Significance
Age-related muscle weakness has major adverse consequences on quality of life, increasing the risk of falls, fractures, and movement impairments. Albeit an increased oxidative state has been shown to contribute to age-dependent reduction in skeletal muscle function, little is known about the mechanisms connecting oxidation and muscle weakness. We show here that genetically enhancing mitochondrial antioxidant activity causes improved skeletal muscle function and voluntary exercise in aged mice. Our findings have broad implications for both the aging and muscle physiology fields, as we present an important molecular mechanism for muscle weakness in aging and skeletal muscle force regulation.
Keywords: aging, skeletal muscle, exercise capacity, muscle weakness, oxidation
Abstract
Age-related skeletal muscle dysfunction is a leading cause of morbidity that affects up to half the population aged 80 or greater. Here we tested the effects of increased mitochondrial antioxidant activity on age-dependent skeletal muscle dysfunction using transgenic mice with targeted overexpression of the human catalase gene to mitochondria (MCat mice). Aged MCat mice exhibited improved voluntary exercise, increased skeletal muscle specific force and tetanic Ca2+ transients, decreased intracellular Ca2+ leak and increased sarcoplasmic reticulum (SR) Ca2+ load compared with age-matched wild type (WT) littermates. Furthermore, ryanodine receptor 1 (the sarcoplasmic reticulum Ca2+ release channel required for skeletal muscle contraction; RyR1) from aged MCat mice was less oxidized, depleted of the channel stabilizing subunit, calstabin1, and displayed increased single channel open probability (Po). Overall, these data indicate a direct role for mitochondrial free radicals in promoting the pathological intracellular Ca2+ leak that underlies age-dependent loss of skeletal muscle function. This study harbors implications for the development of novel therapeutic strategies, including mitochondria-targeted antioxidants for treatment of mitochondrial myopathies and other healthspan-limiting disorders.
Age-dependent muscle weakness is a leading cause of morbidity due to frailty, loss of independence, and physical disability that is associated with increased risk of falls and fractures (1, 2). In geriatric populations age-dependent muscle weakness, characterized both by loss of lean muscle mass (sarcopenia) and reduced skeletal muscle function (3–5), has been estimated to affect 30–50% of 80-y-olds (1, 2, 4).
The ‘free radical theory’ of aging, first proposed in 1956 by Harman (6), states that an underlying mechanism of age-dependent pathology is the accumulation of partially reduced forms of oxygen (7, 8), collectively known as reactive oxygen species (ROS). Mitochondria are a major source of cellular ROS (7, 9) and have been proposed to play a key role in age-dependent loss of skeletal muscle function (3, 7, 10), likely through the production of oxidative damage (11, 12). However, the molecular mechanisms underlying this process have not been fully determined.
Skeletal muscle contraction is dependent upon release of intracellular Ca2+ via the sarcoplasmic reticulum (SR) Ca2+ release channel, ryanodine receptor 1 (RyR1). Following membrane depolarization, voltage-sensing Ca2+ channels in the transverse tubules (Cav1.1) activate RyR1 and the ensuing rise in cytoplasmic [Ca2+] causes muscle contraction via the actin-myosin cross bridge cycle (13). The RyR1 is a homotetrameric protein complex composed of four monomers, kinases, a phosphatase (PP1), phosphodiesterase (PDE4D3), calmodulin, and the RyR1 channel-stabilizing subunit calstabin1 (FK506 binding protein 12, FKBP12) (14). Posttranslational modifications of the channel, including oxidation, cysteine-nitrosylation, and cAMP-dependent protein kinase A-mediated phosphorylation have been linked to impaired Ca2+ handling and perturbed contractility in chronic muscle fatigue, heart failure and muscular dystrophy (13–15). Furthermore, we have recently reported that both oxidation of RyR1 and the subsequent intracellular Ca2+ leak underlie the age-dependent reduction in skeletal muscle specific force (10). Acute induction of RyR1-mediated SR Ca2+ leak with rapamycin, which competes the channel-stabilizing subunit, calstabin1, off from RyR1 (14, 16), resulted in defective mitochondrial function associated with elevated free radical production (10). However, the role of mitochondrial ROS in age-dependent reduction in skeletal muscle function and exercise capacity has not been elucidated.
Recently, there have been numerous efforts to study mitochondria-derived free radicals in health and lifespan by experimentally expressing catalase, which catalyzes the decomposition of hydrogen peroxide to water and oxygen, in the mitochondria. This has been done using in vitro models (17), adeno-associate viral vectors (AAV) (18), and most recently by genetically engineering its overexpression in mice (19). These transgenic mice, MCat mice, in which the human catalase is targeted to and overexpressed in mitochondria, display a 10–20% increase in maximum and median lifespan (19), reduced age-related insulin resistance (20), and attenuated energy imbalance.
Because mitochondrial targeted overexpression of catalase results in reduced mitochondrial ROS (19, 20), we used the MCat mouse model to investigate the relationship between antioxidant activity and skeletal muscle aging and subsequent functional decline. Aged MCat mice displayed improved voluntary exercise, increased skeletal muscle specific force, increased tetanic Ca2+ transients, reduced intracellular Ca2+ leak and increased SR Ca2+ load compared with age-matched wild-type (WT) littermates. RyR1 channels from aged MCat mice were less oxidized, depleted of calstabin1 and exhibited increased single channel open probability (Po). Furthermore, pharmacological application of an antioxidant to aged WT RyR1 reduced SR Ca2+ leak. We have therefore identified mitochondria as a source of ROS involved in the RyR1 oxidation underlying age-associated skeletal muscle dysfunction.
