Abstract
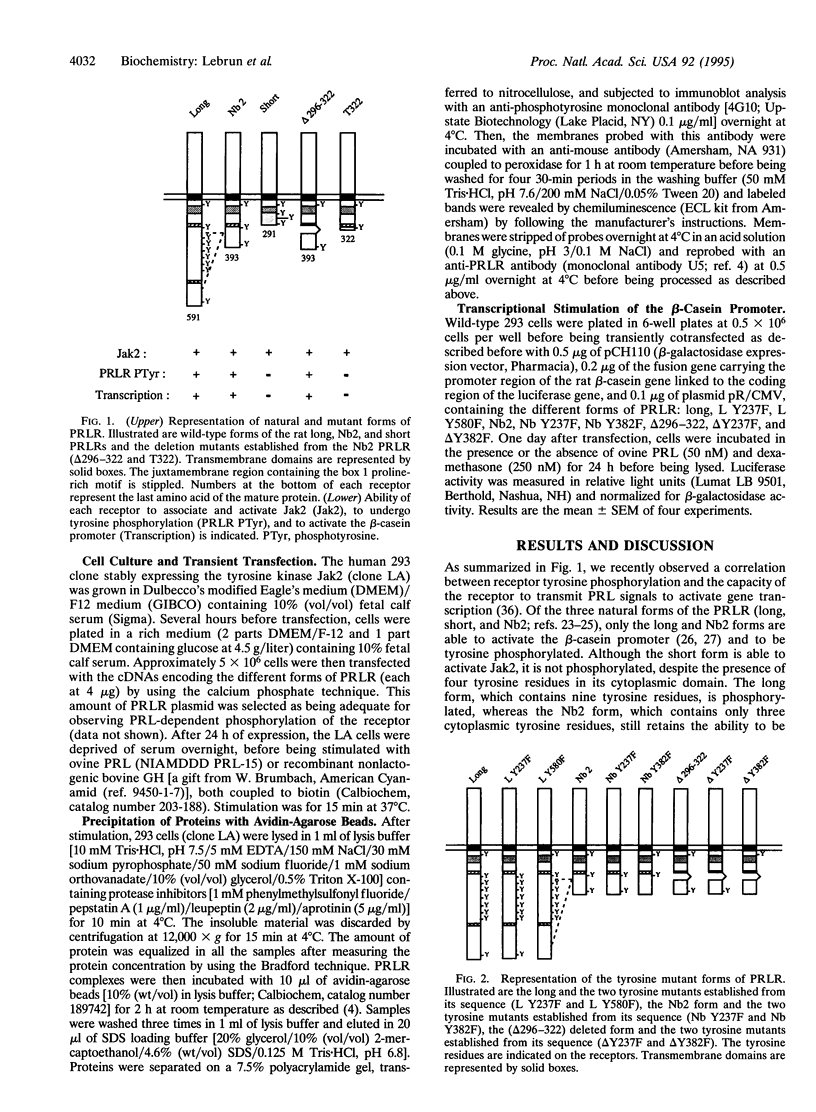
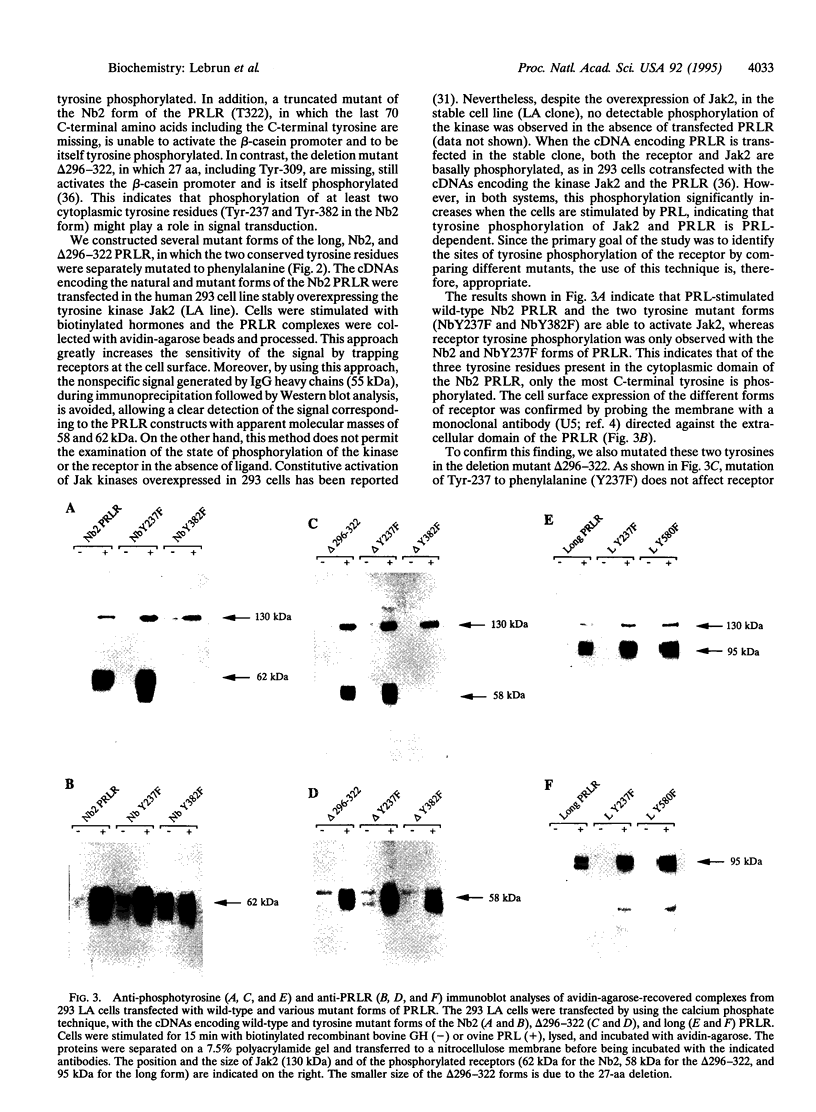
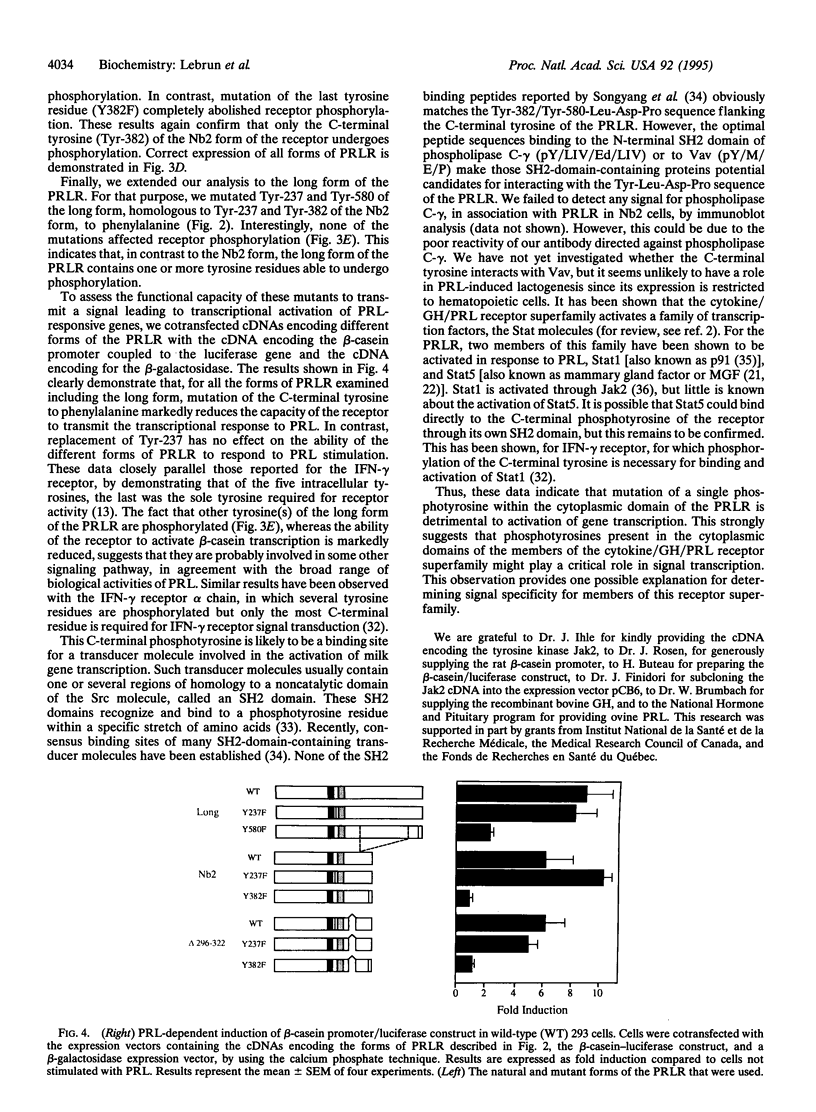
Members of the cytokine/growth hormone/prolactin (PRL) receptor superfamily are associated with cytoplasmic tyrosine kinases of the Jak family. For the PRL receptor (PRLR), after PRL stimulation, both the kinase Jak2 and the receptor undergo tyrosine phosphorylation. To assess the role of tyrosine phosphorylation of the PRLR in signal transduction, several mutant forms of the PRLR in which various tyrosine residues were changed to phenylalanine were constructed and their functional properties were investigated. We identified a single tyrosine residue located at the C terminus of the PRLR to be necessary for in vivo activation of PRL-responsive gene transcription. This clearly indicates that a phosphotyrosine residue in the cytoplasmic domain of a member of the cytokine/growth hormone/PRL receptor superfamily is directly involved in signal transduction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Nishio Y., Inoue M., Wang X. J., Wei S., Matsusaka T., Yoshida K., Sudo T., Naruto M., Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994 Apr 8;77(1):63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Ali S., Edery M., Pellegrini I., Lesueur L., Paly J., Djiane J., Kelly P. A. The Nb2 form of prolactin receptor is able to activate a milk protein gene promoter. Mol Endocrinol. 1992 Aug;6(8):1242–1248. doi: 10.1210/mend.6.8.1406702. [DOI] [PubMed] [Google Scholar]
- Ali S., Pellegrini I., Kelly P. A. A prolactin-dependent immune cell line (Nb2) expresses a mutant form of prolactin receptor. J Biol Chem. 1991 Oct 25;266(30):20110–20117. [PubMed] [Google Scholar]
- Bazan J. F. A novel family of growth factor receptors: a common binding domain in the growth hormone, prolactin, erythropoietin and IL-6 receptors, and the p75 IL-2 receptor beta-chain. Biochem Biophys Res Commun. 1989 Oct 31;164(2):788–795. doi: 10.1016/0006-291x(89)91528-3. [DOI] [PubMed] [Google Scholar]
