Abstract
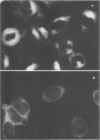
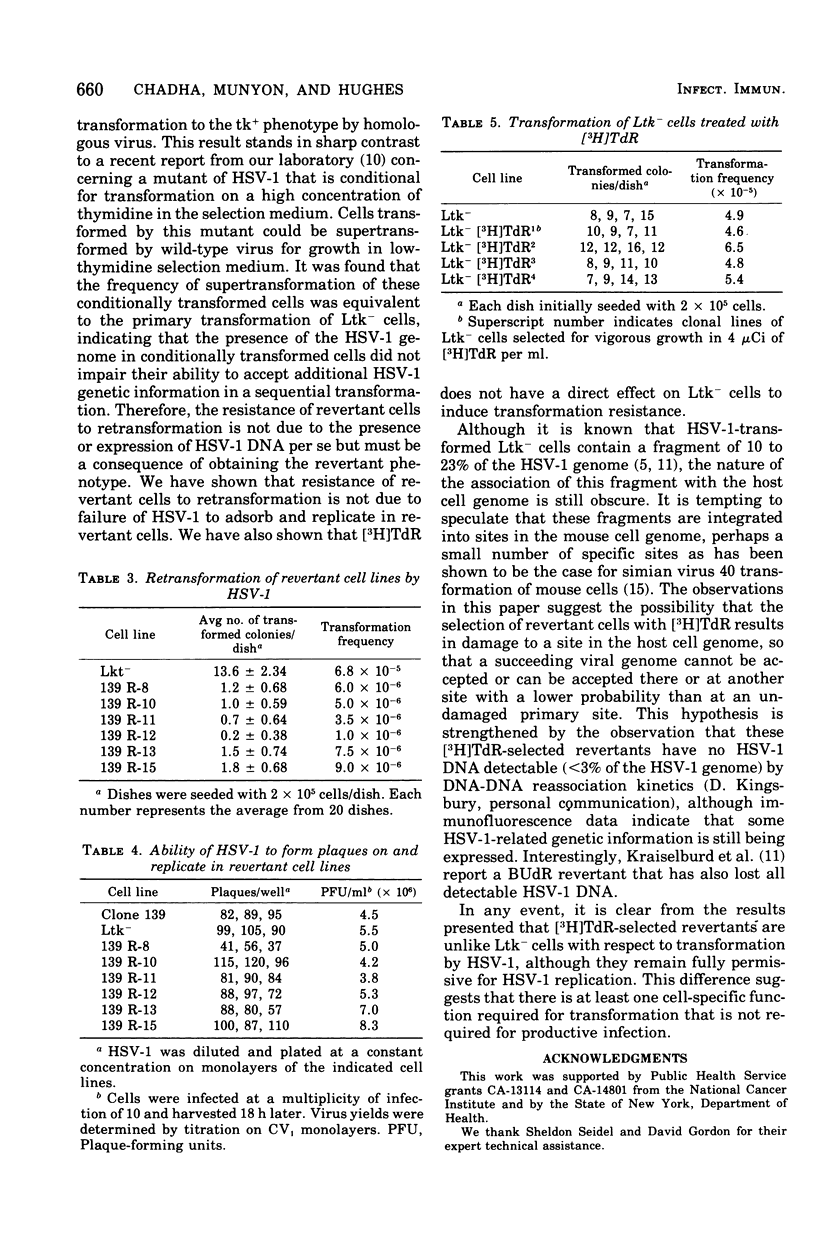
Mouse L cells lacking thymidine kinase (Ltk-) that had been transformed to the thymidine kinase-positive (tk+) phenotype by herpes simplex virus type 1 (HSV-1) were cultured in medium containing tritiated thymidine. Six clonal lines of cells surviving this treatment were found to have the following properties: (i) the cells were tk- and had spontaneous back-reversion frequencies to the tk+ phenotype of 10(-5) or less, (ii) the cells contained HSV antigens, although in lesser amounts than in the parental transformed cells, and (iii) the cells were retransformable to the tk+ phenotype by HSV-1 at frequencies of about 1 to 13% of the frequency of the primary transformation of LtK- cells. HSV-1 plaqued as efficiently on monolayers of these cells and replicated in them to the same extent as it did in Ltk- cells. These results indicate that HSV-1-transformed L cells surviving selection with tritiated thymidine are unlike the parental Ltk- cells in that they are damaged in such a way that the cells are resistant to retransformation by homologous virus, although they remain fully permissive for virus replication.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Clayton D. A. A genetically distinct thymidine kinase in mammalian mitochondria. Exclusive labeling of mitochondrial deoxyribonucleic acid. J Biol Chem. 1973 Apr 25;248(8):2722–2729. [PubMed] [Google Scholar]
- Breslow R. E., Goldsby R. A. Isolation and characterization of thymidine transport mutants of Chinese hamster cells. Exp Cell Res. 1969 Jun;55(3):339–346. doi: 10.1016/0014-4827(69)90567-9. [DOI] [PubMed] [Google Scholar]
- Chadha K. C., Munyon W. H., Zeigel R. F. Expression of type-specific virion structural antigen(s) in L cells transformed by herpes simplex virus types 1 and 2. Virology. 1977 Jan;76(1):433–436. doi: 10.1016/0042-6822(77)90317-8. [DOI] [PubMed] [Google Scholar]
- Chadha K. C., Munyon W. Presence of herpes simplex virus-related antigens in transformed L cells. J Virol. 1975 Jun;15(6):1475–1486. doi: 10.1128/jvi.15.6.1475-1486.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. L., Adelstein S. J., Oxman M. N. Herpes simplex virus as a source of thymidine kinase for thymidine kinase-deficient mouse cells: suppression and reactivation of the viral enzyme. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1912–1916. doi: 10.1073/pnas.70.7.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. B., Kingsbury D. T. Quantitation of the viral DNA present in cells transformed by UV-irradiated herpes simplex virus. J Virol. 1976 Mar;17(3):788–793. doi: 10.1128/jvi.17.3.788-793.