Abstract
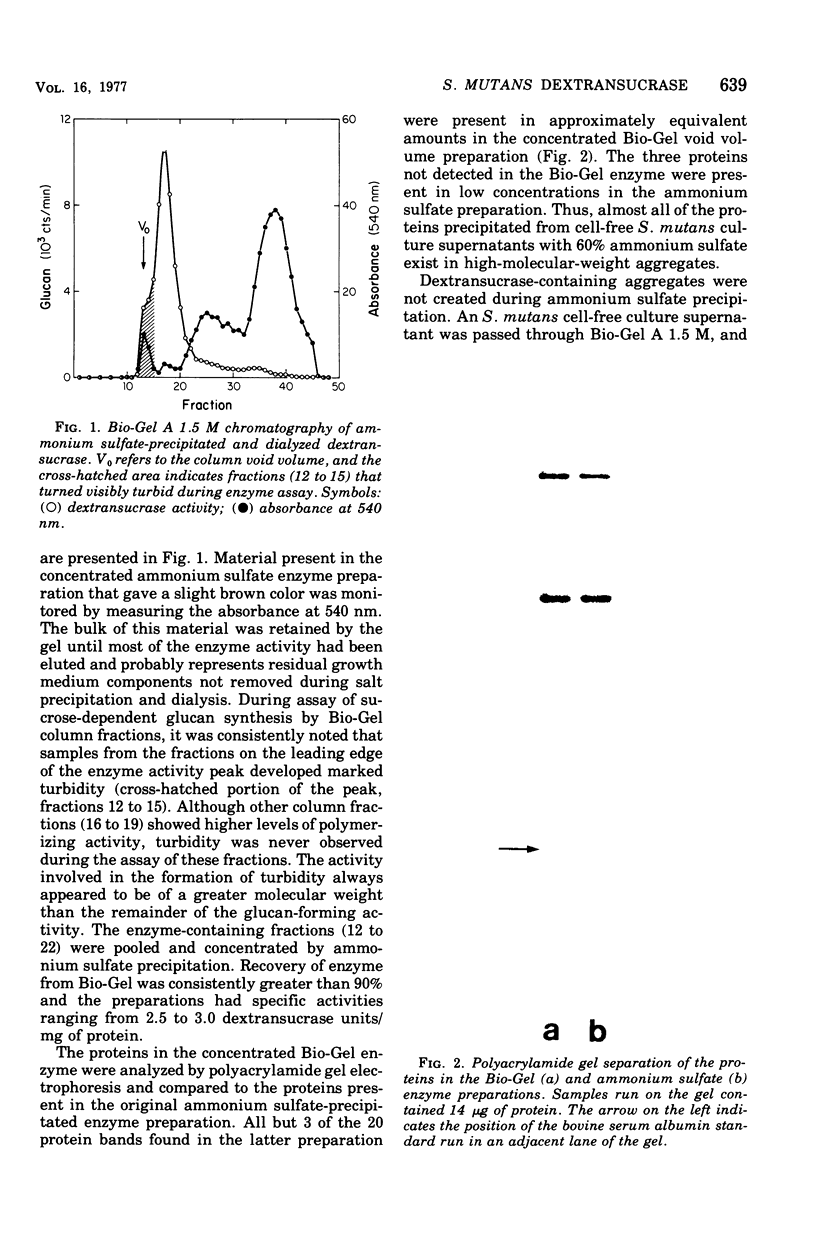
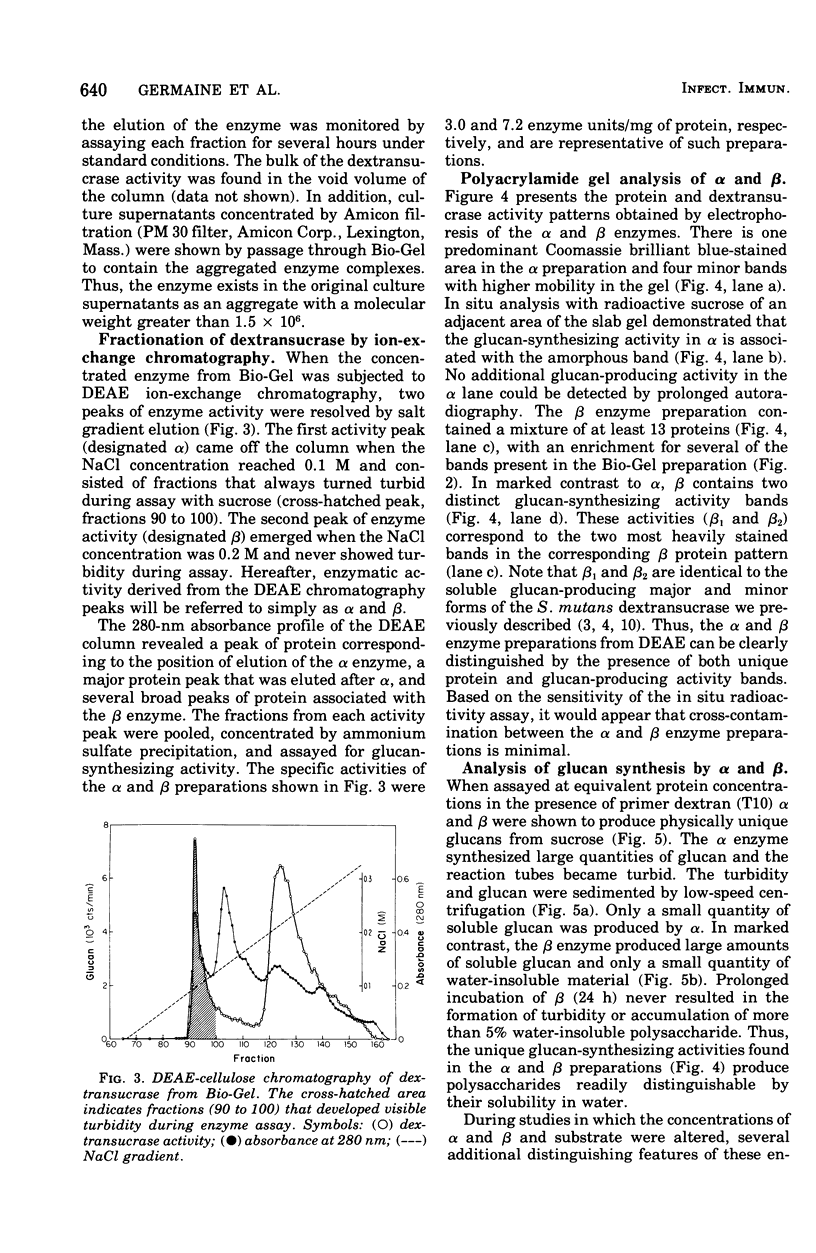
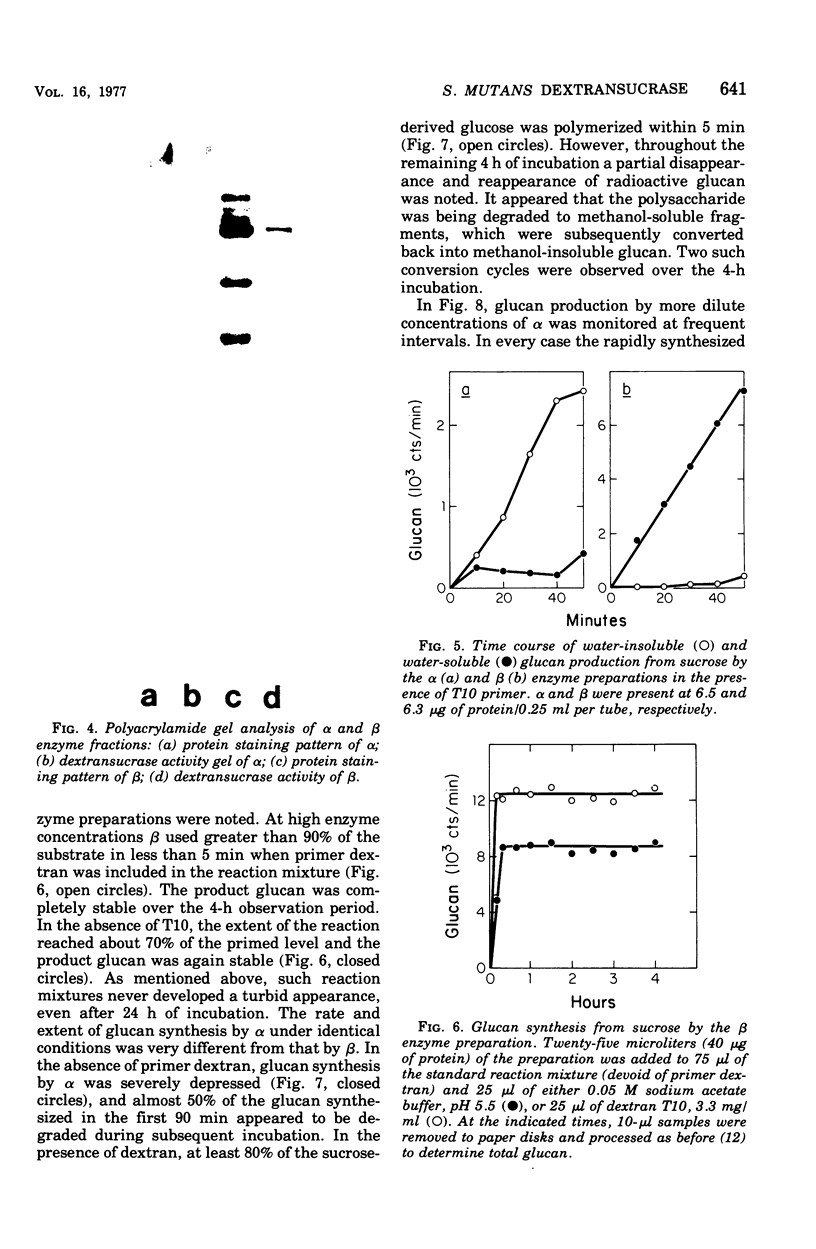
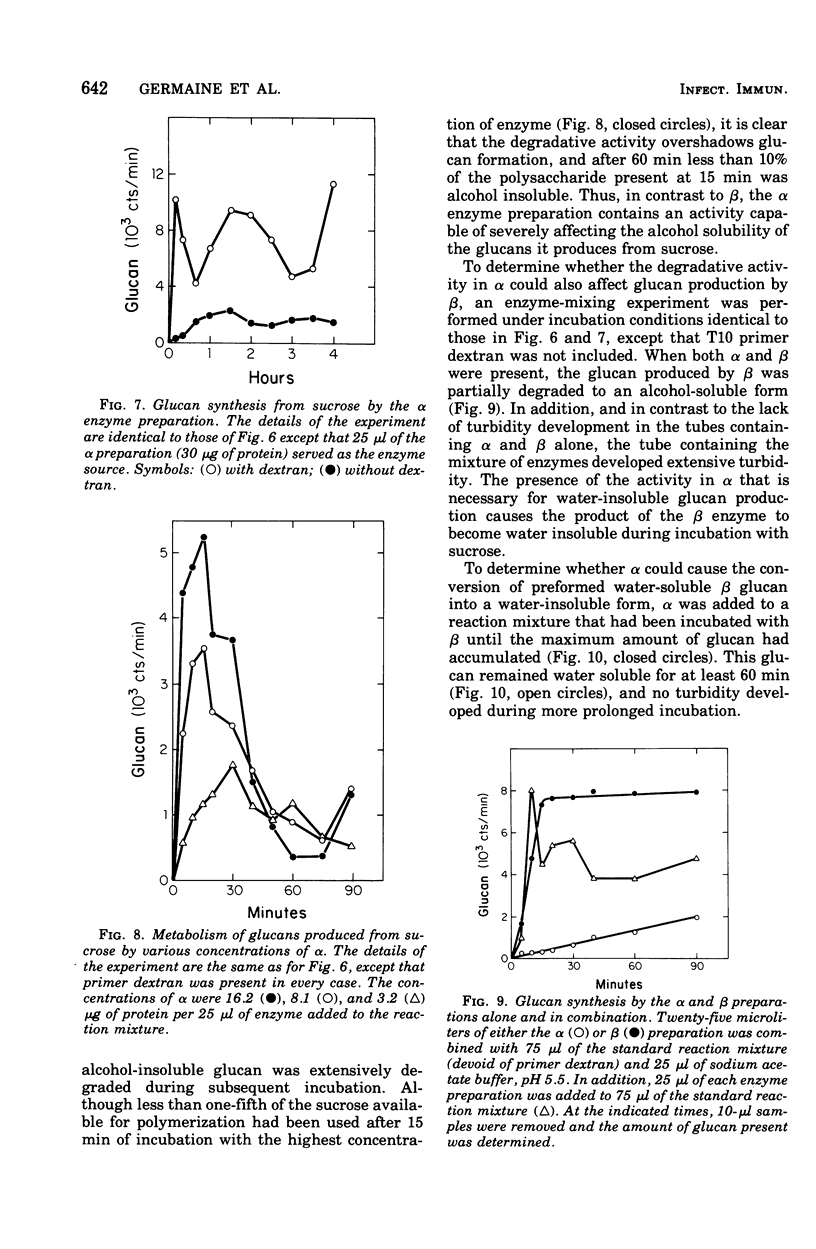
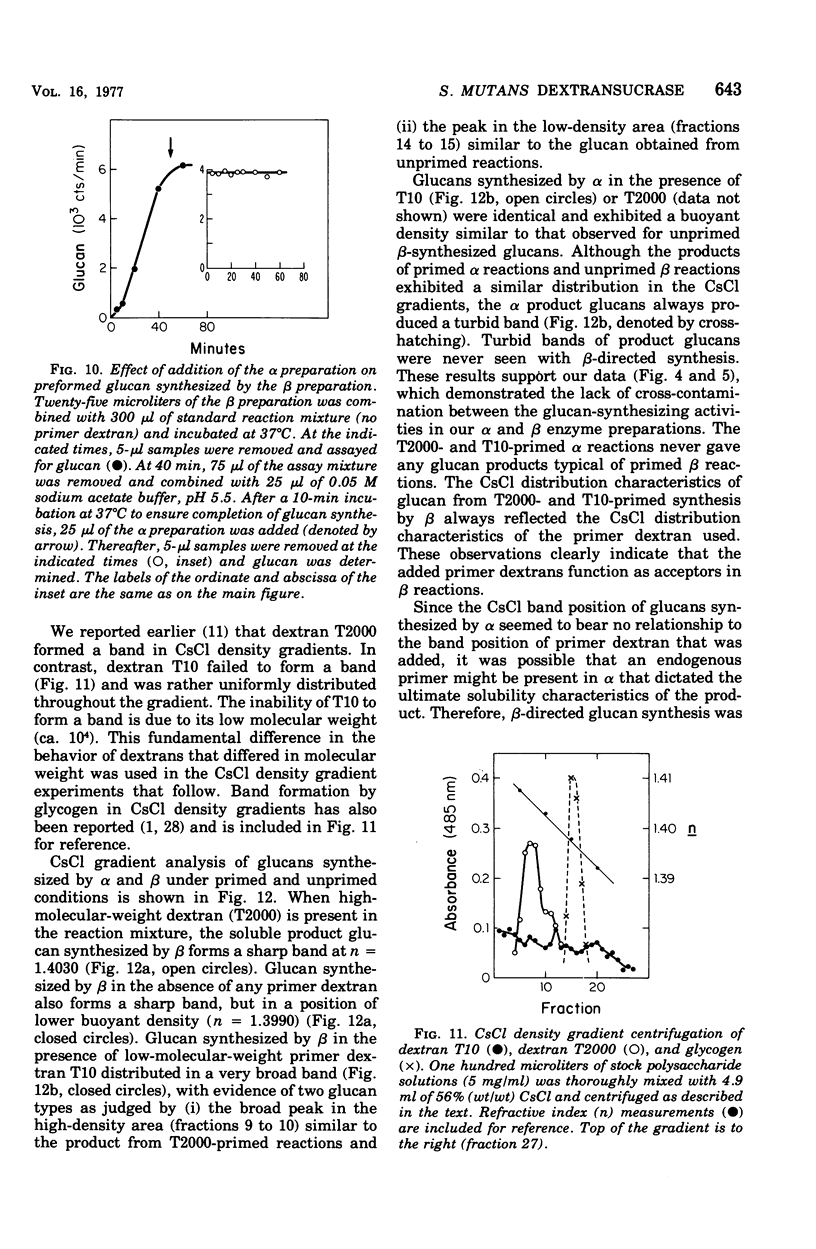
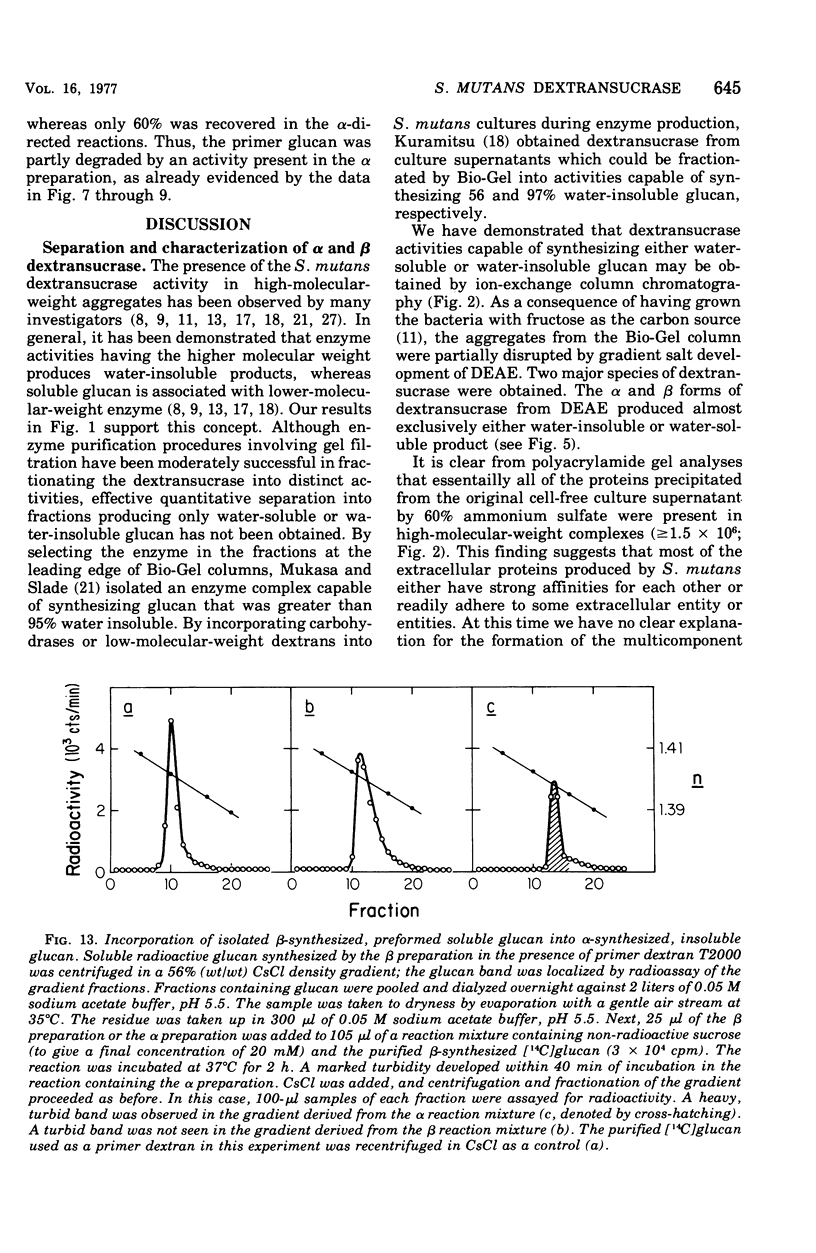
The extracellular enzyme activities of Streptococcus mutans 6715 that synthesize glucans from sucrose were concentrated and partially purified by ammonium sulfate precipitation and gel permeation column chromatography. Polyacrylamide gel analysis demonstrated that all of the major proteins precipitated by ammonium sulfate were quantitatively recovered in the high-molecular-weight, enzyme-containing aggregates found in the void volume of the gel column. Anion-exchange column chromatography was used to fractionate the aggregates into preparations, α and β, which produced water-insoluble and water-soluble glucans, respectively. Polyacrylamide gel analysis showed that α and β contained unique proteins and dextransucrase (EC 2.4.1.5) activities. Studies on the time course of glucan synthesis by α demonstrated that this enzyme preparation contained dextranase activity, which partially degraded nascent alcohol-insoluble glucan into alcohol-soluble products that were subsequently