- Boutin J. M., Jolicoeur C., Okamura H., Gagnon J., Edery M., Shirota M., Banville D., Dusanter-Fourt I., Djiane J., Kelly P. A. Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor gene family. Cell. 1988 Apr 8;53(1):69–77. doi: 10.1016/0092-8674(88)90488-6. [DOI] [PubMed] [Google Scholar]
- Campbell G. S., Argetsinger L. S., Ihle J. N., Kelly P. A., Rillema J. A., Carter-Su C. Activation of JAK2 tyrosine kinase by prolactin receptors in Nb2 cells and mouse mammary gland explants. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5232–5236. doi: 10.1073/pnas.91.12.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Carter-Su C., Stubbart J. R., Wang X. Y., Stred S. E., Argetsinger L. S., Shafer J. A. Phosphorylation of highly purified growth hormone receptors by a growth hormone receptor-associated tyrosine kinase. J Biol Chem. 1989 Nov 5;264(31):18654–18661. [PubMed] [Google Scholar]
- David M., Petricoin E. F., 3rd, Igarashi K., Feldman G. M., Finbloom D. S., Larner A. C. Prolactin activates the interferon-regulated p91 transcription factor and the Jak2 kinase by tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7174–7178. doi: 10.1073/pnas.91.15.7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusanter-Fourt I., Muller O., Ziemiecki A., Mayeux P., Drucker B., Djiane J., Wilks A., Harpur A. G., Fischer S., Gisselbrecht S. Identification of JAK protein tyrosine kinases as signaling molecules for prolactin. Functional analysis of prolactin receptor and prolactin-erythropoietin receptor chimera expressed in lymphoid cells. EMBO J. 1994 Jun 1;13(11):2583–2591. doi: 10.1002/j.1460-2075.1994.tb06548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar M. A., Campbell J. D., Schreiber R. D. Identification of a functionally important sequence in the C terminus of the interferon-gamma receptor. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11706–11710. doi: 10.1073/pnas.89.24.11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster C. M., Shafer J. A., Rozsa F. W., Wang X. Y., Lewis S. D., Renken D. A., Natale J. E., Schwartz J., Carter-Su C. Growth hormone promoted tyrosyl phosphorylation of growth hormone receptors in murine 3T3-F442A fibroblasts and adipocytes. Biochemistry. 1988 Jan 12;27(1):326–334. doi: 10.1021/bi00401a049. [DOI] [PubMed] [Google Scholar]
- Fu X. Y., Zhang J. J. Transcription factor p91 interacts with the epidermal growth factor receptor and mediates activation of the c-fos gene promoter. Cell. 1993 Sep 24;74(6):1135–1145. doi: 10.1016/0092-8674(93)90734-8. [DOI] [PubMed] [Google Scholar]
- Greenlund A. C., Farrar M. A., Viviano B. L., Schreiber R. D. Ligand-induced IFN gamma receptor tyrosine phosphorylation couples the receptor to its signal transduction system (p91). EMBO J. 1994 Apr 1;13(7):1591–1600. doi: 10.1002/j.1460-2075.1994.tb06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Witthuhn B. A., Quelle F. W., Yamamoto K., Thierfelder W. E., Kreider B., Silvennoinen O. Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem Sci. 1994 May;19(5):222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larner A. C., David M., Feldman G. M., Igarashi K., Hackett R. H., Webb D. S., Sweitzer S. M., Petricoin E. F., 3rd, Finbloom D. S. Tyrosine phosphorylation of DNA binding proteins by multiple cytokines. Science. 1993 Sep 24;261(5129):1730–1733. doi: 10.1126/science.8378773. [DOI] [PubMed] [Google Scholar]
- Lebrun J. J., Ali S., Sofer L., Ullrich A., Kelly P. A. Prolactin-induced proliferation of Nb2 cells involves tyrosine phosphorylation of the prolactin receptor and its associated tyrosine kinase JAK2. J Biol Chem. 1994 May 13;269(19):14021–14026. [PubMed] [Google Scholar]
