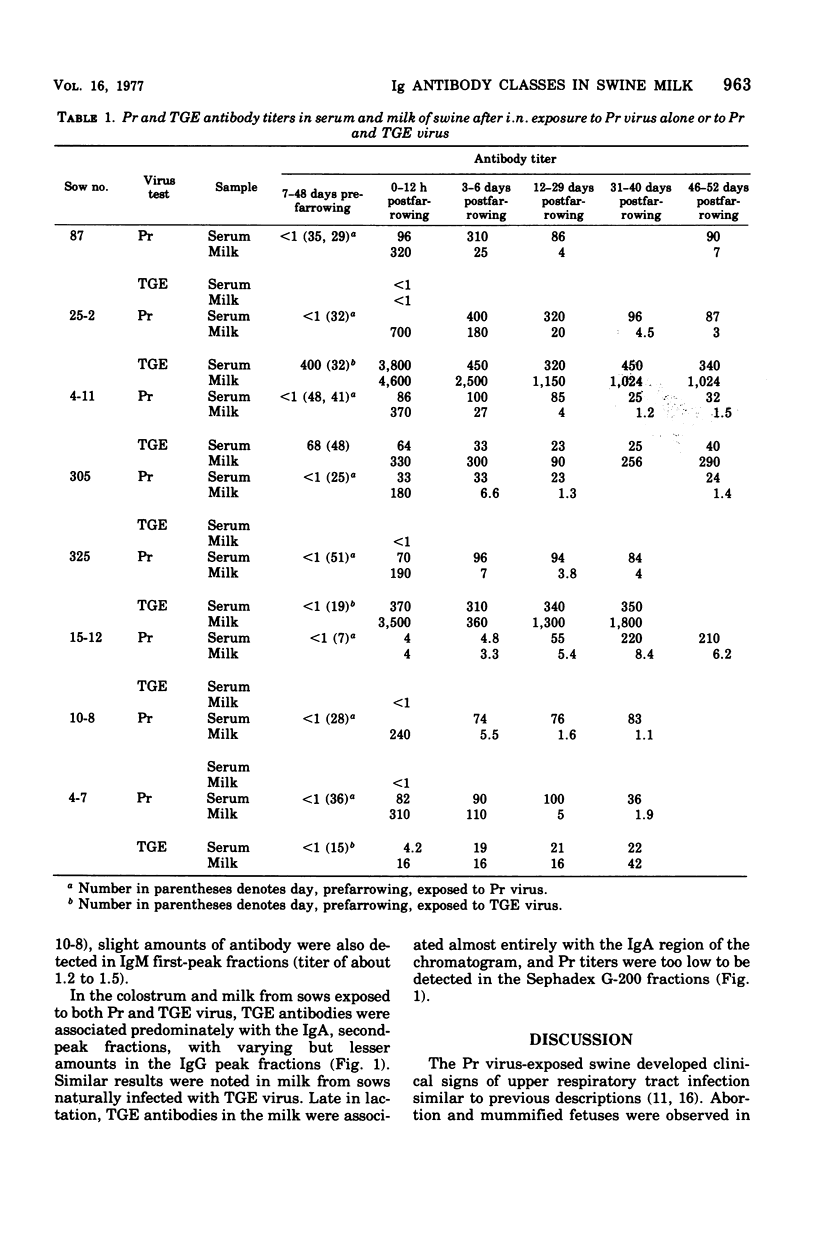
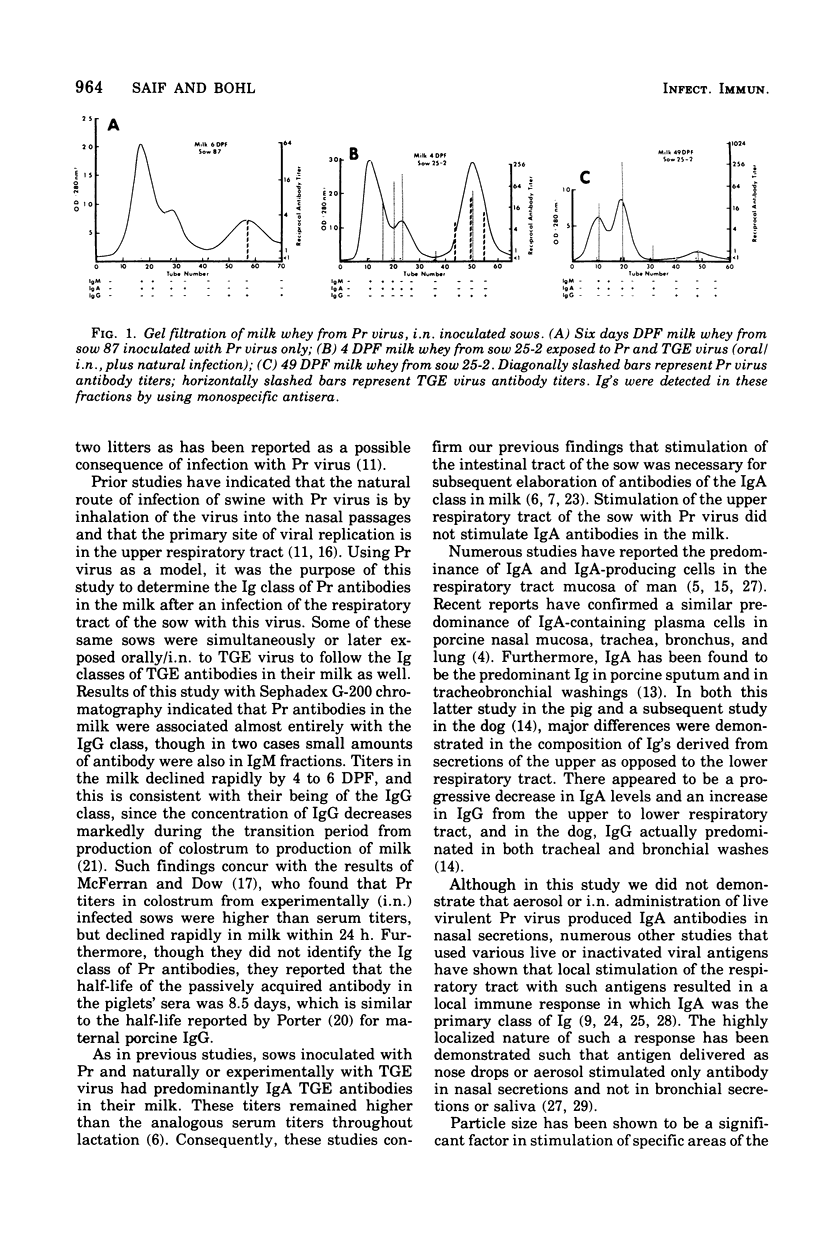
Abstract
Experiments were conducted to evaluate whether infection of the respiratory tract of pregnant swine with pseudorabies (Pr) virus would induce the secretion of immunoglobulin A (IgA) antibodies in their milk as was observed after enteric infection with transmissible gastroenteritis (TGE) virus. The immune response of sows to Pr virus inoculated intranasally and to TGE virus inoculated orally/intranasally or via a natural infection was studied. Emphasis was placed upon titers and Ig classes of Pr and TGE virus-neutralizing antibodies in colostrum and milk. All animals exposed to Pr virus (alone or in combination with TGE virus) developed Pr-neutralizing antibody titers in both serum and milk. Pr antibody titers were generally higher in colostrum than in serum, but the opposite was true in milk compared with serum, with milk titers declining markedly during lactation. In contrast, TGE antibody titers in milk from experimentally or naturally infected sows usually remained higher than the corresponding serum titers and persisted at relatively constant levels throughout lactation. Gel filtration studies of milk indicated that the antibody activity against Pr virus was associated almost entirely with IgG fractions, with small amounts of antibody detectable in IgM fractions in colostrum from two of nine sows. By comparison, TGE antibodies were primarily of the IgA class, with varying but lesser amounts of antibody associated with the IgG class. Such results suggest that viral infection of the intestinal tract of the sow, but not the upper respiratory tract, stimulates the secretion of IgA antibodies in the milk.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlstedt S., Carlsson B., Hanson L. A., Goldblum R. M. Antibody production by human colostral cells. I. Immunoglobulin class, specificity, and quantity. Scand J Immunol. 1975 Sep;4(5-6):535–539. doi: 10.1111/j.1365-3083.1975.tb02659.x. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Bohl E. H., Gupta R. K., Olquin M. V., Saif L. J. Antibody responses in serum, colostrum, and milk of swine after infection or vaccination with transmissible gastroenteritis virus. Infect Immun. 1972 Sep;6(3):289–301. doi: 10.1128/iai.6.3.289-301.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohl E. H., Saif L. J., Gupta R. K., Frederick G. T. Secretory antibodies in milk of swine against transmissible gastroenteritis virus. Adv Exp Med Biol. 1974;45(0):337–342. doi: 10.1007/978-1-4613-4550-3_40. [DOI] [PubMed] [Google Scholar]