reincorporated into insoluble product. The β enzyme preparation contained no detectable dextranase activity. Mixing experiments in the absence of primer dextran demonstrated that the dextranase activity present in α could modify glucan production by β. CsCl density gradient analysis of product glucans demonstrated that exogenous primer dextrans were used as acceptor molecules by both the α and β enzyme preparations, and that water-soluble glucans synthesized by β could be converted into water-insoluble glucans by α. It is proposed that the structural heterogeneity of the native glucans produced from sucrose by S. mutans is a result of the concerted action of glucan-forming dextransucrases and endohydrolytic dextranase activity.
Full text
PDF





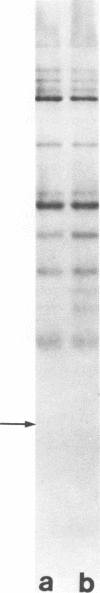
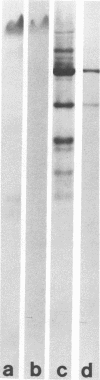
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunk C. F., Hanawalt P. C. Glycogen satellite bands in isopycnic CsCl gradients. Exp Cell Res. 1966 May;42(2):406–408. doi: 10.1016/0014-4827(66)90311-9. [DOI] [PubMed] [Google Scholar]
- Ceska M., Granath K., Norrman B., Guggenheim B. Structural and enzymatic studies on glucans synthesized with glucosyltransferases of some strains of oral streptococci. Acta Chem Scand. 1972;26(6):2223–2230. doi: 10.3891/acta.chem.scand.26-2223. [DOI] [PubMed] [Google Scholar]
