Abstract
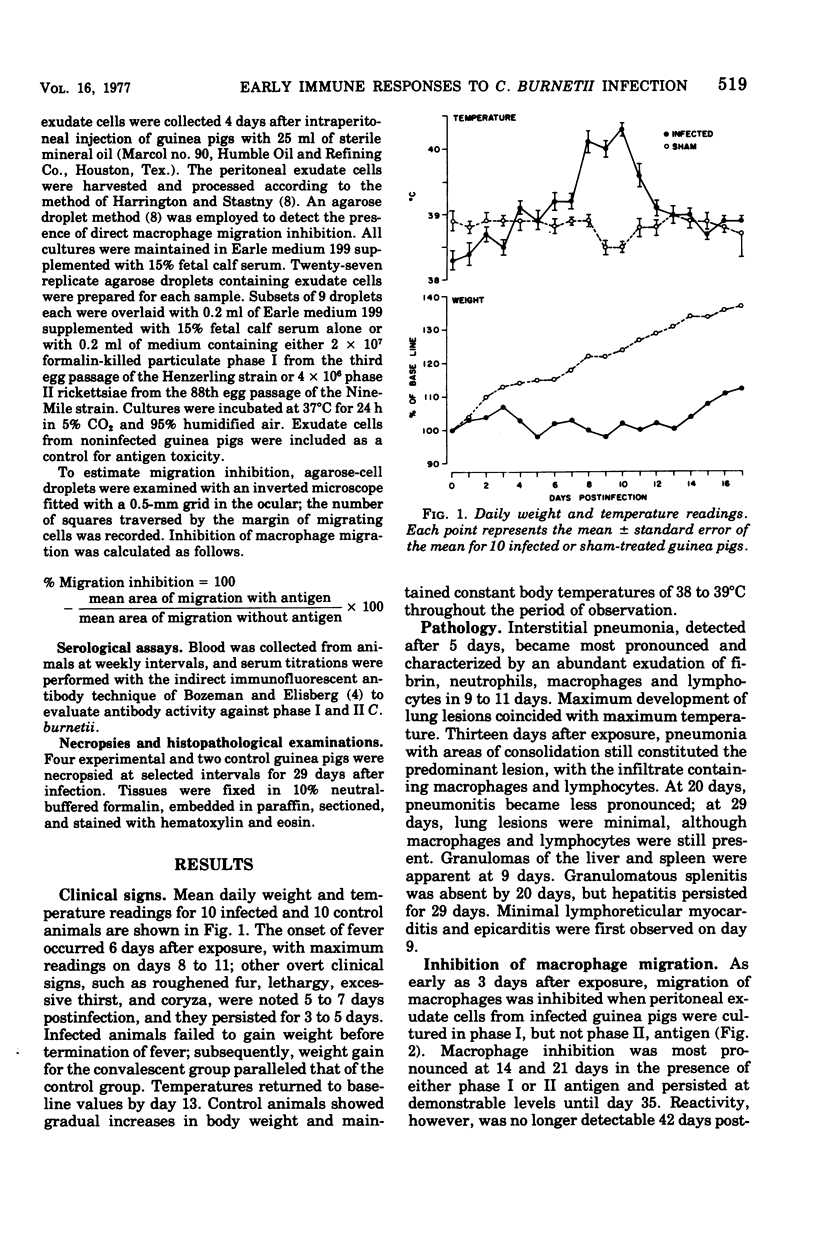
The development of humoral and cell-mediated immune responses was studied in guinea pigs infected with Coxiella burnetti administered in small-particle aerosols. Direct macrophage migration inhibition was observed in cultured peritoneal exudate cells as early as 3 days after exposure. Maximum inhibition of macrophages cultured with phase I or II antigen occurred 14 to 21 days postexposure and persisted through 35 days. This inhibitory action was no longer detectable at 42 days. Serum antibody to the phase II antigen of C. burnetii was detected at 14 days, and serum antibody to phase I antigen was detected at 21 days, 18 days after the cell-mediated immune response.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABINANTI F. R., MARMION B. P. Protective or neutralizing antibody in Q fever. Am J Hyg. 1957 Sep;66(2):173–195. doi: 10.1093/oxfordjournals.aje.a119894. [DOI] [PubMed] [Google Scholar]
- BOZEMAN F. M., ELISBERG B. L. Serological diagnosis of scrub typhus by indirect immunofluorescence. Proc Soc Exp Biol Med. 1963 Mar;112:568–573. doi: 10.3181/00379727-112-28107. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Bennett B. Migration inhibitory factor associated with delayed-type hypersensitivity. Fed Proc. 1968 Jan-Feb;27(1):13–15. [PubMed] [Google Scholar]
- FISET P. Phase variation of Rickettsia (Coxiella) burneti; study of the antibody response in guinea pigs and rabbits. Can J Microbiol. 1957 Apr;3(3):435–445. doi: 10.1139/m57-046. [DOI] [PubMed] [Google Scholar]
- Fowles R. E., Fajardo I. M., Leibowitch J. L., David J. R. The enhancement of macrophage bacteriostasis by products of activated lymphocytes. J Exp Med. 1973 Oct 1;138(4):952–964. doi: 10.1084/jem.138.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington J. T., Jr, Stastny P. Macrophage migration from an agarose droplet: development of a micromethod for assay of delayed hypersensitivity. J Immunol. 1973 Mar;110(3):752–759. [PubMed] [Google Scholar]
- Heggers J. P., Billups L. H., Hinrichs D. J., Mallavia L. P. Pathophysiologic features of Q fever-infected guinea pigs. Am J Vet Res. 1975 Jul;36(7):1047–1052. [PubMed] [Google Scholar]
- Heggers J. P., Mallavia L. P., Hinrichs D. J. The cellular immune response to antigens of Coxiella burneti. Can J Microbiol. 1974 May;20(5):657–662. doi: 10.1139/m74-101. [DOI] [PubMed] [Google Scholar]
- Hinrichs D. J., Jerrells T. R. In vitro evaluation of immunity to Coxiella burnetii. J Immunol. 1976 Sep;117(3):996–1003. [PubMed] [Google Scholar]