- Lesueur L., Edery M., Ali S., Paly J., Kelly P. A., Djiane J. Comparison of long and short forms of the prolactin receptor on prolactin-induced milk protein gene transcription. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):824–828. doi: 10.1073/pnas.88.3.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütticken C., Wegenka U. M., Yuan J., Buschmann J., Schindler C., Ziemiecki A., Harpur A. G., Wilks A. F., Yasukawa K., Taga T. Association of transcription factor APRF and protein kinase Jak1 with the interleukin-6 signal transducer gp130. Science. 1994 Jan 7;263(5143):89–92. doi: 10.1126/science.8272872. [DOI] [PubMed] [Google Scholar]
- Murakami M., Narazaki M., Hibi M., Yawata H., Yasukawa K., Hamaguchi M., Taga T., Kishimoto T. Critical cytoplasmic region of the interleukin 6 signal transducer gp130 is conserved in the cytokine receptor family. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11349–11353. doi: 10.1073/pnas.88.24.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal K. D., Yu-Lee L. Y. Differential signal transduction of the short, Nb2, and long prolactin receptors. Activation of interferon regulatory factor-1 and cell proliferation. J Biol Chem. 1994 Oct 21;269(42):26076–26082. [PubMed] [Google Scholar]
- Ruff-Jamison S., Chen K., Cohen S. Induction by EGF and interferon-gamma of tyrosine phosphorylated DNA binding proteins in mouse liver nuclei. Science. 1993 Sep 24;261(5129):1733–1736. doi: 10.1126/science.8378774. [DOI] [PubMed] [Google Scholar]
- Rui H., Kirken R. A., Farrar W. L. Activation of receptor-associated tyrosine kinase JAK2 by prolactin. J Biol Chem. 1994 Feb 18;269(7):5364–5368. [PubMed] [Google Scholar]
- Sadowski H. B., Shuai K., Darnell J. E., Jr, Gilman M. Z. A common nuclear signal transduction pathway activated by growth factor and cytokine receptors. Science. 1993 Sep 24;261(5129):1739–1744. doi: 10.1126/science.8397445. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford D. A., Trowbridge I. S. Ligand-stimulated tyrosine phosphorylation of the IL-2 receptor beta chain and receptor-associated proteins. Cell Regul. 1991 Jan;2(1):73–85. doi: 10.1091/mbc.2.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirota M., Banville D., Ali S., Jolicoeur C., Boutin J. M., Edery M., Djiane J., Kelly P. A. Expression of two forms of prolactin receptor in rat ovary and liver. Mol Endocrinol. 1990 Aug;4(8):1136–1143. doi: 10.1210/mend-4-8-1136. [DOI] [PubMed] [Google Scholar]