- Chludzinski A. M., Germaine G. R., Schachtele C. F. Purification and properties of dextransucrase from Streptococcus mutans. J Bacteriol. 1974 Apr;118(1):1–7. doi: 10.1128/jb.118.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chludzinski A. M., Germaine G. R., Schachtele C. F. Streptoccus mutans dextransucrase: purification, properties, and requirement for primer dextran. J Dent Res. 1976 Apr;55(Spec No):C75–C86. doi: 10.1177/002203457605500329011. [DOI] [PubMed] [Google Scholar]
- Dewar M. D., Walker G. J. Metabolism of the polysaccharides of human dental plaque. I. Dextranase activity of streptococci, and the extracellular polysaccharides synthesized from sucrose. Caries Res. 1975;9(1):21–35. doi: 10.1159/000260139. [DOI] [PubMed] [Google Scholar]
- Ebert K. H., Brosche M. Origin of branches in native dextrans. Biopolymers. 1967 Jun;5(5):423–429. doi: 10.1002/bip.1967.360050503. [DOI] [PubMed] [Google Scholar]
- Ebisu S., Misaki A., Kato K., Kotani S. The structure of water-insoluble glucans of cariogenic Streptococcus mutans, formed in the absence and presence of dextranase. Carbohydr Res. 1974 Dec;38:374–381. doi: 