Results
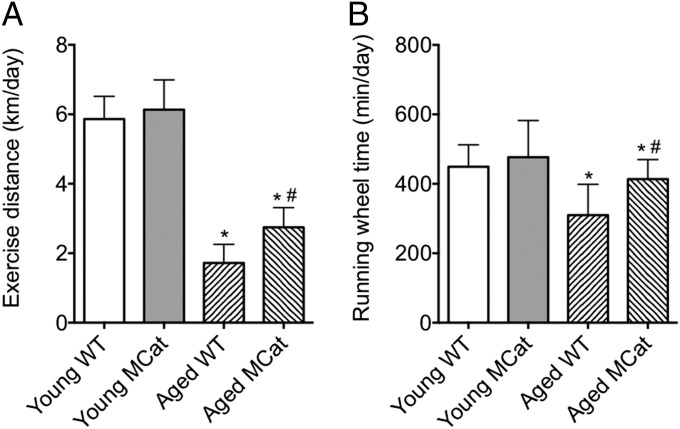
Six-month-old and 24-mo-old MCat and WT littermates were housed individually for 3 wk in cages equipped with running wheels, and voluntary running performance was recorded. Aged MCat mice exhibited significantly increased running distance relative to age-matched WT mice (Fig. 1A). This finding correlated with increased time spent on running wheels (Fig. 1B).
Fig. 1.
Improved exercise capacity in aged MCat mice. Mice were housed in individual cages equipped with running wheels for three weeks. Exercise distance (A) and running wheel time (B) were recorded. Data are mean ± SEM (*P < 0.01 vs. young WT; #P < 0.05 vs. aged WT; n: young WT = 7, young MCat = 8, aged WT = 8, aged MCat = 8, ANOVA).
To better characterize MCat mice versus WT controls, we performed Masson’s trichrome staining on the tibialis anterior muscle. There was no significant difference in the amount of muscle fibrosis when comparing age-matched MCat vs. WT littermates (Fig. S1 A and B), nor was there a difference in muscle cross-sectional area (Fig. S1C). Interestingly, extensor digitorum longus (EDL) muscle weight was lower in aged MCat than in age-matched WT littermates (Fig. S1D).
Because MCat mice overexpress human catalase in their mitochondria, mitochondrial integrity was analyzed with electron microscopy. EDL muscle from 24-mo-old WT mice exhibited a significant decrease in cristae density relative to young WT mice (Fig. S2 A and B). Such a decrease was not observed in aged MCat mice, indicating healthier mitochondria in these mice. Following this trend, mitochondrial ATP synthesis was significantly increased in skeletal muscle mitochondria from aged MCat mice relative to age-matched WT littermates (Fig. S2C). Furthermore, aged MCat flexor digitorum brevis (FDB) muscle fibers exhibited reduced mitochondrial ROS levels compared with aged WT (Fig. S2D).
To ensure that genetically enhancing mitochondrial catalase reduced oxidative stress on proteins in skeletal muscle we measured advanced oxidation protein products (AOPP). AOPP are uremic toxins created during oxidative stress through the reaction of chlorinated oxidants, including chloramines and hypochlorous acid, with proteins (21). The AOPP content of aged MCat mice was significantly lower than that of WT littermates (Fig. S2E). Consistent with these data, the oxidative stress in skeletal muscle nuclear and mitochondrial DNA has been previously reported to be significantly lower in aged (26–29 mo) MCat mice relative to aged WT mice (19). Similarly, the incidence of mitochondrial DNA deletions associated with oxidative damage is lower in aged (18–22 mo old and 33 mo old) MCat mice relative to age-matched WT littermates (19).
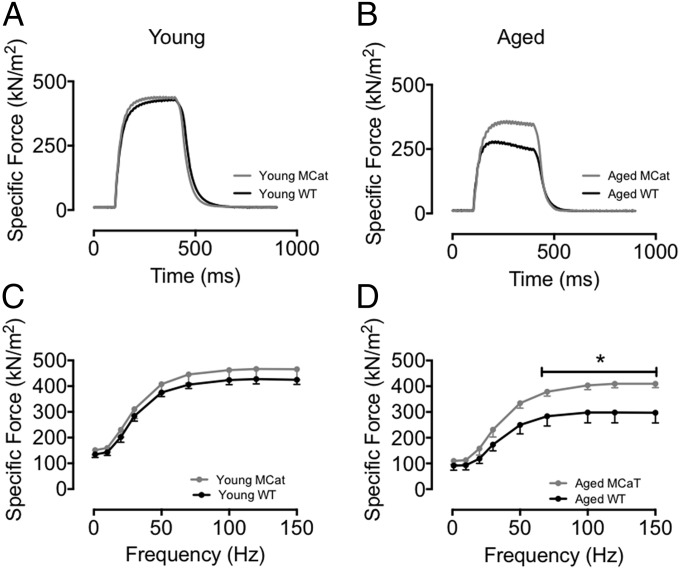
Because muscle force production is an essential determinant of exercise capacity (22), we hypothesized that this parameter would be affected by the decreased oxidative stress conferred by mitochondrial overexpression of catalase. To test the hypothesis that mitochondrial ROS contribute to age-dependent reduction in skeletal muscle force generating capacity we measured force in EDL muscles from young and aged WT and MCat mice. Isolated EDL muscles were electrically stimulated to contract and force production was measured and normalized to cross-sectional area (yielding a measure of muscle specific force; Fig. 2 A–D). There were no significant difference in specific force between young WT and MCat muscles. However, EDL muscle from aged MCat mice exhibited significantly higher specific force than muscles from WT littermates (Fig. 2 A–D).
