Abstract
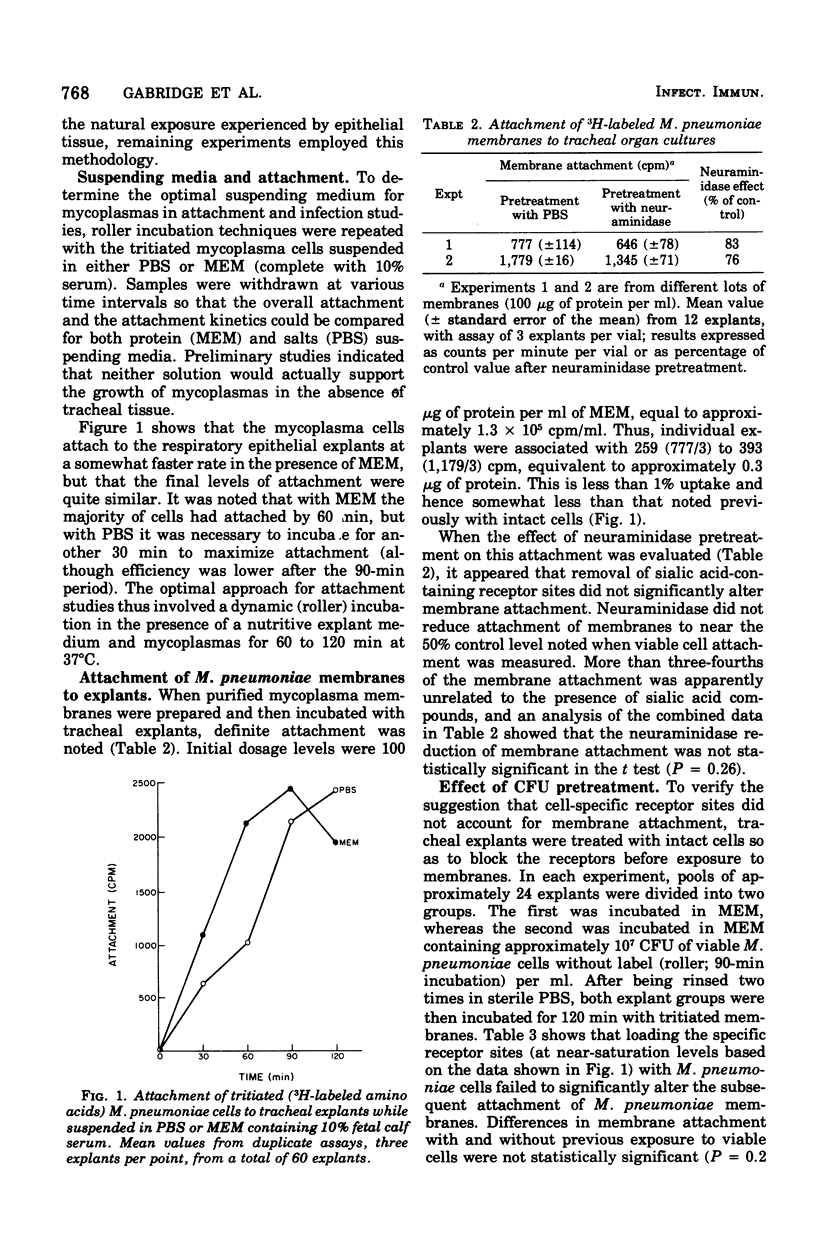
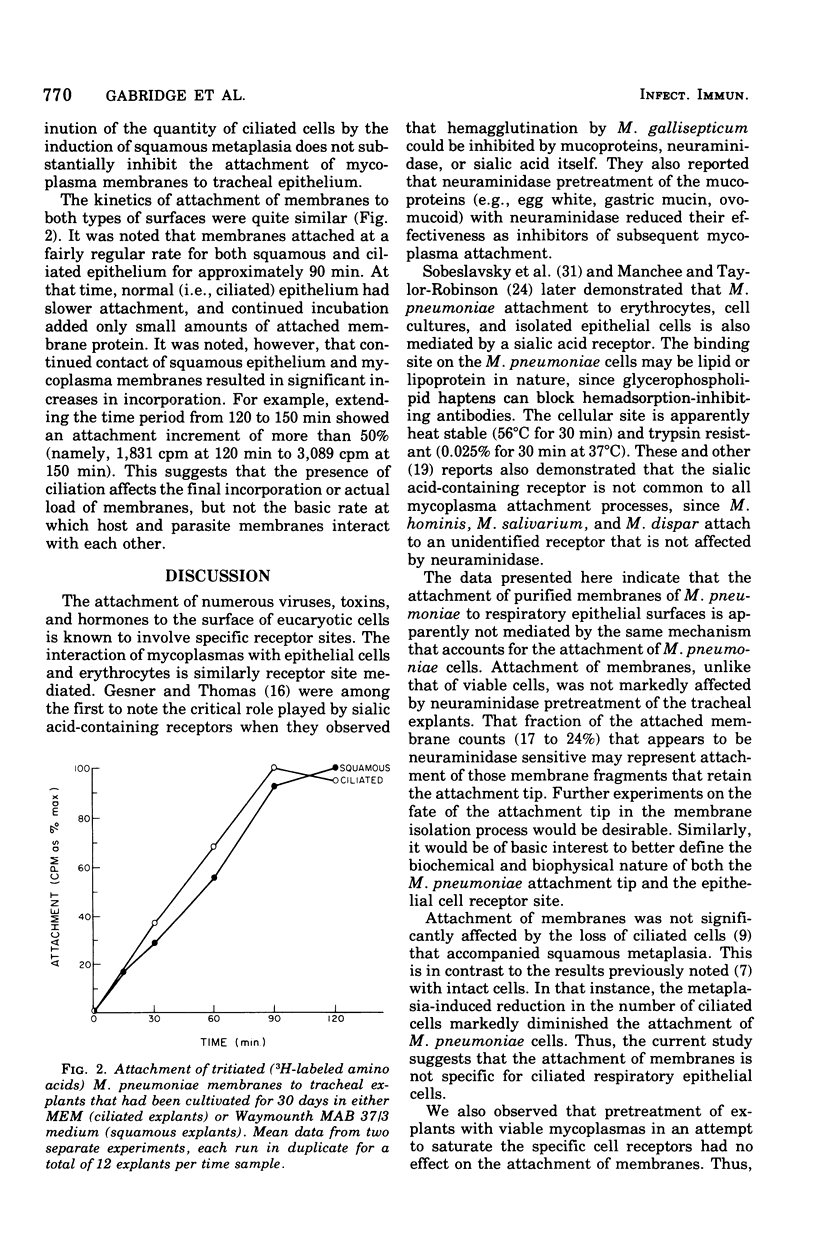
Hamster trachea organ cultures were exposed to isolated membranes of Mycoplasma pneumoniae, PI 1428. Attachment, monitored by the uptake of tritiated membranes, was relatively insensitive to neuraminidase pretreatment, unlike the attachment of viable cells. Membrane attachment was optimal when explants were incubated with 50 to 100 micrograms of membrane protein per ml in minimal essential medium broth while gently being rotated (1 rpm) in a roller apparatus for 90 to 120 min at 37 degrees C. Saturation of the receptor sites with viable cells failed to inhibit subsequent membrane attachment. Induction of squamous metaplasia by extended cultivation of tracheal explants in a vitamin A-free medium reduced the content of ciliated cells without significantly affecting total cell viability, but did not alter the attachment of M. pneumoniae membranes. Collectively, the data indicate that the mechanism of attachment of M. pneumoniae membranes to respiratory epithelium is distinct from the receptor site-mediated attachment of M. pneumoniae cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., McClure W. O. An improved scintillation cocktail of high-solubilizing power. Anal Biochem. 1973 Jan;51(1):173–179. doi: 10.1016/0003-2697(73)90465-x. [DOI] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A. Relationships Between Mycoplasma pneumoniae and Human Respiratory Epithelium. Infect Immun. 1971 May;3(5):694–701. doi: 10.1128/iai.3.5.694-701.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS G. A., FOGH J. Fine structure of pleuropneumonia-like organisms in pure culture and in infected tissue culture cells. J Bacteriol. 1960 Feb;79:267–276. doi: 10.1128/jb.79.2.267-276.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt J. A., Gabridge M. G. Effect of squamous metaplasia on infection of hamster trachea organ cultures with Mycoplasma pneumoniae. Infect Immun. 1977 Feb;15(2):647–655. doi: 10.1128/iai.15.2.647-655.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Agee C. C., Cameron A. M. Differential distribution of ciliated epithelial cells in the trachea of hamsters: implications for studies of pathogenesis. J Infect Dis. 1977 Jan;135(1):9–19. doi: 10.1093/infdis/135.1.9. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Johnson C. K., Cameron A. M. Cytotoxicity of Mycoplasma pneumoniae Membranes. Infect Immun. 1974 Nov;10(5):1127–1134. doi: 10.1128/iai.10.5.1127-1134.