- Bohl E. H., Saif L. J. Passive immunity in transmissible gastroenteritis of swine: immunoglobulin characteristics of antibodies in milk after inoculating virus by different routes. Infect Immun. 1975 Jan;11(1):23–32. doi: 10.1128/iai.11.1.23-32.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley P. A., Bourne F. J., Brown P. J. The respiratory tract immune system in the pig. I. Distribution of immunoglobulin-containing cells in the respiratory tract mucosa. Vet Pathol. 1976;13(2):81–89. doi: 10.1177/030098587601300201. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Fjellanger I., Gjeruldsen S. T. Localization of immunoglobulins in human nasal mucosa. Immunochemistry. 1967 Jan;4(1):57–60. doi: 10.1016/0019-2791(67)90197-8. [DOI] [PubMed] [Google Scholar]
- Cate T. R., Rossen R. D., Douglas R. G., Jr, Butler W. T., Couch R. B. The role of nasal secretion and serum antibody in the rhinovirus common cold. Am J Epidemiol. 1966 Sep;84(2):352–363. doi: 10.1093/oxfordjournals.aje.a120648. [DOI] [PubMed] [Google Scholar]
- Goldblum R. M., Ahlstedt S., Carlsson B., Hanson L. A., Jodal U., Lidin-Janson G., Sohl-Akerlund A. Antibody-forming cells in human colostrum after oral immunisation. Nature. 1975 Oct 30;257(5529):797–798. doi: 10.1038/257797a0. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The gut-associated lymphoid system: nature and properties of the large dividing cells. Eur J Immunol. 1974 Jun;4(6):435–443. doi: 10.1002/eji.1830040610. [DOI] [PubMed] [Google Scholar]
- Holmgren N. Immunoglobulins in normal porcine tracheobronchial secretions. Acta Vet Scand. 1973;14(3):366–380. doi: 10.1186/BF03547425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltreider H. B., Chan M. K. The class-specific immunoglobulin composition of fluids obtained from various levels of the canine respiratory tract. J Immunol. 1976 Feb;116(2):423–429. [PubMed] [Google Scholar]
- MCFERRAN J. B., DOW C. THE DISTRIBUTION OF THE VIRUS OF AUJESZKY'S DISEASE (PSEUDORABIES VIRUS) IN EXPERIMENTALLY INFECTED SWINE. Am J Vet Res. 1965 May;26:631–635. [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Prignot J. Studies on the proteins of human bronchial secretions. Biochim Biophys Acta. 1965 Dec 16;111(2):466–478. doi: 10.1016/0304-4165(65)90056-5. [DOI] [PubMed] [Google Scholar]
- McFerran J. B., Dow C. The effect of colostrum derived antibody on mortality and virus excretion following experimental infection of piglets with Aujeszky's disease virus. Res Vet Sci. 1973 Sep;15(2):208–214. [PubMed] [Google Scholar]