Fig. 2.
Preserved skeletal muscle function in aged MCat mice. (A and B) Tetanic contractions (70 Hz) in isolated EDL muscles from MCat and WT littermates (force normalized to cross-sectional area). (C and D) Average specific force in EDL muscles from the same mice as in A and B. Data are mean ± SEM (n: young WT = 4, young MCat = 4, aged WT = 8; aged MCat = 7; t test was performed for each individual point: *P < 0.05 vs. aged WT).
An additional marked feature of skeletal muscle that may account for changes in exercise capacity is its susceptibility to fatigue. Measurement of EDL muscle fatigability was thus accomplished by repeatedly stimulating isolated EDL muscles to tetanic contraction and recording force. The degree of force reduction during fatigue was not different between aged WT and MCat muscles (Fig. S3 A and B). Furthermore, skeletal muscle twitch contraction was not different among these groups (Fig. S3C).
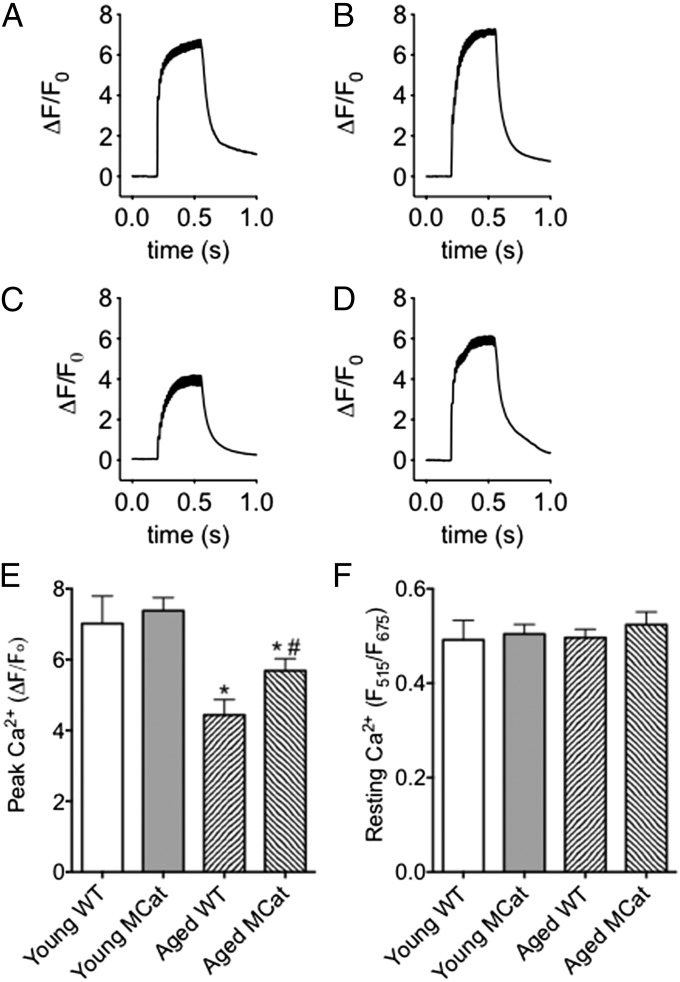
Appropriate SR Ca2+ release is essential to skeletal muscle contraction, and we thus studied tetanic Ca2+ transients in enzymatically dissociated FDB muscle fibers loaded with the fluorescent Ca2+ indicator, Fluo-4 AM. Cells were electrically stimulated to produce tetanic contractions and fluorescence was recorded. Ca2+ transients in aged WT and MCat myocytes were markedly reduced relative to young cells. However, this age-dependent reduction in Ca2+ transients was significantly improved in aged MCat myocytes (Fig. 3 A–E). These changes in Ca2+ transients were found in the absence of a significant difference in resting Ca2+. Ca2+ content was measured ratiometrically in cells simultaneously loaded with Fluo-4 and Fura-Red and paced to tetanic stimulation (Fig. S4A). These results are consistent with our in vivo and ex vivo observations on exercise performance and improved muscle function in aged MCat mice (Figs. 1 and 2).
Fig. 3.
Improved tetanic Ca2+ in skeletal muscle from aged MCat mice. (A–D) Representative traces of normalized Fluo-4 fluorescence in FDB muscle fibers during a 70 Hz tetanic stimulation in young WT (A), young MCat (B), aged WT (C), and aged MCat (D). (E) Peak Ca2+ responses in FDB fibers stimulated at 70 Hz (fibers taken from the same animals as in A–D, n = 15–21 cells from at least three mice in each group). (F) Resting cytosolic Ca2+ (measured ratiometrically). Data are mean ± SEM (*P < 0.05 vs. young WT; #P < 0.05 vs. aged WT, ANOVA).