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Murphy W. H. Toxic membrane fractions from Mycoplasma fermentans. Infect Immun. 1971 Dec;4(6):678–682. doi: 10.1128/iai.4.6.678-682.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Polisky R. B. Quantitative reduction of 2,3,4-triphenyl tetrazolium chloride by hamster trachea organ cultures: effects of Mycoplasma pneumoniae cells and membranes. Infect Immun. 1976 Jan;13(1):84–91. doi: 10.1128/iai.13.1.84-91.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Schneider P. R. Cytotoxic effect of Mycoplasma fermentans on mouse thymocytes. Infect Immun. 1975 Mar;11(3):460–465. doi: 10.1128/iai.11.3.460-465.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Singer S. E., Esposito R. A. Cultivation of mycoplasmas in a modified tissue culture medium. Appl Environ Microbiol. 1976 Jun;31(6):986–989. doi: 10.1128/aem.31.6.986-989.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Singer S. E., Esposito R. A. Gradient, polyacrylamide gel electrophoresis of proteins from cytotoxic mycoplasma membranes. Biochem Biophys Res Commun. 1976 May 3;70(1):271–279. doi: 10.1016/0006-291x(76)91138-4. [DOI] [PubMed] [Google Scholar]
- Gesner B., Thomas L. Sialic acid binding sites: role in hemagglutination by Mycoplasma gallisepticum. Science. 1966 Feb 4;151(3710):590–591. doi: 10.1126/science.151.3710.590. [DOI] [PubMed] [Google Scholar]
- Grant C. W., McConnell H. M. Fusion of phospholipid vesicles with viable Acholeplasma laidlawii. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1238–1240. doi: 10.1073/pnas.70.4.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMMELER K., ARMSTRONG D., TOMASSINI N. CYTOPATHOGENIC MYCOPLASMAS ASSOCIATED WITH TWO HUMAN TUMORS. II. MORPHOLOGICAL ASPECTS. J Bacteriol. 1965 Aug;90:511–516. doi: 10.1128/jb.90.2.511-516.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges G. R., Fass R. J., Saslaw S. Central nervous system disease associated with Mycoplasma pneumoniae infection. Arch Intern Med. 1972 Aug;130(2):277–282. [PubMed] [Google Scholar]
- Hodges G. R., Perkins R. L. Landry-Guillain-Barré syndrome associated with Mycoplasma pneumoniae infection. JAMA. 1969 Dec 15;210(11):2088–2090. [PubMed] [Google Scholar]
