The capacity of the nervous system to perceive sensory information, form new memories, navigate through space, and make decisions relies on complex communication within and between brain areas. Anatomical studies have identified the routes by which information flows within the six layers of the neocortex and between hierarchically organized cortical regions. However, the details of how cortical communication is accomplished are still unclear. Several have suggested that rhythmic neuronal activity may play a key role in interareal communication (1–4). In particular, coherence between spiking activity and the local network in the γ-frequency band (30–90 Hz) has been proposed to be a signature of feedforward communication, signaling directed from an area earlier in the cortical hierarchy to a higher-order area (5–7). By contrast, lower-frequency rhythms in both α (8–12 Hz) (7) and β (13–30 Hz) (8, 9) have been proposed to play a role in top-down or feedback communication. However, causal evidence for these rhythmic distinctions in the routing of information has been lacking. In PNAS, van Kerkoerle et al. (10) present an important advance in this research direction by directly testing the hypothesis that rhythmic activity in the α band signifies feedback processing and rhythmic activity in the γ band signifies feedforward processing.
Areas associated with visual processing encompass roughly 55% of the neocortex of the rhesus macaque brain (11), and connectivity within and between visual cortical areas is often used as the archetype of cortical interaction. Dating back to the seminal studies of Hubel and Wiesel, we have understood visual processing in the brain to occur in hierarchical stages, with a progressive increase in the complexity of the neuronal representation at each successive stage of the system. Neurons in early visual areas (V1 and V2) are selective for simple features of stimuli and are sensitive to where these stimuli are positioned within the visual field, whereas neurons in higher-order visual areas (TE and TEO) are less influenced by stimulus location and are selective for complex combinations of features that make up whole objects. Apart from these differences in physiological responses, it has been suggested that anatomical criteria could be used to identify the hierarchical stages in the cortical areas devoted to visual processing (11–13). Specifically, the neocortex typically contains six layers of neurons, with each layer made up of a distinct distribution of neuronal cell types and having distinct connections both within a particular brain region and across regions. Characteristically, ascending (feedforward) projections originate in layer 2 and arrive predominately in layer 4, with a weaker input onto layer 6 neurons. Descending (feedback) projections are thought to both originate and terminate in layers outside of layer 4, particularly layers 1, 2, and 5.
The next question, then, concerns how neurons communicate via these anatomical pathways in the service of cognition. The prevailing model of cortical communication is based on the idea that information is encoded through action potentials, and each neuron communicates by sending action potentials to each of the neurons to which it makes an axonal termination. Given the myriad potential pathways by which one neuron can connect with another, it is clear that cognition requires the flexible routing of information through the relatively stable anatomical infrastructure. This is sometimes described as the problem of selective gating: how are relevant neural inputs and pathways selected for any particular cognitive task and revised on a moment-by-moment basis? One possibility is that the precise timing of action potentials, apart from their rate, plays a critical role in effective cortical communication. Synchronization of neuronal activity in time may serve to enhance the impact of projection neurons on downstream areas, where neurons converge on a common target. This feedforward coincidence detection may involve increased temporal summation of excitatory postsynaptic potentials, resulting in an increased likelihood that downstream neurons will fire. In addition, network oscillations can align rhythmic inhibition among neuronal groups, ensuring that the interactions between groups are the strongest when their phases are well aligned with each other. Recent studies have suggested that effective communication uses coherent neuronal activity to route information (5, 6), and this idea was made explicit in the communication-through-coherence (CTC) hypothesis (1). However, whether rhythmic neuronal activity in distinct frequency ranges could serve as a marker to distinguish feedforward and feedback activity was still unclear. van Kerkoerle et al. (10) provide a comprehensive test of this hypothesis.