10.1016/s0008-6215(00)82375-7. [DOI] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Purification and properties of dextransucrase and invertase from Streptococcus mutans. J Bacteriol. 1974 Jun;118(3):796–804. doi: 10.1128/jb.118.3.796-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Some Immunochemical Properties of Dextransucrase and Invertase from Streptococcus mutans. Infect Immun. 1974 Nov;10(5):985–990. doi: 10.1128/iai.10.5.985-990.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Chludzinski A. M., Schachtele C. F. Streptococcus mutans dextransucrase: requirement for primer dextran. J Bacteriol. 1974 Oct;120(1):287–294. doi: 10.1128/jb.120.1.287-294.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F., Chludzinski A. M. Rapid filter paper assay for the dextransucrase activity from Streptococcus mutans. J Dent Res. 1974 Nov-Dec;53(6):1355–1360. doi: 10.1177/00220345740530061101. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F. Streptococcus mutans dextransucrase: mode of interaction with high-molecular-weight dextran and role in cellular aggregation. Infect Immun. 1976 Feb;13(2):365–372. doi: 10.1128/iai.13.2.365-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J. Presence of an invertase-like enzyme and a sucrose permeation system in strains of Streptococcus mutans. Caries Res. 1972;6(2):122–131. doi: 10.1159/000259784. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., Burckhardt J. J. Isolation and properties of a dextranase from streptococcus mutans OMZ 176. Helv Odontol Acta. 1974 Oct;18(2):101–113. [PubMed] [Google Scholar]
- Hehre E. J., Genghof D. S., Okada G. The alpha-amylases as glycosylases, with wider catalytic capacities than envisioned or explained by their representation as hydrolases. Arch Biochem Biophys. 1971 Jan;142(1):382–393. doi: 10.1016/0003-9861(71)90297-9. [DOI] [PubMed] [Google Scholar]
- Hehre E. J., Okada G., Genghof D. S. Configurational specificity: unappreciated key to understanding enzymic reversions and de novo glycosidic bond synthesis. I. Reversal of hydrolysis by alpha-, beta- and glucoamylases with donors of correct anomeric form. Arch Biochem Biophys. 1969 Dec;135(1):74–89. [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of cell-associated dextransucrase activity from glucose-grown cells of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):227–235. doi: 10.1128/iai.10.1.227-235.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of extracellular glucosyltransferase activity of Steptococcus mutans. Infect Immun. 1975 Oct;12(4):738–749. doi: 10.1128/iai.12.4.738-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L. W., Edwards J. R. Detailed structure of a dextran from a cariogenic bacterium. Carbohydr Res. 1972 Sep;24(1):216–217. doi: 10.1016/s0008-6215(00)82285-5. [DOI] [PubMed] [Google Scholar]
- MARTINEZSEGOVIA Z. M., SOKOL F., GRAVES I. L., ACKERMANN W. W. SOME PROPERTIES OF NUCLEIC ACIDS EXTRACTED WITH PHENOL. Biochim Biophys Acta. 1965 Feb 8;95:329–340. doi: 10.1016/0005-2787(65)90497-1. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of the Adherence of Streptococcus mutans to Smooth Surfaces III. Purification and Properties of the Enzyme Complex Responsible for Adherence. Infect Immun. 1974 Nov;10(5):1135–1145. doi: 10.1128/iai.10.5.1135-1145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbrun E. Extracellular polysaccharides synthesized by glucosyltransferases of oral streptococci. Composition and susceptibility to hydrolysis. Caries Res. 1972;6(2):132–147. doi: 10.1159/000259785. [DOI] [PubMed] [Google Scholar]
- Nisizawa T., Imai S., Akada H., Hinoide M., Araya S. Extracellular glucans produced by oral streptococci. Arch Oral Biol. 1976;21(3):207–213. doi: 10.1016/0003-9969(76)90131-x. [DOI] [PubMed] [Google Scholar]