A major event in skeletal muscle excitation-contraction coupling is Ca2+ reuptake by the SR Ca2+ ATPase 1 (SERCA1). SERCA1 pumps Ca2+ back into the SR following intracellular Ca2+ release, lowering the cytosolic [Ca2 +] to baseline levels of ∼100 nM, thereby causing relaxation. SERCA1 is tightly regulated by its redox state, and its activity is reduced in aged murine skeletal muscle (23). Thus, we hypothesized that enhanced SERCA activity mechanistically underlies the enhancement of skeletal muscle function in aged MCat muscle. However, activity of SERCA1 in aged WT skeletal muscle was not significantly different from that in aged MCat littermates (Fig. S5A). Furthermore, there was no significant difference in SERCA1 tyrosine nitration in MCat vs. age-matched WT littermates (Fig. S5 B and C). Overall SERCA1 expression in WT vs. MCat littermates was consistent throughout (Fig. S5 D and E).
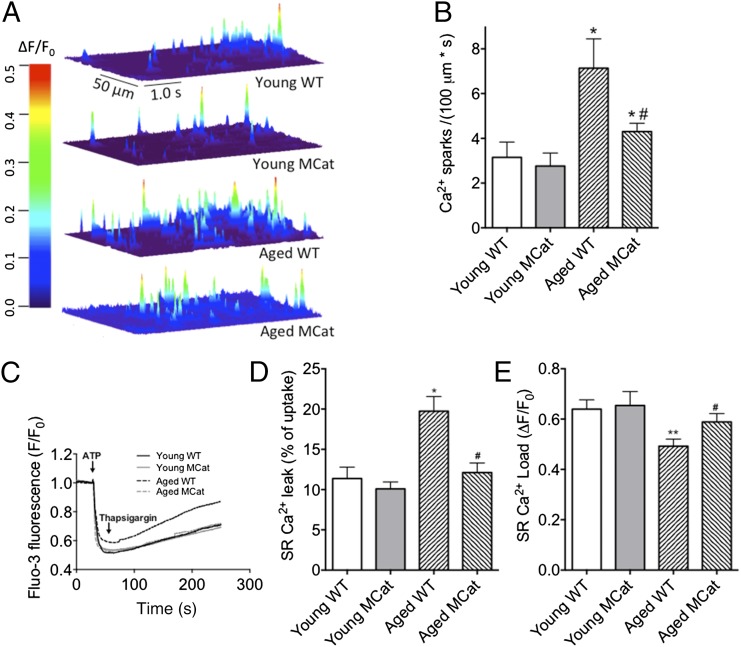
We and others have shown that SR Ca2+ leak is associated with impaired exercise capacity, defective Ca2+ handling, and dysfunctional skeletal muscle performance (15, 24). To test the hypothesis that RyR1-mediated SR Ca2+ leak is decreased in aged MCat mice, we measured Ca2+ sparks in permeabilized FDB muscles (25). We found a significant reduction in Ca2+ spark frequency in aged MCat muscles compared with WT littermates (Fig. 4 A and B). Additionally, SR Ca2+ leak was measured in skeletal muscle microsomes preloaded with Fluo-3. Energized Ca2+ load was initiated by adding 0.5 mM ATP and the time course of Ca2+ uptake was detected spectrophotometrically. After the Ca2+ uptake had reached a plateau, 1 mM thapsigargin was added to inhibit SERCA activity, and the resultant Ca2+ leak was monitored. We detected reduced SR Ca2+ leak using this alternate method of detection in SR vesicles isolated from aged MCat muscles relative to aged WT littermates (Fig. 4 C and D). Application of the RYR-specific drug, ryanodine, demonstrated RyR1 specificity (Fig. S4B).
Fig. 4.
Reduced SR Ca2+ leak and increased SR Ca2+ load in muscle from aged MCat mice. (A) Representative images of line scans of Fluo-4 fluorescence from permeabilized FDB muscle fibers showing Ca2+ spark activity. The heat diagram indicates the normalized change in fluorescence intensity (ΔF/F0). (B) Bar graph showing average Ca2+ spark frequency (n = 15–25 cells from at least three mice in each group). (C) Representative time course of Ca2+ leak from SR microsomes following Ca2+ uptake. (D) Ca2+ leak as calculated by the percentage of uptake. (E) SR Ca2+ load (measured by applying 1 mM 4-CmC). Data are mean ± SEM (*P < 0.05, **P < 0.01 vs. young WT; #P < 0.05 vs. aged WT, ANOVA).
Depletion of the SR Ca2+ store is a consequence of increased SR Ca2+ leak in aged skeletal muscle (26). Therefore, we hypothesized that reducing oxidative stress by genetically enhancing mitochondrial catalase activity would prevent this Ca2+ depletion in MCat mice. Although SR Ca2+ load was reduced in aged WT and MCat relative to their young counterparts, aged MCat muscle exhibited significantly higher SR Ca2+ load than aged WT (Fig. 4E). Thus, it is likely that the reduced SR Ca2+ leak measured in aged MCat mice (Fig. 4 A–D) results in increased SR Ca2+ load, which enhances tetanic Ca2+ (Fig. 3 A–D) and skeletal muscle force production (Fig. 2 A–D).