- Michalek S. M., McGhee J. R., Mestecky J., Arnold R. R., Bozzo L. Ingestion of Streptococcus mutans induces secretory immunoglobulin A and caries immunity. Science. 1976 Jun 18;192(4245):1238–1240. doi: 10.1126/science.1273589. [DOI] [PubMed] [Google Scholar]
- Montgomery P. C., Cohn J., Lally E. T. The induction and characterization of secretory IgA antibodies. Adv Exp Med Biol. 1974;45(0):453–462. doi: 10.1007/978-1-4613-4550-3_54. [DOI] [PubMed] [Google Scholar]
- Porter P., Noakes D. E., Allen W. D. Secretory IgA and antibodies to Escherichia coli in porcine colostrum and milk and their significance in the alimentary tract of the young pig. Immunology. 1970 Feb;18(2):245–257. [PMC free article] [PubMed] [Google Scholar]
- Porter P. Porcine colostral IgA and IgM antibodies to Escherichia coli and their intestinal absorption by the neonatal piglet. Immunology. 1969 Oct;17(4):617–626. [PMC free article] [PubMed] [Google Scholar]
- Rudzik O., Perey D. Y., Bienenstock J. Differential IgA repopulation after transfer of autologous and allogeneic rabbit Peyer's patch cells. J Immunol. 1975 Jan;114(1 Pt 1):40–44. [PubMed] [Google Scholar]
- Saif L. J., Bohl E. H., Gupta R. K. Isolation of porcine immunoglobulins and determination of the immunoglobulin classes of transmissible gastroenteritis viral antibodies. Infect Immun. 1972 Oct;6(4):600–609. doi: 10.1128/iai.6.4.600-609.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. B., Purcell R. H., Bellanti J. A., Chanock R. M. Protective effect of antibody to parainfluenza type 1 virus. N Engl J Med. 1966 Nov 24;275(21):1145–1152. doi: 10.1056/NEJM196611242752101. [DOI] [PubMed] [Google Scholar]
- Steele E. J., Chaicumpa W., Rowley D. Isolation and biological properties of three classes of rabbit antibody to Vibrio cholerae. J Infect Dis. 1974 Aug;130(2):93–103. doi: 10.1093/infdis/130.2.93. [DOI] [PubMed] [Google Scholar]
- Waldman R. H., Ganguly R. Immunity to infections on secretory surfaces. J Infect Dis. 1974 Oct;130(4):419–440. doi: 10.1093/infdis/130.4.419. [DOI] [PubMed] [Google Scholar]
- Waldman R. H., Mann J. J., Small P. A., Jr Immunization against influenza. Prevention of illness in man by aerosolized inactivated vaccine. JAMA. 1969 Jan 20;207(3):520–524. doi: 10.1001/jama.207.3.520. [DOI] [PubMed] [Google Scholar]
- Waldman R. H., Wood S. H., Torres E. J., Small P. A., Jr Influenza antibody response following aerosal administration of inactivated virus. Am J Epidemiol. 1970 Jun;91(6):574–585. [PubMed] [Google Scholar]