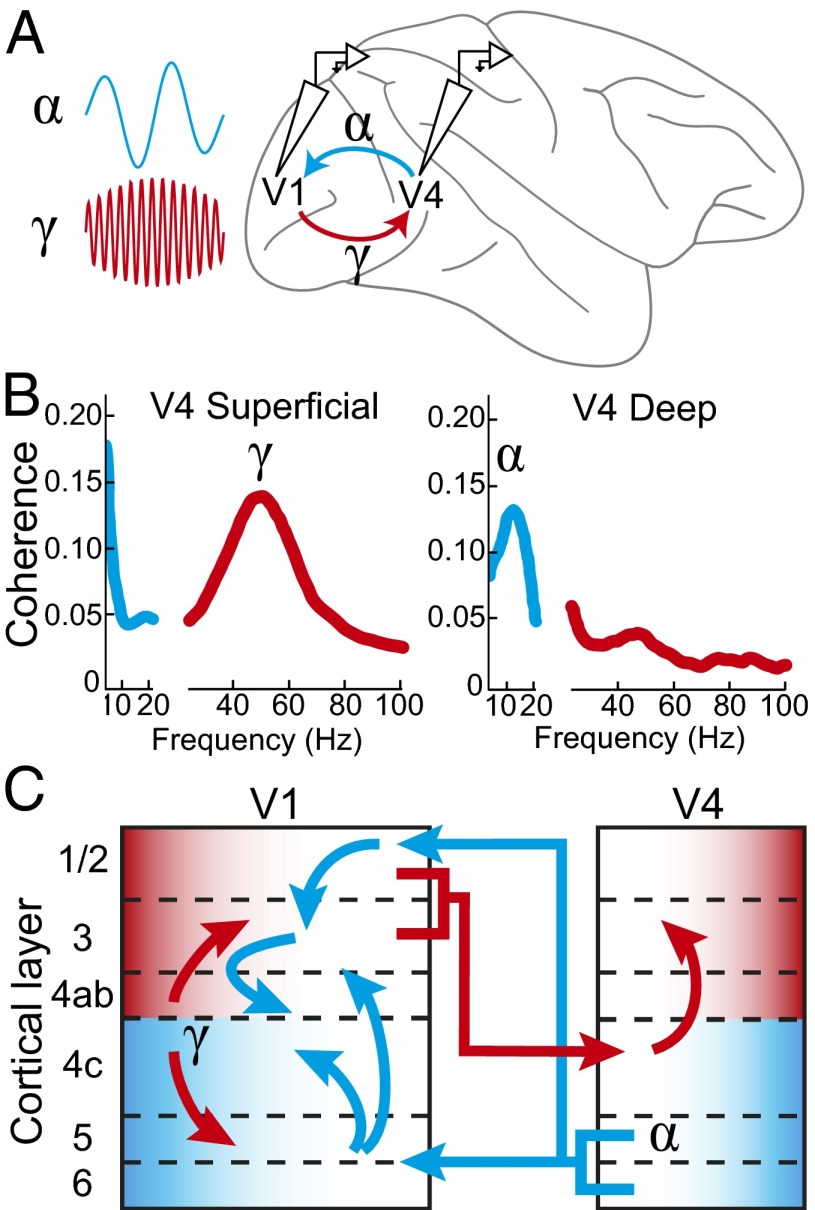
In a first set of experiments, van Kerkoerle et al. (10) take advantage of laminar electrodes arrays to examine connectivity within area V1 (Fig. 1A). Across almost 500 recording sites, the authors identify the laminar profile of cortical oscillations through analyses of multiunit neuronal activity (MUA), the local field potential (LFP), and the current source density (CSD). These analyses revealed prominent peaks in LFP power in the α and γ bands, with the α power concentrated in the deep layers of V1 (layers 5/6), and the γ power concentrated in upper layer 4 and the superficial layers (layers 2/3). These findings are consistent with previous reports of laminar differences in γ and α power (7) (Fig. 1B) and extend these previous findings by providing significantly greater spatial resolution for the localization of frequency bands to specific cortical layers. This enhanced spatial resolution through the use of a laminar electrode, with fine spacing between the contact points, allowed for computation of the CSD, which revealed the temporal and spatial profile of these oscillations. Using several convergent analyses, van Kerkoerle et al. demonstrate that α oscillatory activity is directed from the superficial and deep layers toward layer 4 and that the opposite pattern exists for γ-band oscillations, in which activity is directed from layer 4 out to the superficial and deep layers. The authors then extend this finding to identify the role of rhythmic oscillations in coupling between cortical regions with simultaneous recordings from areas V1 and V4. Here, they observed directionality in the interactions from V4 toward V1 for the α rhythm, supporting the idea that α is a signature of feedback processing. By contrast, the directionality from V1 to V4 was stronger for γ, supporting the hypothesis that γ oscillations provide a marker for feedforward processing (Fig. 1C). Additional experiments investigating the interareal effects of microstimulation on rhythmicity in V1 and V4 and experiments using pharmacological techniques to preferentially block feedforward or feedback neural activity provide further support for their main conclusions. Together, these findings provide strong experimental evidence that rhythmic neuronal activity in the α- and γ-frequency bands can serve as markers for feedback and feedforward cortical communication, respectively.
Fig. 1.
Circuitry of alpha and gamma rhythms in visual cortex. (A) van Kerkoerle et al. simultaneously recorded multiunit activity and field potentials from regions with overlapping receptive fields in V1 and V4. A feedforward γ rhythm (40–90 Hz; V1–V4) and feedback α rhythm (8–12 Hz; V4–V1) were identified. (B) γ rhythms in superficial layers of V4 and α rhythms in deep layers of V4 have previously been identified (7). That finding is depicted here with plots of intralaminar coherence vs. frequency. (C) Through Granger causality analysis, γ rhythms (red arrows) were found to originate in layer 4 of V1 and spread to superficial and deep layers of V1. The γ rhythm then was directed from superficial layers of V1–V4. Feedback α rhythms from the deep layers of V4 were directed toward deep and superficial layers of V1 (blue arrows). Dominant field potential power is represented by laminar shading (red for γ and blue for α).