Preserved RyR1-calstabin1 interaction is linked to reduced SR Ca2+ leak (10, 14). Furthermore, RyR1 oxidation and cysteine nitrosylation decrease the binding affinity of calstabin1 for RyR1 (27, 28), eventually resulting in leaky channels associated with intracellular Ca2+ leak and increased Ca2+ sparks. Oxidation-dependent posttranslational modifications of RyR1 affect skeletal muscle force generating capacity and this is a key mechanism in age-dependent muscle weakness (10). We therefore examined whether age-dependent oxidative remodeling of the RyR1 macromolecular complex is reduced in MCat mice. RyR1 from aged and young EDL muscles were immunoprecipitated and immunoblotted for components of the RyR1 complex and concomitant redox modifications (10, 14). Age-dependent RyR1 oxidation and cysteine-nitrosylation were both reduced in MCat skeletal muscle, and there was more calstabin1 associated with channels from aged mutant animals compared with WT littermates (Fig. 5 A and B). Overall expression of neither RyR1 nor calstabin1 was altered in aged WT relative to aged MCat muscles (Fig. S5 D and E). The relative free thiol content was measured using the specific free thiol-labeling agent, monobromobimane (mBB), in the presence of the pharmacological antioxidant DTT (29). The free thiol content of aged MCat muscle was significantly higher than that of aged WT littermates, indicating reduced RyR1 Cys-oxidation in the aged MCat muscle (Fig. S6 A and B).
Fig. 5.
Skeletal muscle RyR1 isolated from aged MCat mice is remodeled and exhibits reduced single-channel open probability (Po). (A) Representative immunoblots from triplicate experiments of immunoprecipitated RyR1 from aged murine EDL. (B) Bar graphs showing quantification of the immunoblots in A; DNP: 2,4-dinitrophenylhydrazone. (C) Representative RyR1 single-channel current traces. Channel openings are shown as upward deflections and the closed (c-) state of the channel is indicated by horizontal bars in the beginning of each trace. Tracings from over 2 min of recording for each condition showing channel activity at two time scales (5 s in upper trace and 500 ms in lower trace) as indicated by dimension bars, and the respective Po (open probability), To (average open time), and Tc (average closed time) are shown above each trace. The activity of the channel indicated by the thick black bar is shown on the expanded time scale (the 500 ms trace below). (D) Bar graph summarizing Po at 150 nM cytosolic [Ca2+] in young WT (n = 6), aged WT (n = 5), young MCat (n = 7), and aged MCat (n = 5) channels. Data are mean ± SEM (*P < 0.05, **P < 0.01 vs. young WT, #P < 0.05, #P < 0.01 vs. aged WT, ANOVA).
Of interest, reduced RyR1 cysteine nitrosylation in an increased antioxidative environment such as that found in 2-y-old MCat muscle is consistent with the emerging evidence indicating an interplay between Ca2+ and oxidative/nitrosative stress (30). Moreover, it has been reported that reactive nitrogen species can substantially modulate catalase and other antioxidant enzymes in skeletal muscle (8, 31, 32). Thus, catalase overexpression may down-regulate cellular levels of nitroxide free radicals, thereby impacting cysteine nitrosylation of RyR1.
The relative effects of calstabin1 depletion, nitrosylation and oxidation on RyR1 activity were dissected with a ligand-binding assay using the RyR1-specific probe, ryanodine, as has been previously published (33). Preferential binding to open RyR1 provides an indirect measure of RyR1 activity (34). Treatment of skeletal SR microsomes with NOC12, a nitric oxide (NO) donor, rapamycin, and the oxidant H2O2 increased [3H]ryanodine binding, an indication that oxidation, nitrosylation and calstabin1 depletion from RyR1 each independently cause increased RyR1 activity. Incubation of nitrosylated and/or oxidized samples (35) with calstabin1 +/− the RyR stabilizing rycal drug, S107, significantly reduced RyR1 activity (Fig. S7 A–C).
To assess the single channel properties of RyR1 in its remodeled state, SR membranes were prepared from EDL muscles and fused to planar lipid membrane bilayers, and Ca2+ fluxes through RyR1 channels were recorded (10, 36). The open probability (Po) of skeletal muscle RyR1 channels from young mice was low, as expected for normal skeletal muscle RyR1 channels (Fig. 5 C and D). In contrast, skeletal muscle RyR1 channels from aged WT mice exhibited a significantly increased Po relative to those from aged MCat mice (Fig. 5 C and D).
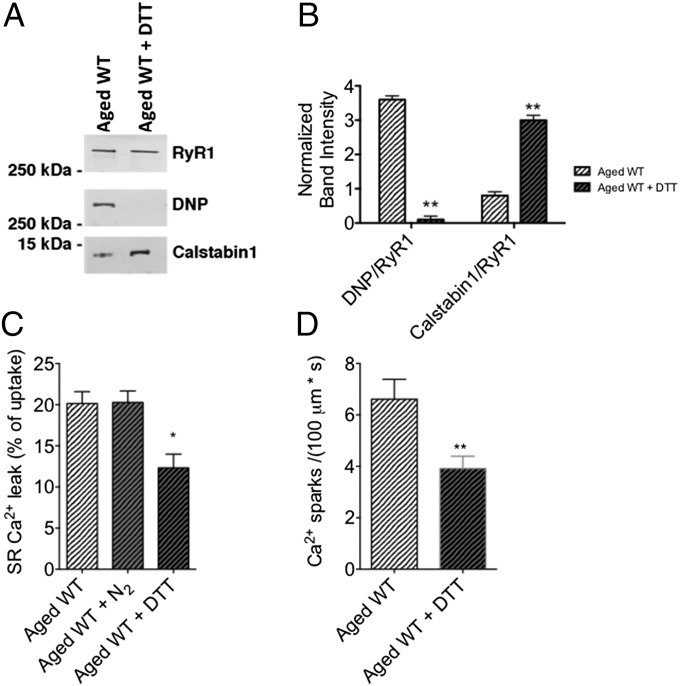
Finally, we used a pharmacological approach to demonstrate the causative role of RyR1 oxidation in the described skeletal muscle phenotype. Application of the antioxidant, DTT, to aged murine skeletal muscle caused a significant reduction in the DNP signal associated with immunoblotted RyR1 (Fig. 6 A and B). SR Ca2+ leak (Fig. 6C) and RyR1 Ca2+ sparks (Fig. 6D) were both reduced in aged WT muscle after application of DTT. Therefore, the aged MCat muscle phenotype is likely a result of the antioxidant activity of mitochondrial catalase overexpression.