- Shuai K., Ziemiecki A., Wilks A. F., Harpur A. G., Sadowski H. B., Gilman M. Z., Darnell J. E. Polypeptide signalling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nature. 1993 Dec 9;366(6455):580–583. doi: 10.1038/366580a0. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O., Ihle J. N., Schlessinger J., Levy D. E. Interferon-induced nuclear signalling by Jak protein tyrosine kinases. Nature. 1993 Dec 9;366(6455):583–585. doi: 10.1038/366583a0. [DOI] [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., McGlade J., Olivier P., Pawson T., Bustelo X. R., Barbacid M., Sabe H., Hanafusa H., Yi T. Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol Cell Biol. 1994 Apr;14(4):2777–2785. doi: 10.1128/mcb.14.4.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakao H., Gouilleux F., Groner B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J. 1994 May 1;13(9):2182–2191. doi: 10.1002/j.1460-2075.1994.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Uhler M. D., Billestrup N., Norstedt G., Talamantes F., Nielsen J. H., Carter-Su C. Evidence for association of the cloned liver growth hormone receptor with a tyrosine kinase. J Biol Chem. 1992 Aug 25;267(24):17390–17396. [PubMed] [Google Scholar]
- Welte T., Garimorth K., Philipp S., Doppler W. Prolactin-dependent activation of a tyrosine phosphorylated DNA binding factor in mouse mammary epithelial cells. Mol Endocrinol. 1994 Aug;8(8):1091–1102. doi: 10.1210/mend.8.8.7527899. [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Lodish H. F. In vitro phosphorylation of the erythropoietin receptor and an associated protein, pp130. Mol Cell Biol. 1992 Feb;12(2):706–715. doi: 10.1128/mcb.12.2.706. [DOI] [PMC free article] [PubMed] [Google Scholar]