- Ono S., Hiromi K., Takahashi K. Calorimetric studies on hydrolysis of glucosides. I. Heats of hydrolysis of maltose and phenyl alpha-maltoside. J Biochem. 1965 Jun;57(6):799–807. doi: 10.1093/oxfordjournals.jbchem.a128147. [DOI] [PubMed] [Google Scholar]
- Richards G. N., Streamer M. Studies on dextranases. IV. Mode of action of dextranase D1 on oligosaccharides. Carbohydr Res. 1974 Feb;32(2):251–260. doi: 10.1016/s0008-6215(00)82103-5. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Harlander S. K., Germaine G. R. Streptococcus mutans dextransucrase: availability of disaggregated enzyme after growth in a chemically defined medium. Infect Immun. 1976 May;13(5):1522–1524. doi: 10.1128/iai.13.5.1522-1524.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidebotham R. L. Dextrans. Adv Carbohydr Chem Biochem. 1974;30:371–444. doi: 10.1016/s0065-2318(08)60268-1. [DOI] [PubMed] [Google Scholar]
- Sidebotham R. L., Weigel H., Bowen W. H. Studies on dextrans and destranases. IX. Dextrans elaborated by cariogenic organisms. Carbohydr Res. 1971 Sep;19(2):151–159. doi: 10.1016/s0008-6215(00)81615-8. [DOI] [PubMed] [Google Scholar]