Fig. 6.
Antioxidant application to aged WT skeletal muscle reduces age-associated SR Ca2+ leak. (A) Representative immunoblot of immunoprecipitated RyR1 from aged murine skeletal muscle. For DTT treatment, SR vesicles were preincubated with 1 mM DTT. (B) Bar graphs showing quantification of the immunoblots in A. (C) Bar graph representing Ca2+ leak in SR microsomes of skeletal muscles from aged WT mice. For N2 treatment, solutions was prebubbled with 100% N2 for 1 h. (D) Bar graph representing average Ca2+ spark frequency in permeabilized FDB muscle fibers from aged WT mice. Data are mean ± SEM (n = 19–22 cells from three mice per group; *P < 0.05 vs. aged WT; **P < 0.01 vs. aged WT, ANOVA).
To rule out the potential influence of oxygen tension, which has been reported to affect RyR1 function (37), we determined that pretreating microsomes with N2 gas had no significant effect on SR Ca2+ leak in aged skeletal muscle (Fig. 6C). These data are supported by a more recent study investigating the effects of pO2 on the activation of RyR1 by NO (38). Although another group found that RyR1 activity is incrementally increased from low (1%) to ambient (20%) O2, these experiments were conducted on muscle from young mice. RyR1 from aged muscle are highly oxidized (10) and thus a change from low to ambient O2 levels should not have a significant effect on the oxidation state of the already oxidized channel. Given the fact that young RyR1 activity can increase upon exposure to ambient O2 levels, the difference between young and aged RyR1 would further increase in the case of low O2 exposure (38).
Taken together, our data indicate that reducing oxidative stress by genetically enhancing mitochondrial catalase activity in skeletal muscle improves muscle function in aged mice by reducing the loss of calstabin1 from the channel complexes, thus improving channel function. This enhanced channel function results in improved tetanic Ca2+ and skeletal muscle specific force in aged mice.
Discussion
In the present study we use a genetic model with enhanced mitochondrial antioxidant activity (MCat mouse model) to investigate the effects of increased antioxidative capacity on age-dependent loss of skeletal muscle function and Ca2+ signaling. Our results indicate that MCat mice exhibit reduced age-dependent loss of muscle function. We thus provide compelling evidence for a direct role of mitochondrial free radicals in promoting the pathological intracellular Ca2+ leak that underlies age-dependent loss of skeletal muscle function.
Although it has been determined that ectopic catalase overexpression in mitochondria using AAV-9 confers enhanced treadmill performance (18), as measured by exhaustion-limited running distance, neither the underlying mechanism of this observation, nor the effects on age-dependent changes have been reported. Importantly, although RyR1 oxidation has been causally implicated in the reduction of specific force generating capacity in mammalian skeletal muscle (10), the source of these oxidative changes has not been fully established. In the present study we show that mitochondrial ROS is a functionally consequential source of these age-dependent oxidative changes to RyR1. Indeed, mitochondrial targeted overexpression of catalase improves both whole organism (exercise capacity), and skeletal muscle (specific force) performance, and prevents age-dependent reduction in Ca2+ transients, reduces age-related biochemical modifications of the SR Ca2+ release channel, and decreases SR Ca2+ leak. Furthermore, application of a pharmacological antioxidant to aged skeletal muscle reduces age-dependent SR Ca2+ leak.
A growing body of evidence indicates that RyR is tightly regulated by posttranslational modifications involving remodeling of the RyR macromolecular complex (27, 28, 39, 40). Our laboratory has previously shown that RyR1 channels are oxidized, cysteine-nitrosylated and depleted of calstabin1 in muscular dystrophy (14) and in senescence (10), and that these modifications have functional consequences on the Ca2+ release channel (15). Intriguingly, here we show that not only age-dependent RyR1 oxidation, but also cysteine nitrosylation is reduced in MCat mice. This finding is consistent with reports that uncovered the capacity of reactive nitrogen species to regulate catalase activity in skeletal muscle (31, 32). Thus, catalase overexpression may down-regulate cellular levels of nitroxide free radicals, thereby impacting cysteine nitrosylation of RyR1. The redox-specific posttranslational modifications that were attenuated in aged MCat mice were consistent with reduced RyR1-mediated SR Ca2+ leak. This is in agreement with studies in which prolonged exposure to NO donors has been shown to increase the SR Ca2+ leak and resting cytosolic Ca2+ in voltage-clamped mouse FDB fibers (41). Additionally, inhibiting RyR1-mediated SR Ca2+ leak results in rescue of age-dependent increase in spontaneous releases of SR Ca2+ (Ca2+ sparks) in permeabilized FDB muscle fibers, as shown in aged MCat muscle fibers in the present study.