- Jerrells T. R., Mallavia L. P., Hinrichs D. J. Detection of long-term cellular immunity to Coxiella burneti as assayed by lymphocyte transformation. Infect Immun. 1975 Feb;11(2):280–286. doi: 10.1128/iai.11.2.280-286.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazár J., Skultétyová E., Brezina R. Phagocytosis of Coxiella burneti by macrophages. Acta Virol. 1975 Sep;19(5):426–431. [PubMed] [Google Scholar]
- Kishimoto R. A., Veltri B. J., Canonico P. G., Shirey F. G., Walker J. S. Electron microscopic study on the interaction between normal guinea pig peritoneal macrophages and Coxiella burnetii. Infect Immun. 1976 Oct;14(4):1087–1096. doi: 10.1128/iai.14.4.1087-1096.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto R. A., Veltri B. J., Shirey F. G., Canonico P. G., Walker J. S. Fat of Coxiella burnetti in macrophages from immune guinea pigs. Infect Immun. 1977 Feb;15(2):601–607. doi: 10.1128/iai.15.2.601-607.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto R. A., Walker J. S. Interaction between Coxiella burnetii and guinea pig peritoneal macrophages. Infect Immun. 1976 Aug;14(2):416–421. doi: 10.1128/iai.14.2.416-421.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostiala A. A., McGregor D. D. The mediator of cellular immunity. IX. The relationship between cellular hypersensitivity and acquired cellular resistance in rats infected with Listeria monocytogenes. J Exp Med. 1975 Jun 1;141(6):1249–1260. doi: 10.1084/jem.141.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Karnovsky M. L., David J. R. Alterations of macrophage functions by mediators from lymphocytes. J Exp Med. 1971 Jun 1;133(6):1356–1376. doi: 10.1084/jem.133.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROESSLER W. G., KAUTTER D. A. Modifications to the Henderson apparatus for studying air-borne infections. Evaluations using aerosols of Listeria monocytogenes. J Infect Dis. 1962 Jan-Feb;110:17–22. doi: 10.1093/infdis/110.1.17. [DOI] [PubMed] [Google Scholar]
- STOKER M. G., FISET P. Phase variation of the Nine Mile and other strains of Rickettsia burneti. Can J Microbiol. 1956 May;2(3):310–321. doi: 10.1139/m56-036. [DOI] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Cellular immunity in vitro. I. Immunologically mediated enhancement of macrophage bactericidal capacity. J Exp Med. 1971 Jun 1;133(6):1377–1389. doi: 10.1084/jem.133.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Enhancement of macrophage bactericidal capacity by antigenically stimulated immune lymphocytes. Cell Immunol. 1972 Jun;4(2):163–174. doi: 10.1016/0008-8749(72)90015-9. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Blanden R. V., Langman R. E. Early appearance of sensitized lymphocytes in mice infected with Listeria monocytogenes. J Immunol. 1974 Feb;112(2):496–501. [PubMed] [Google Scholar]