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. B., Munyon W., Buchsbaum R., Chawda R. Virus type-specific thymidine kinase in cells biochemically transformed by herpes simplex virus types 1 and 2. J Virol. 1974 Jan;13(1):140–145. doi: 10.1128/jvi.13.1.140-145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff R., Rapp F. Properties of hamster embryo fibroblasts transformed in vitro after exposure to ultraviolet-irradiated herpes simplex virus type 2. J Virol. 1971 Oct;8(4):469–477. doi: 10.1128/jvi.8.4.469-477.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Locker H., Cox B., Roizman B., Rapp F. Herpes simplex virus DNA in transformed cells: sequence complexity in five hamster cell lines and one derived hamster tumor. J Virol. 1976 Jun;18(3):885–893. doi: 10.1128/jvi.18.3.885-893.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. G., Munyon W. H. Mutant of herpes simplex virus type 1 conditionally able to transform thymidine kinaseless L cells to a tk+ phenotype. J Virol. 1976 Jun;18(3):867–872. doi: 10.1128/jvi.18.3.867-872.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraiselburd E., Gage L. P., Weissbach A. Presence of a herpes simplex virus DNA fragment in an L cell clone obtained after infection with irradiated herpes simplex virus I. J Mol Biol. 1975 Oct 5;97(4):533–542. doi: 10.1016/s0022-2836(75)80057-x. [DOI] [PubMed] [Google Scholar]
- Lin S. S., Munyon W. Expression of the viral thymidine kinase gene in herpes simplex virus-transformed L cells. J Virol. 1974 Nov;14(5):1199–1208. doi: 10.1128/jvi.14.5.1199-1208.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon W., Buchsbaum R., Paoletti E., Mann J., Kraiselburd E., Davis D. Electrophoresis of thymidine kinase activity synthesized by cells transformed by herpes simplex virus. Virology. 1972 Sep;49(3):683–689. doi: 10.1016/0042-6822(72)90525-9. [DOI] [PubMed] [Google Scholar]
- Munyon W., Kraiselburd E., Davis D., Mann J. Transfer of thymidine kinase to thymidine kinaseless L cells by infection with ultraviolet-irradiated herpes simplex virus. J Virol. 1971 Jun;7(6):813–820. doi: 10.1128/jvi.7.6.813-820.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad I., Zouzias D., Basilico C. Simian virus 40 integration sites in the genome of virus-transformed mouse cells. J Virol. 1975 Oct;16(4):897–904. doi: 10.1128/jvi.16.4.897-904.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thouless M. E., Chadha K. C., Munyon W. H. Serological specificity of thymidine kinase activity in herpes simplex virus-transformed L cells. Virology. 1976 Jan;69(1):350–351. doi: 10.1016/0042-6822(76)90225-7. [DOI] [PubMed] [Google Scholar]