We conclude that mitochondrial ROS have a causative role in mediating age-dependent redox modifications of RyR1 and consequently play a key role in the regulation of age-dependent loss of skeletal muscle function. Not only do our results have substantial translational implications for the development of novel therapeutic strategies, such as mitochondria-targeted antioxidants for treatment of mitochondrial myopathies, ROS mediated muscular dysfunctions and other healthspan limiting disorders (12, 42), we also present a molecular mechanism for age-dependent skeletal muscle weakness and regulation of musculoskeletal force generation.
Materials and Methods
See SI Materials and Methods for additional and detailed descriptions.
Ethical Approval.
The use and maintenance of mice was in accordance with Columbia University Institutional Animal Care and Use Committee regulations and with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (43).
Statistics.
In all of the experiments mice were coded to ‘blind’ investigators with respect to genotype. The sample size (n in each group) for each experiment is stated in the figure legends. Data are expressed as mean ± SE (SEM), unless otherwise indicated. To determine statistical significance, we used two-way ANOVA and comparison t test, as appropriate. Bonferroni post hoc testing was performed where applicable. Minimum statistically significant differences were established at P < 0.05.
Supplementary Material
Acknowledgments
We thank Peter S. Rabinovitch (University of Washington) for generously providing the MCat mouse founders. We also thank Bi-Xing Chen (Columbia University) for technical support. This study was supported by American Heart Association Grants AHA13POST16810041 (to G.S.) and AHA11PRE7810019 (to A.U.), by the Swedish Heart Lung Foundation (to D.C.A.), and by grants from the National Heart, Lung, and Blood Institute and from the Ellison Foundation (to A.R.M.).
Footnotes
Conflict of interest statement: A.R.M. is a consultant for ARMGO, which is targeting RyR channels for therapeutic purposes.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1412754111/-/DCSupplemental.
References
- 1.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boockvar KS, Meier DE. Palliative care for frail older adults: “There are things I can’t do anymore that I wish I could . . .”. JAMA. 2006;296(18):2245–2253. doi: 10.1001/jama.296.18.2245. [DOI] [PubMed] [Google Scholar]
- 3.Short KR, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA. 2005;102(15):5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roubenoff R, Castaneda C. Sarcopenia-understanding the dynamics of aging muscle. JAMA. 2001;286(10):1230–1231. doi: 10.1001/jama.286.10.1230. [DOI] [PubMed] [Google Scholar]
- 5.Sardu C, Marfella R, Santulli G. Impact of diabetes mellitus on the clinical response to cardiac resynchronization therapy in elderly people. J Cardiovasc Transl Res. 2014;7(3):362–368. doi: 10.1007/s12265-014-9545-9. [DOI] [PubMed] [Google Scholar]
- 6.Harman D. Aging: A theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 7.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Santulli G, Iaccarino G. Pinpointing beta adrenergic receptor in ageing pathophysiology: Victim or executioner? Evidence from crime scenes. Immun Ageing. 2013;10(1):10. doi: 10.1186/1742-4933-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin GM, Loeb LA. Ageing: Mice and mitochondria. Nature. 2004;429(6990):357–359. doi: 10.1038/429357a. [DOI] [PubMed] [Google Scholar]
- 10.Andersson DC, et al. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011;14(2):196–207. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finley LW, et al. Skeletal muscle transcriptional coactivator PGC-1α mediates mitochondrial, but not metabolic, changes during calorie restriction. Proc Natl Acad Sci USA. 2012;109(8):2931–2936. doi: 10.1073/pnas.1115813109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santulli G, Ciccarelli M, Trimarco B, Iaccarino G. Physical activity ameliorates cardiovascular health in elderly subjects: The functional role of the β adrenergic system. Front Physiol. 2013;4:209. doi: 10.3389/fphys.2013.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: Cellular mechanisms. Physiol Rev. 2008;88(1):287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 14.Bellinger AM, et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med. 2009;15(3):325–330. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellinger AM, et al. Remodeling of ryanodine receptor complex causes “leaky” channels: A molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci USA. 2008;105(6):2198–2202. doi: 10.1073/pnas.0711074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahern GP, Junankar PR, Dulhunty AF. Subconductance states in single-channel activity of skeletal muscle ryanodine receptors after removal of FKBP12. Biophys J. 1997;72(1):146–162. doi: 10.1016/S0006-3495(97)78654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai J, Rodriguez AM, Melendez JA, Cederbaum AI. Overexpression of catalase in cytosolic or mitochondrial compartment protects HepG2 cells against oxidative injury. J Biol Chem. 1999;274(37):26217–26224. doi: 10.1074/jbc.274.37.26217. [DOI] [PubMed] [Google Scholar]