- Staat R. H., Schachtele C. F. Evaluation of dextranase production by the cariogenic bacterium Streptococcus mutans. Infect Immun. 1974 Feb;9(2):467–469. doi: 10.1128/iai.9.2.467-469.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Yoshikawa Y., Hiromi K., Ono S. Calorimetric studies on hydrolysis of glucosides. II. Heats of hydrolysis of alpha-1,6 glucosidic linkages. J Biochem. 1965 Sep;58(3):251–254. doi: 10.1093/oxfordjournals.jbchem.a128194. [DOI] [PubMed] [Google Scholar]
- Takeshita M., Hehre E. J. The capacity of alpha-amylases to catalyze the nonhydrolytic degradation of starch and glycogen with formation of novel glycosylation products. Arch Biochem Biophys. 1975 Aug;169(2):627–637. doi: 10.1016/0003-9861(75)90207-6. [DOI] [PubMed] [Google Scholar]
- Walker G. J., Builder J. E. Metabolism of the reserve polysaccharide of Streptococcus mitis. Properties of alpha-(1-->6)-glucosidase, its separation from transglucosylase, and the action of the two enzymes on branched oligosaccharides. Biochem J. 1967 Dec;105(3):937–942. doi: 10.1042/bj1050937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. J., Dewar M. D. The action pattern of Penicillium lilacinum dextranase. Carbohydr Res. 1975 Feb;39(2):303–315. doi: 10.1016/s0008-6215(00)86140-6. [DOI] [PubMed] [Google Scholar]