Lee LT, et al. Insulin resistance, obesity, and metabolic syndrome among non-diabetic pre- and post-menopausal women in North Taiwan. Int J Obes (Lond) 2006;30:912–917 [DOI] [PubMed] [Google Scholar]
- 13.Ferrannini E, Balkau B, Coppack S, et al. Insulin resistance, insulin response, and obesity as indicators of metabolic risk. J Clin Endocrinol Metab 2007;92:2885–2892 [DOI] [PubMed] [Google Scholar]
- 14.Oka R, Kobayashi J, Inazu A, et al. Contribution of visceral adiposity and insulin resistance to metabolic risk factors in Japanese men. Metabolism 2010;59:748–754 [DOI] [PubMed] [Google Scholar]
- 15.Mykkanen L, Haffner SM, Ronnemaa T, et al. Low insulin sensitivity is associated with clustering of cardiovascular disease risk factors. Am J Epidemiol 1997;146:315–321 [DOI] [PubMed] [Google Scholar]
- 16.Park SH, Lee WY, Rhee EJ, et al. The relative risks of metabolic syndrome according to the degree of insulin resistance in apparently healthy Korean adults. Clin Sci (Lond) 2005;108:553–559 [DOI] [PubMed] [Google Scholar]
- 17.Liese AD, Mayer-Davis EJ, Tyroler HA, et al. Development of the multiple metabolic syndrome in the ARIC cohort: Joint contribution of insulin, BMI, and WHR. Atherosclerosis Risk in Communities. Ann Epidemiol 1997;7:407–416 [DOI] [PubMed] [Google Scholar]
- 18.Everson SA, Goldberg DE, Helmrich SP, et al. Weight gain and the risk of developing insulin resistance syndrome. Diabetes Care 1998;21:1637–1643 [DOI] [PubMed] [Google Scholar]
- 19.Srinivasan SR, Myers L, Berenson GS. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood: The Bogalusa Heart Study. Diabetes 2002;51:204–209 [DOI] [PubMed] [Google Scholar]
- 20.Palaniappan L, Carnethon MR, Wang Y, et al. Predictors of the incident metabolic syndrome in adults: The Insulin Resistance Atherosclerosis Study. Diabetes Care 2004;27:788–793 [DOI] [PubMed] [Google Scholar]
- 21.Chen W, Srinivasan SR, Li S, Xu J, et al. Clustering of long-term trends in metabolic syndrome variables from childhood to adulthood in Blacks and Whites: The Bogalusa Heart Study. Am J Epidemiol 2007;166:527–533 [DOI] [PubMed] [Google Scholar]
- 22.Mirmiran P, Noori N, Azizi F. A prospective study of determinants of the metabolic syndrome in adults. Nutr Metab Cardiovasc Dis 2008;18:567–573 [DOI] [PubMed] [Google Scholar]
- 23.Cameron AJ, Boyko EJ, Sicree RA, et al. Central obesity as a precursor to the metabolic syndrome in the AusDiab study and Mauritius. Obesity (Silver Spring) 2008;16:2707–2716 [DOI] [PubMed] [Google Scholar]
- 24.Haffner SM, Valdez RA, Hazuda HP, et al. Prospective analysis of the insulin-resistance syndrome (syndrome X). Diabetes 1992;41:715–722 [DOI] [PubMed] [Google Scholar]
- 25.Srinivasan SR, Myers L, Berenson GS. Rate of change in adiposity and its relationship to concomitant changes in cardiovascular risk variables among biracial (black-white) children and young adults: The Bogalusa Heart Study. Metabolism 2001;50:299–305 [DOI] [PubMed] [Google Scholar]
- 26.Case CC, Jones PH, Nelson K, et al. Impact of weight loss on the metabolic syndrome. Diabetes Obes Metab 2002;4:407–414 [DOI] [PubMed] [Google Scholar]
- 27.Hillier TA, Fagot-Campagna A, Eschwege E, et al. Weight change and changes in the metabolic syndrome as the French population moves towards overweight: The D.E.S.I.R. cohort. Int J Epidemiol 2006;35:190–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garnett SP, Baur LA, Srinivasan S, et al. Body mass index and waist circumference in midchildhood and adverse cardiovascular disease risk clustering in adolescence. Am J Clin Nutr 2007;86:549–555 [DOI] [PubMed] [Google Scholar]
- 29.Maison P, Byrne CD, Hales CN, et al. Do different dimensions of the metabolic syndrome change together over time? Evidence supporting obesity as the central feature. Diabetes Care 2001;24:1758–1763 [DOI] [PubMed] [Google Scholar]
- 30.Sung KC, Seo MH, Rhee EJ, et al. Elevated fasting insulin predicts the future incidence of metabolic syndrome: A 5-year follow-up study. Cardiovasc Diabetol 2011;10:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kannel WB, Feinleib M, McNamara PM, et al. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 1979;110:281–290 [DOI] [PubMed] [Google Scholar]
- 32.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the Metabolic Syndrome. A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 33.Meigs JB, Mittleman MA, Nathan DM, et al. Hyperinsulinemia, hyperglycemia, and impaired hemostasis: The Framingham Offspring Study. JAMA 2000;283:221–228 [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. The World Health Report: Conquering Suffering, Enriching Humanity. Geneva: World Health Organization; 1997 [Google Scholar]
- 35.Laakso M. How good a marker is insulin level for insulin resistance? Am J Epidemiol 1993;137:959–965 [DOI] [PubMed] [Google Scholar]
- 36.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 37.Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000;23:57–63 [DOI] [PubMed] [Google Scholar]
- 38.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998;21:2191–2192 [DOI] [PubMed] [Google Scholar]
- 39.Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: A key component of the obesity-diabetes link. Diabetes 1994;43:1271–1278 [DOI] [PubMed] [Google Scholar]
- 40.Adiels M, Olofsson SO, Taskinen MR, et al. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol 2008;28:1225–1236 [DOI] [PubMed] [Google Scholar]
- 41.Hall JE, Brands MW, Henegar JR. Mechanisms of hypertension and kidney disease in obesity. Ann NY Acad Sci 181999;892:91–107 [DOI] [PubMed] [Google Scholar]
- 42.Semplicini A, Ceolotto G, Massimino M, et al. Interactions between insulin and sodium homeostasis in essential hypertension. Am J Med Sci 1994;307(Suppl 1):S43–S6 [PubMed] [Google Scholar]
- 43.Kern W, Peters A, Born J, et al. Changes in blood pressure and plasma catecholamine levels during prolonged hyperinsulinemia. Metabolism 2005;54:391–396 [DOI] [PubMed] [Google Scholar]
- 44.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991;14:173–194 [DOI] [PubMed] [Google Scholar]
- 45.Gale EA. The myth of the metabolic syndrome. Diabetologia 2005;48:1679–1683 [DOI] [PubMed] [Google Scholar]
- 46.Kahn R, Buse J, Ferrannini E, et al. The metabolic syndrome: Time for a critical appraisal Joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2005;48:1684–1699 [DOI] [PubMed] [Google Scholar]
- 47.Yudkin JS. Insulin resistance and the metabolic syndrome—or the pitfalls of epidemiology. Diabetologia 2007;50:1576–1586 [DOI] [PubMed] [Google Scholar]
- 48.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007;132:2169–2180 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.