- Howard C. J., Gourlay R. N., Collins J. Serological comparison and haemagglutinating activity of Mycoplasma dispar. J Hyg (Lond) 1974 Dec;73(3):457–466. doi: 10.1017/s0022172400042820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Interaction of virulent Mycoplasma pneumoniae with hamster tracheal organ cultures. Infect Immun. 1976 Jul;14(1):217–224. doi: 10.1128/iai.14.1.217-224.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Studies on the nature of receptors involved in attachment of tissue culture cells to mycoplasmas. Br J Exp Pathol. 1969 Feb;50(1):66–75. [PMC free article] [PubMed] [Google Scholar]
- Okada H., Cyong J. C. Generation of cytotoxic lipid substance in cell-mediated cytotoxicity. Jpn J Exp Med. 1975 Dec;45(6):533–534. [PubMed] [Google Scholar]
- Organick A. B., Siegesmund K. A., Lutsky I. I. Pneumonia due to mycoplasma in gnotobiotic mice. II. Localization of Mycoplasma pulmonis in the lungs of infected gnotobiotic mice by electron microscopy. J Bacteriol. 1966 Oct;92(4):1164–1176. doi: 10.1128/jb.92.4.1164-1176.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piercy D. W. Synovitis induced by intra-articular inoculation of inactivated Mycoplasma mycoides in calves. J Comp Pathol. 1970 Oct;80(4):549–558. doi: 10.1016/0021-9975(70)90052-6. [DOI] [PubMed] [Google Scholar]
- Pollack J. D., Razin S., Cleverdon R. C. Localization of Enzymes in Mycoplasma. J Bacteriol. 1965 Sep;90(3):617–622. doi: 10.1128/jb.90.3.617-622.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D. A., Hu P. C., Wilson M., Collier A. M., Baseman J. B. Attachment of Mycoplasma pneumoniae to respiratory epithelium. Infect Immun. 1976 Mar;13(3):959–966. doi: 10.1128/iai.13.3.959-966.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobeslavsky O., Prescott B., Chanock R. M. Adsorption of Mycoplasma pneumoniae to neuraminic acid receptors of various cells and possible role in virulence. J Bacteriol. 1968 Sep;96(3):695–705. doi: 10.1128/jb.96.3.695-705.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalheim O. H., Proctor S. J., Gallagher J. E. Growth and effects of ureaplasmas (T mycoplasmas) in bovine oviductal organ cultures. Infect Immun. 1976 Mar;13(3):915–925. doi: 10.1128/iai.13.3.915-925.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuber M., Bader J. Action of polymyxin B on bacterial membranes: phosphatidylglycerol- and cardiolipin-induced susceptibility to polymyxin B in Acholeplasma laidlawii B. Antimicrob Agents Chemother. 1976 Jan;9(1):26–35. doi: 10.1128/aac.9.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D., Davidson M., Thomas L. The interaction of mycoplasmas with mammalian cells. I. HeLa cells, neutrophils, and eosinophils. J Exp Med. 1966 Sep 1;124(3):521–532. doi: 10.1084/jem.124.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]


