Abstract
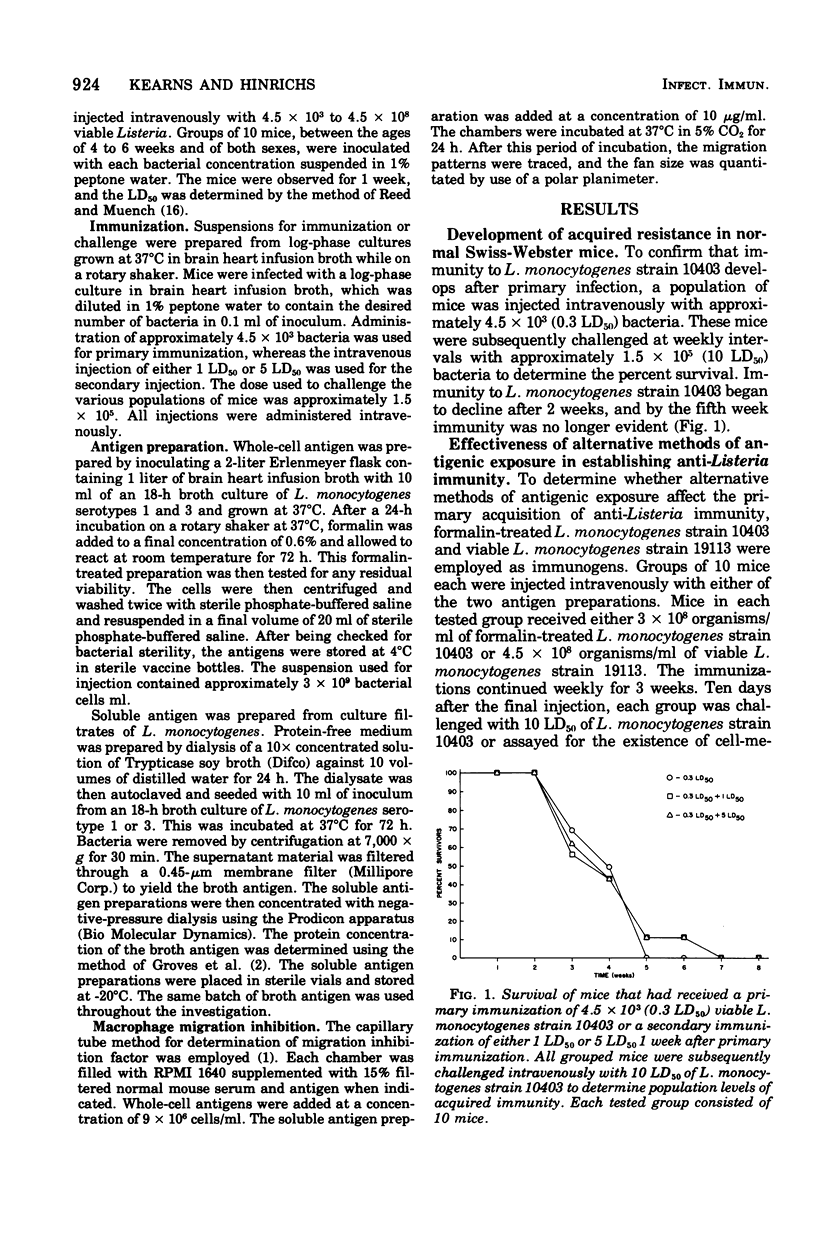
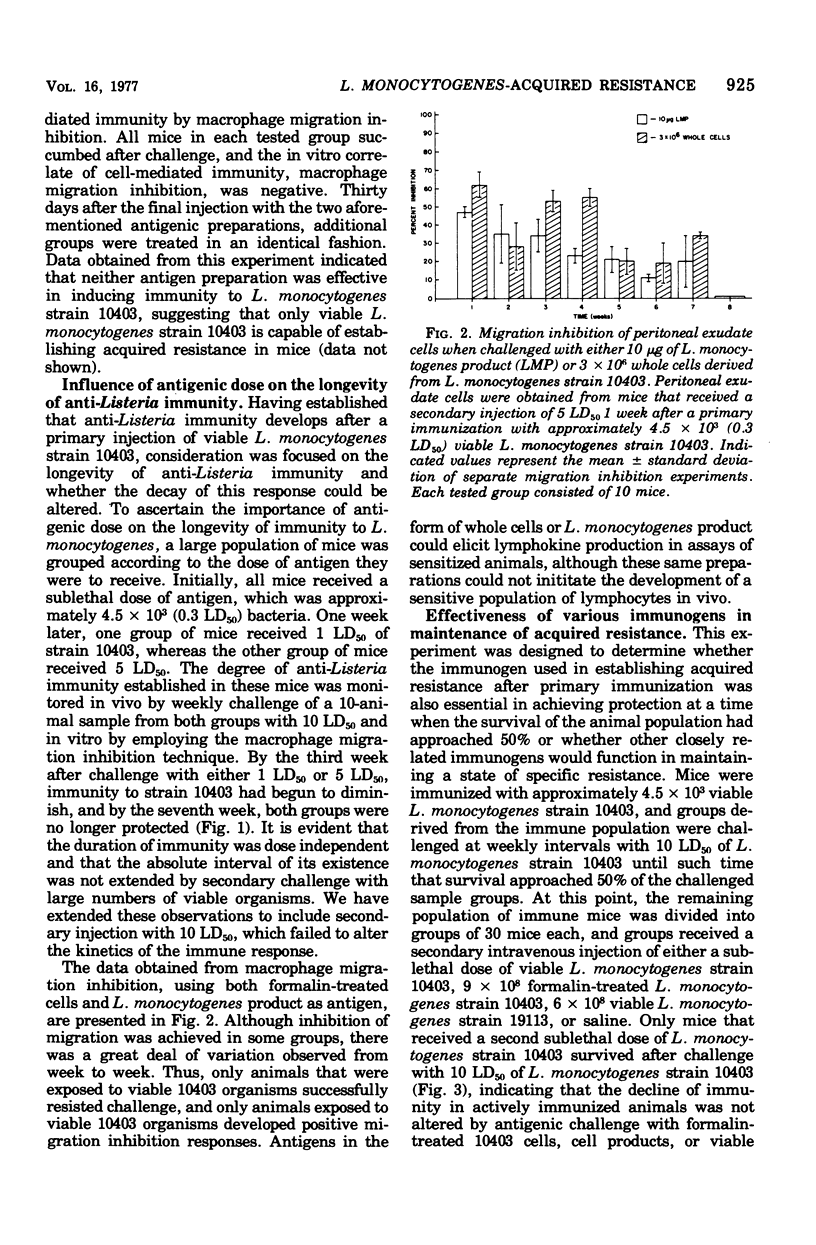
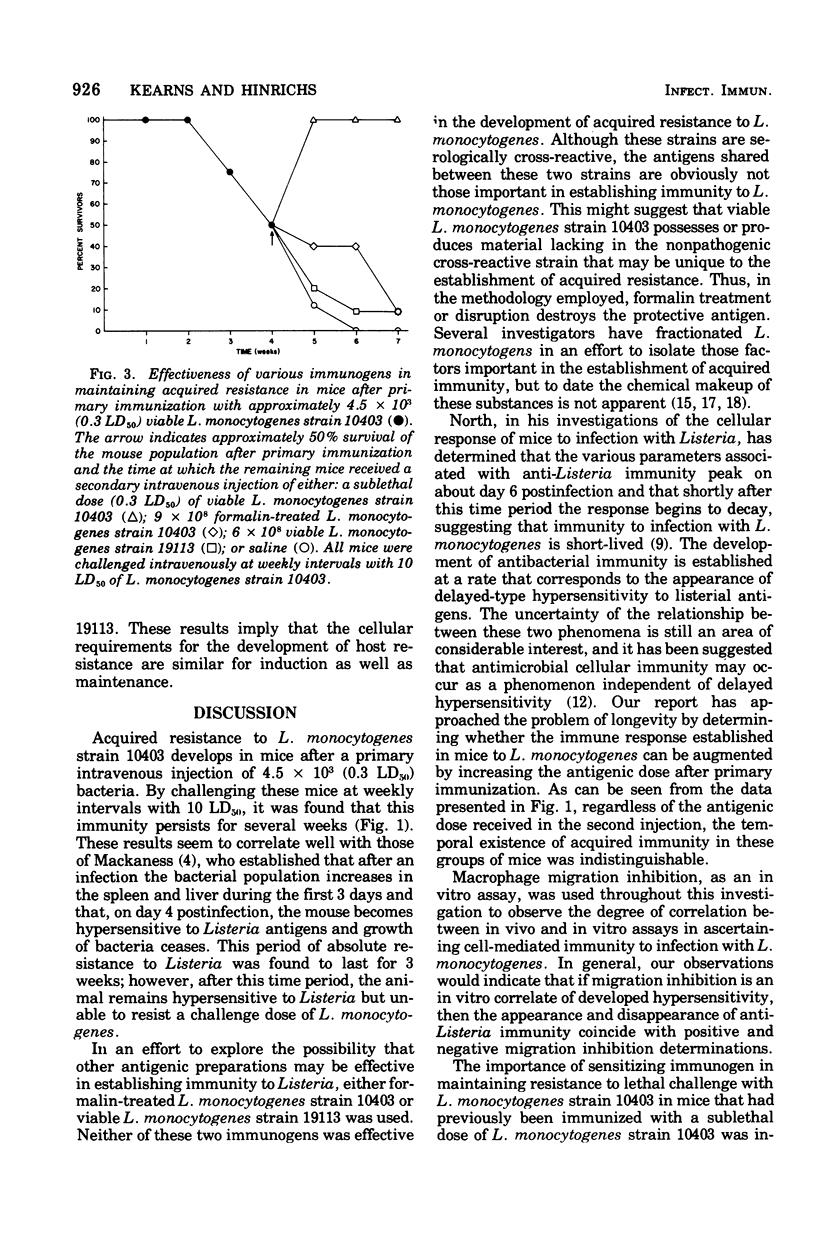
In the mouse system, acquired resistance to Listeria monocytogenes can only be demonstrated after immunization with viable microorganisms. A successful state of immunity cannot be elicited with formalin-killed organisms or bacterial cell-derived products. Viable, serologically cross-reactive organisms (not mouse pathogenic) do not induce a state of immunity as measured by acquired resistance. The duration of immunity, once established, is dose independent, and the absolute interval of its existence is not extended by secondary challenge with large numbers of viable organisms. The decline of immunity in actively immunized animals is not altered by antigenic challenge with formalin-killed cells or cell products. This indicates that the cellular requirements for the development of host resistance are similar for induction as well as maintenance. In vitro measurements of cellular immunity by migration inhibition indicate that formalin-killed organisms as well as cell products were recognized by actively sensitized lymphocytes obtained from immune animals.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVID J. R., AL-ASKARI S., LAWRENCE H. S., THOMAS L. DELAYED HYPERSENSITIVITY IN VITRO. I. THE SPECIFICITY OF INHIBITION OF CELL MIGRATION BY ANTIGENS. J Immunol. 1964 Aug;93:264–273. [PubMed] [Google Scholar]