- 18.Li D, et al. Ectopic catalase expression in mitochondria by adeno-associated virus enhances exercise performance in mice. PLoS ONE. 2009;4(8):e6673. doi: 10.1371/journal.pone.0006673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schriner SE, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308(5730):1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 20.Lee HY, et al. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab. 2010;12(6):668–674. doi: 10.1016/j.cmet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson DR, et al. Albumin-based microbubbles bind up-regulated scavenger receptors following vascular injury. J Biol Chem. 2010;285(52):40645–40653. doi: 10.1074/jbc.M110.134809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rantanen T, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281(6):558–560. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 23.Viner RI, Williams TD, Schöneich C. Peroxynitrite modification of protein thiols: Oxidation, nitrosylation, and S-glutathiolation of functionally important cysteine residue(s) in the sarcoplasmic reticulum Ca-ATPase. Biochemistry. 1999;38(38):12408–12415. doi: 10.1021/bi9909445. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, et al. Uncontrolled calcium sparks act as a dystrophic signal for mammalian skeletal muscle. Nat Cell Biol. 2005;7(5):525–530. doi: 10.1038/ncb1254. [DOI] [PubMed] [Google Scholar]
- 25.Isaeva EV, Shkryl VM, Shirokova N. Mitochondrial redox state and Ca2+ sparks in permeabilized mammalian skeletal muscle. J Physiol. 2005;565(Pt 3):855–872. doi: 10.1113/jphysiol.2005.086280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romero-Suarez S, et al. Muscle-specific inositide phosphatase (MIP/MTMR14) is reduced with age and its loss accelerates skeletal muscle aging process by altering calcium homeostasis. Aging (Albany, NY Online) 2010;2(8):504–513. doi: 10.18632/aging.100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aracena-Parks P, et al. Identification of cysteines involved in S-nitrosylation, S-glutathionylation, and oxidation to disulfides in ryanodine receptor type 1. J Biol Chem. 2006;281(52):40354–40368. doi: 10.1074/jbc.M600876200. [DOI] [PubMed] [Google Scholar]
- 28.Eu JP, Xu L, Stamler JS, Meissner G. Regulation of ryanodine receptors by reactive nitrogen species. Biochem Pharmacol. 1999;57(10):1079–1084. doi: 10.1016/s0006-2952(98)00360-8. [DOI] [PubMed] [Google Scholar]
- 29.Sun J, Xu L, Eu JP, Stamler JS, Meissner G. Classes of thiols that influence the activity of the skeletal muscle calcium release channel. J Biol Chem. 2001;276(19):15625–15630. doi: 10.1074/jbc.M100083200. [DOI] [PubMed] [Google Scholar]
- 30.Hidalgo C, Donoso P. Crosstalk between calcium and redox signaling: From molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10(7):1275–1312. doi: 10.1089/ars.2007.1886. [DOI] [PubMed] [Google Scholar]
- 31.Trebak M, Ginnan R, Singer HA, Jourd’heuil D. Interplay between calcium and reactive oxygen/nitrogen species: An essential paradigm for vascular smooth muscle signaling. Antioxid Redox Signal. 2010;12(5):657–674. doi: 10.1089/ars.2009.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawler JM, Song W. Specificity of antioxidant enzyme inhibition in skeletal muscle to reactive nitrogen species donors. Biochem Biophys Res Commun. 2002;294(5):1093–1100. doi: 10.1016/S0006-291X(02)00602-2. [DOI] [PubMed] [Google Scholar]
- 33.Meissner G, Rios E, Tripathy A, Pasek DA. Regulation of skeletal muscle Ca2+ release channel (ryanodine receptor) by Ca2+ and monovalent cations and anions. J Biol Chem. 1997;272(3):1628–1638. doi: 10.1074/jbc.272.3.1628. [DOI] [PubMed] [Google Scholar]
- 34.Sutko JL, Airey JA, Welch W, Ruest L. The pharmacology of ryanodine and related compounds. Pharmacol Rev. 1997;49(1):53–98. [PubMed] [Google Scholar]
- 35.Mei Y, et al. Stabilization of the skeletal muscle ryanodine receptor ion channel-FKBP12 complex by the 1,4-benzothiazepine derivative S107. PLoS ONE. 2013;8(1):e54208. doi: 10.1371/journal.pone.0054208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brillantes AB, et al. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994;77(4):513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 37.Eu JP, Sun J, Xu L, Stamler JS, Meissner G. The skeletal muscle calcium release channel: Coupled O2 sensor and NO signaling functions. Cell. 2000;102(4):499–509. doi: 10.1016/s0092-8674(00)00054-4. [DOI] [PubMed] [Google Scholar]
- 38.Cheong E, Tumbev V, Stoyanovsky D, Salama G. Effects of pO2 on the activation of skeletal muscle ryanodine receptors by NO: A cautionary note. Cell Calcium. 2005;38(5):481–488. doi: 10.1016/j.ceca.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Xia R, Stangler T, Abramson JJ. Skeletal muscle ryanodine receptor is a redox sensor with a well defined redox potential that is sensitive to channel modulators. J Biol Chem. 2000;275(47):36556–36561. doi: 10.1074/jbc.M007613200. [DOI] [PubMed] [Google Scholar]
- 40.Durham WJ, et al. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell. 2008;133(1):53–65. doi: 10.1016/j.cell.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pouvreau S, Allard B, Berthier C, Jacquemond V. Control of intracellular calcium in the presence of nitric oxide donors in isolated skeletal muscle fibres from mouse. J Physiol. 2004;560(Pt 3):779–794. doi: 10.1113/jphysiol.2004.072397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schapira AH. Mitochondrial diseases. Lancet. 2012;379(9828):1825–1834. doi: 10.1016/S0140-6736(11)61305-6. [DOI] [PubMed] [Google Scholar]
- 43. National Research Council Institute for Laboratory Animal Research (1996) Guide for the Care and Use of Laboratory Animals (National Academies, Washington, DC) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.