Although these results advance our understanding of interaction within and between cortical areas, they also raise important questions for further research. A significant body of previous work has identified β-band activity as a mechanism for top-down signaling (2).
van Kerkoerle et al. suggest that both γ and α frequencies can be used for long-range communication, and they differ only in the directionality of that communication.
Similar to the findings in van Kerkoerle et al. (10), this β-band feedback signal is found predominantly in the deep layers (14) and is thought to influence γ power in the superficial layers (9). Although β-band activity has classically been associated with motor functions (15), there are wide-ranging hypothesis about the function of this rhythm (16), and the distinct roles of α- and β-band activity are currently unclear. Other work has suggested that synchronization in higher (γ) frequencies might be more efficient for local communication, whereas synchronization in lower (α, β) frequencies might better support long-range communication. This notion is supported by the idea that the lower frequencies are better adapted to synchronization when there are long conduction delays, as is required for communication across distant brain regions (17). However, the results presented in van Kerkoerle et al. suggest that both γ and α frequencies can be used for long-range communication, and they differ only in the directionality of that communication. A potential resolution to this apparent conflict comes from research demonstrating that within the γ band, activity at specific frequencies may differentially affect cognition. Although γ likely depends on local network properties (18), Roberts et al. (6) recently demonstrated that contrast-induced differences in the peak γ frequency, on the order of 18–45 Hz, are coherent across visual areas V1 and V2, thus providing support for the idea that coherent fluctuations in the γ rhythm can support long-range communication and that neural communication through coherence does not depend on a fixed γ frequency (1).
In summary, van Kerkoerle et al. provide a comprehensive examination of the hypothesis that feedforward and feedback communication are accomplished via coherent neural activity in distinct frequency bands. Although these findings advance our understanding of cortical communication and highlight the importance of a temporal code, they are fully compatible with the idea that changes in the rates at which neurons fire action potentials also affect information transfer. What is apparent is that we can best advance our understanding of cortical communication and information transfer through a focus on both laminar and multiarea interactions.
Footnotes
The authors declare no conflict of interest.
See companion article on page 14332.
References
- 1.Fries P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Cannon J, et al. Neurosystems: Brain rhythms and cognitive processing. Eur J Neurosci. 2014;39(5):705–719. doi: 10.1111/ejn.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colgin LL, et al. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462(7271):353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- 4.Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14(11):506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosman CA, et al. Attentional stimulus selection through selective synchronization between monkey visual areas. Neuron. 2012;75(5):875–888. doi: 10.1016/j.neuron.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts MJ, et al. Robust gamma coherence between macaque V1 and V2 by dynamic frequency matching. Neuron. 2013;78(3):523–536. doi: 10.1016/j.neuron.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Buffalo EA, Fries P, Landman R, Buschman TJ, Desimone R. Laminar differences in gamma and alpha coherence in the ventral stream. Proc Natl Acad Sci USA. 2011;108(27):11262–11267. doi: 10.1073/pnas.1011284108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315(5820):1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Whittington MA, Kopell NJ. Top-down beta rhythms support selective attention via interlaminar interaction: A model. PLOS Comput Biol. 2013;9(8):e1003164. doi: 10.1371/journal.pcbi.1003164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Kerkoerle T, et al. Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex. Proc Natl Acad Sci USA. 2014;111:14332–14341. doi: 10.1073/pnas.1402773111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1(1):1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 12.Maunsell JH, van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 1983;3(12):2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rockland KS, Pandya DN. Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res. 1979;179(1):3–20. doi: 10.1016/0006-8993(79)90485-2. [DOI] [PubMed] [Google Scholar]
- 14.Roopun AK, et al. A beta2-frequency (20-30 Hz) oscillation in nonsynaptic networks of somatosensory cortex. Proc Natl Acad Sci USA. 2006;103(42):15646–15650. doi: 10.1073/pnas.0607443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker SN. Oscillatory interactions between sensorimotor cortex and the periphery. Curr Opin Neurobiol. 2007;17(6):649–655. doi: 10.1016/j.conb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engel AK, Fries P. Beta-band oscillations—signalling the status quo? Curr Opin Neurobiol. 2010;20(2):156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci USA. 2000;97(4):1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buia C, Tiesinga P. Attentional modulation of firing rate and synchrony in a model cortical network. J Comput Neurosci. 2006;20(3):247–264. doi: 10.1007/s10827-006-6358-0. [DOI] [PubMed] [Google Scholar]