- Groves W. E., Davis F. C., Jr, Sells B. H. Spectrophotometric determination of microgram quantities of protein without nucleic acid interference. Anal Biochem. 1968 Feb;22(2):195–210. doi: 10.1016/0003-2697(68)90307-2. [DOI] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIKI K., MACKANESS G. B. THE PASSIVE TRANSFER OF ACQUIRED RESISTANCE TO LISTERIA MONOCYTOGENES. J Exp Med. 1964 Jul 1;120:93–103. doi: 10.1084/jem.120.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cellular kinetics associated with the development of acquired cellular resistance. J Exp Med. 1969 Aug 1;130(2):299–314. doi: 10.1084/jem.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Deissler J. F. Nature of "memory" in T-cell mediated antibacterial immunity: cellular parameters that distinguish between the active immune response and a state of "memory". Infect Immun. 1975 Oct;12(4):761–767. doi: 10.1128/iai.12.4.761-767.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Nature of "memory" in T-cell-mediated antibacterial immunity: anamnestic production of mediator T cells. Infect Immun. 1975 Oct;12(4):754–760. doi: 10.1128/iai.12.4.754-760.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osebold J. W., Pearson L. D., Medin N. I. Relationship of antimicrobial cellular immunity to delayed hypersensitivity in Listeriosis. Infect Immun. 1974 Feb;9(2):354–362. doi: 10.1128/iai.9.2.354-362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson L. D., Osebold J. W. Effects of antithymocyte and antimacrophage sera on the survival of mice infected with Listeria monocytogenes. Infect Immun. 1973 Mar;7(3):479–486. doi: 10.1128/iai.7.3.479-486.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson L. D., Osebold J. W. Effects of antithymocyte sera and antimacrophage sera on cell-mediated immune reactions in Listeria-infected mice. Infect Immun. 1974 Jan;9(1):127–133. doi: 10.1128/iai.9.1.127-133.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J. C., Unanue E. R. Effects of bacterial products on lymphocytes and macrophages: their possible role in natural resistance to listeria infetion in mice. J Immunol. 1974 Sep;113(3):984–992. [PubMed] [Google Scholar]
- Rodriguez G. E., McClatchy J. K., Campbell P. A. Induction of Resistance by Listeria monocytogenes Cell Wall Fraction. Infect Immun. 1974 Nov;10(5):1163–1169. doi: 10.1128/iai.10.5.1163-1169.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique I. H., Srivastava K. K. Cell wall and cytoplasmic components of Listeria monocytogenes. Am J Vet Res. 1973 Apr;34(4):567–570. [PubMed] [Google Scholar]


